Modern Chemistry Chapter 13 Ions in Aqueous Solutions








- Slides: 14

Modern Chemistry Chapter 13 • Ions in Aqueous Solutions and Colligative Properties

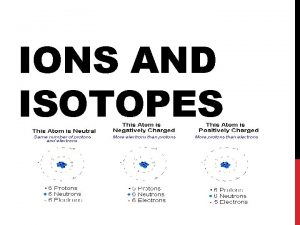
Section 1 Compounds in Aqueous Solutions • dissociation is the separation of ions that occurs when an ionic compound dissolves – see sample problem A on page 436 – do practice problem #1 on page 436

Solubitity • precipitation reactions occur when combinations of ions in a solution have an extremely low solubility and a precipitate forms – see table 1 on page 437 of the textbook for general solubility guidelines

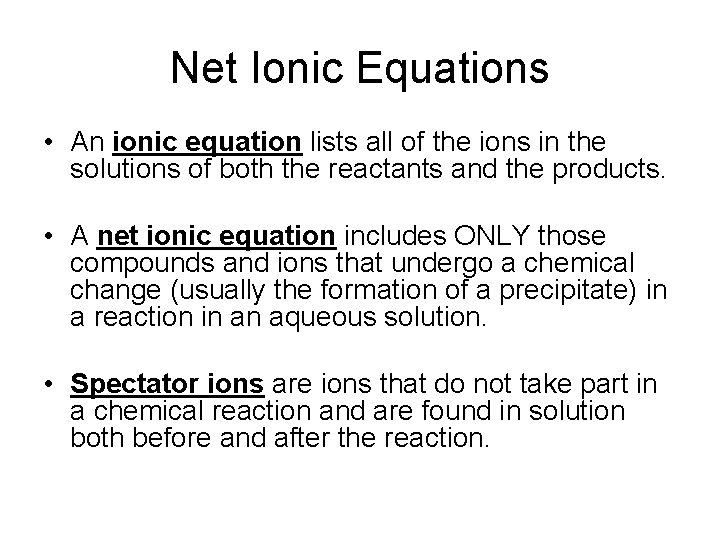
Net Ionic Equations • An ionic equation lists all of the ions in the solutions of both the reactants and the products. • A net ionic equation includes ONLY those compounds and ions that undergo a chemical change (usually the formation of a precipitate) in a reaction in an aqueous solution. • Spectator ions are ions that do not take part in a chemical reaction and are found in solution both before and after the reaction.

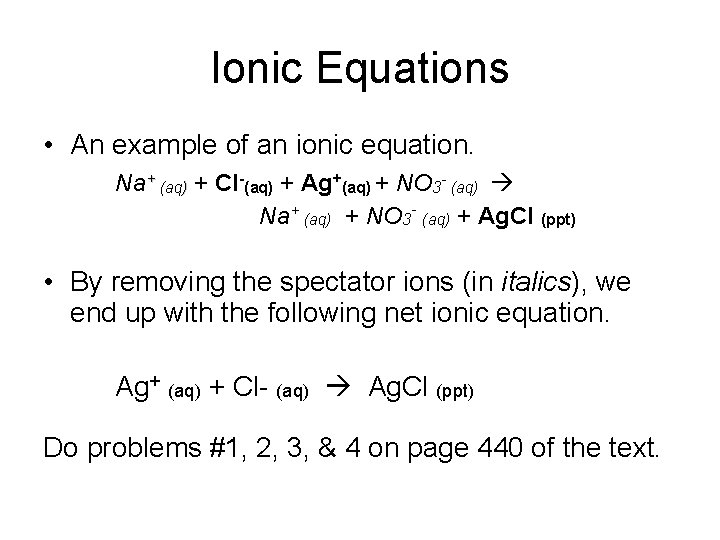
Ionic Equations • An example of an ionic equation. Na+ (aq) + Cl-(aq) + Ag+(aq) + NO 3 - (aq) Na+ (aq) + NO 3 - (aq) + Ag. Cl (ppt) • By removing the spectator ions (in italics), we end up with the following net ionic equation. Ag+ (aq) + Cl- (aq) Ag. Cl (ppt) Do problems #1, 2, 3, & 4 on page 440 of the text.

