Chapter 15 Water and Aqueous Systems 1 Section













































- Slides: 64

Chapter 15 Water and Aqueous Systems 1

Section 15. 1 Water and It’s Properties • Objectives – Explain the high surface tension and low vapor pressure of water in terms of the structure of the water molecule and hydrogen bonding 2

Water in Liquid State • The Water Molecule – Water is a simple three atom molecule – Each O-H bond is a highly polar covalent bond because of the high electronegativity of the oxygen – Due to the bent shape, the O-H bond polarities do not cancel. This means water as a whole is polar molecule 3

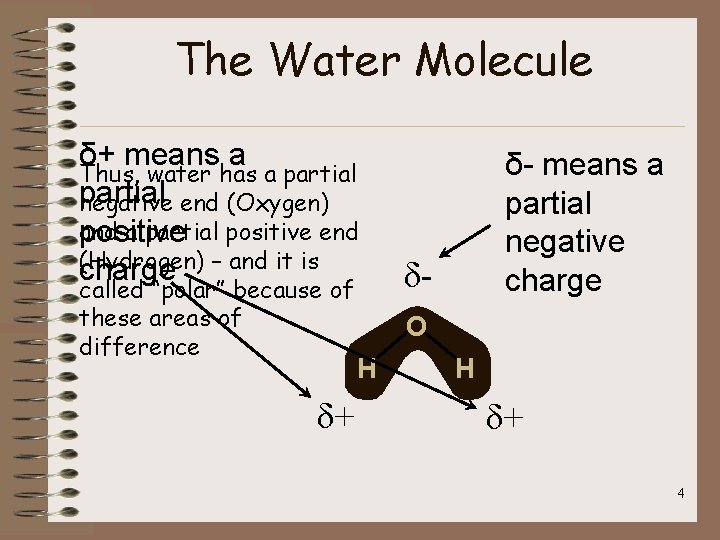
The Water Molecule δ+ means a Thus, water has a partial negative end (Oxygen) and a partial positive end positive (Hydrogen) – and it is charge called “polar” because of these areas of difference δ+ δ- means a partial negative charge δO H H δ+ 4

The Water Molecule • • • – – Water’s bent shape and ability to hydrogen bond gives it many special properties Water molecules are attracted to one another by dipole interactions This hydrogen bonding gives water: High surface tension Low vapor pressure 5

High Surface Tension • Liquid water acts like it has a “skin” – In a glass water bulges over the top • Water forms round drops – Spray water on greasy surface • All because water has ability to form hydrogen bonds 6

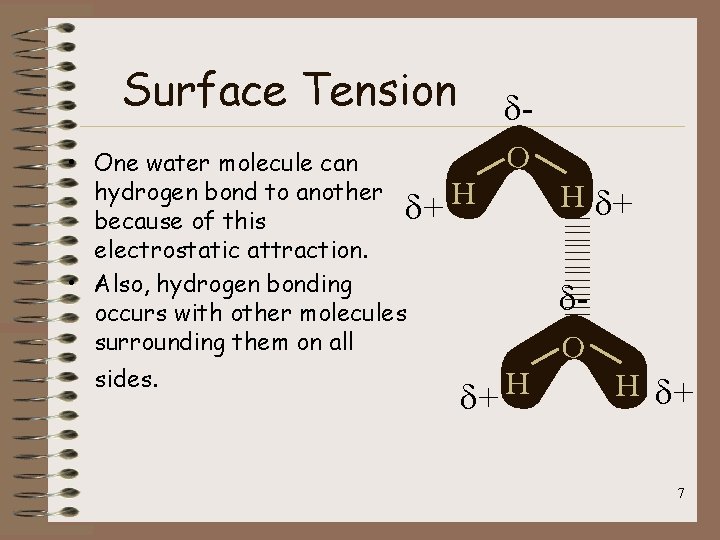
Surface Tension • One water molecule can hydrogen bond to another d+ because of this electrostatic attraction. • Also, hydrogen bonding occurs with other molecules surrounding them on all sides. d. O H H d+ d. O H d+ 7

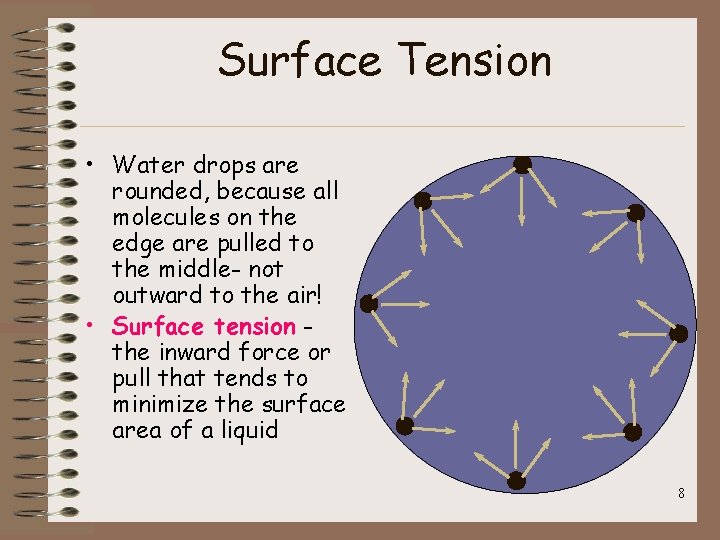
Surface Tension • Water drops are rounded, because all molecules on the edge are pulled to the middle- not outward to the air! • Surface tension the inward force or pull that tends to minimize the surface area of a liquid 8

Low Vapor Pressure • Hydrogen bonding also explains water’s unusually low vapor pressure • Remember vapor pressure of a liquid is the result of molecules escaping from the surface of the liquid and entering the vapor phase – Hold water molecules together, so they do not escape easily – This is a good thing, because lakes and oceans would evaporate very quickly due to their large surface area – REMEMBER REFERENCE TABLE H 9

Section 15. 2 – Homogeneous Aqueous Solutions • Objectives: – Distinguish between a solvent and a solute – Describe what happens in the solution process – Explain why all ionic compounds are electrolytes – Demonstrate how the formula for a hydrate is written 10