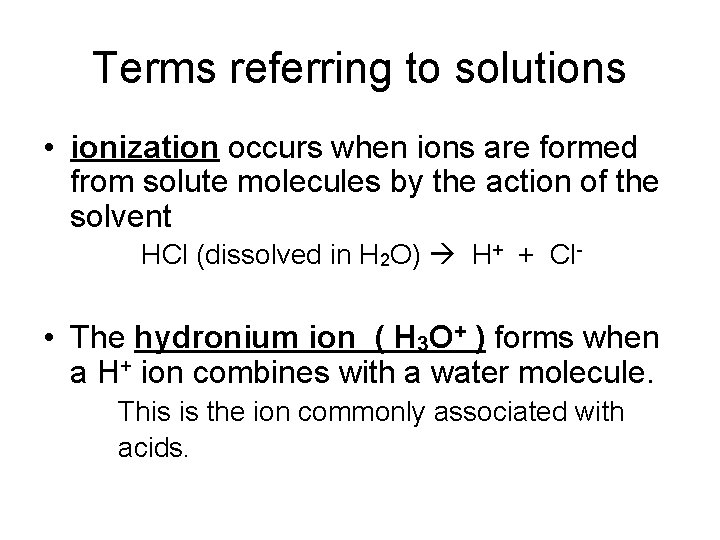
Terms referring to solutions • ionization occurs when ions are formed from solute molecules by the action of the solvent HCl (dissolved in H 2 O) H+ + Cl- • The hydronium ion ( H 3 O+ ) forms when a H+ ion combines with a water molecule. This is the ion commonly associated with acids.

Terms • A strong electrolyte is any compound whose dilute aqueous solutions conduct electricity well due to all or almost all of the dissolved compound forming ions. • A weak electrolyte is any compound whose dilute solutions conduct electricity poorly due to only small amounts of the dissolved compound forming ions. – Do section review questions #1 & 2 on page 443.

Historical Chemistry • Read the Historical Chemistry feature on “The Riddle of Electrolysis”. • Answer the two questions at the end of the feature. • What role might electrolysis play in the production of fuel for hydrogen fuel cells?

Section 2 Colligative Properties • Colligative properties are properties that depend on the concentration of solute particles, not their identity. • A nonvolatile substance is one that has little tendency to become a gas under its existing conditions.

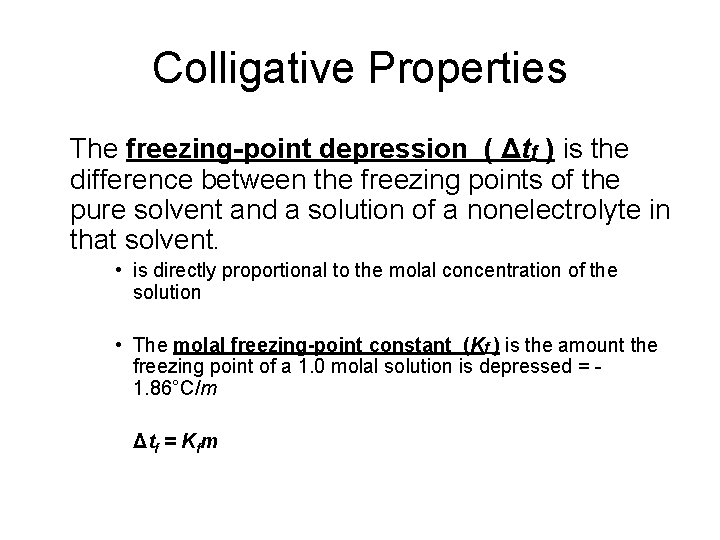
Colligative Properties The freezing-point depression ( Δtf ) is the difference between the freezing points of the pure solvent and a solution of a nonelectrolyte in that solvent. • is directly proportional to the molal concentration of the solution • The molal freezing-point constant (Kf ) is the amount the freezing point of a 1. 0 molal solution is depressed = 1. 86°C/m Δtf = Kfm

Colligative Properties • boiling-point elevation ( Δtb ) is the difference between the boiling points of a pure solvent and a nonelectrolyte solution of that solvent. • molal boiling-point constant ( Kb ) is the boiling point elevation of a solvent in a 1. 0 molal solution of a nonvolatile, nonelectrolyte solute Kb = 0. 51°C/m Do problems #1 -4 on page 451.

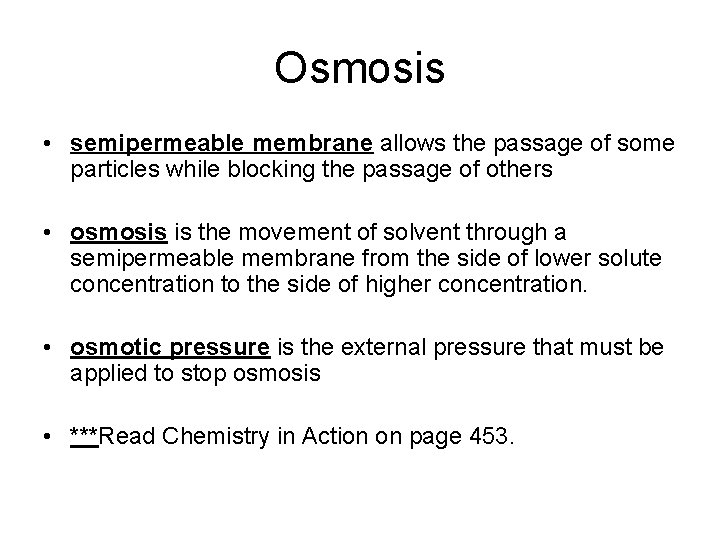
Osmosis • semipermeable membrane allows the passage of some particles while blocking the passage of others • osmosis is the movement of solvent through a semipermeable membrane from the side of lower solute concentration to the side of higher concentration. • osmotic pressure is the external pressure that must be applied to stop osmosis • ***Read Chemistry in Action on page 453.

Electrolytes & Colligative Properties • Because electrolytes dissolve in aqueous solution to produce more than one ion per molecule, electrolytes produce colligative properties that are almost equal to the molality of the dissolved ions. 1 mole Na. Cl 1 mole Na+ + 1 mole Cl- = 2 moles of ions Do section Review problems #1, 3, & 5 on page 456.

Chemistry Chapter 13 Test Review • multiple choice (20) – – – – – relationship of moles of ions to molecules recognize net ionic equations definition of precipitation reaction use solubility guidelines to identify precipitates definitions of dissociation & ionization the hydronium ion formula & its anions definitions of weak & strong electrolytes colligative properties how nonelectrolytes, and nonvolatile solutes affect colligative properties – calculate molality using freezing point depression & molal freezing point constant
 Modern chemistry chapter 13
Modern chemistry chapter 13 Freezing point chapter 13
Freezing point chapter 13 Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Concentrated solution
Concentrated solution Chapter 4 reactions in aqueous solutions worksheet answers
Chapter 4 reactions in aqueous solutions worksheet answers Which solution
Which solution What do the roman numerals in a cation's name indicate?
What do the roman numerals in a cation's name indicate? Modern chemistry solutions
Modern chemistry solutions Reactions in aqueous solutions
Reactions in aqueous solutions Balancing aqueous solutions
Balancing aqueous solutions Aqueous solutions module
Aqueous solutions module Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions General properties of aqueous solutions
General properties of aqueous solutions Aqueous solution practice problems
Aqueous solution practice problems Electrical conductivity of aqueous solutions
Electrical conductivity of aqueous solutions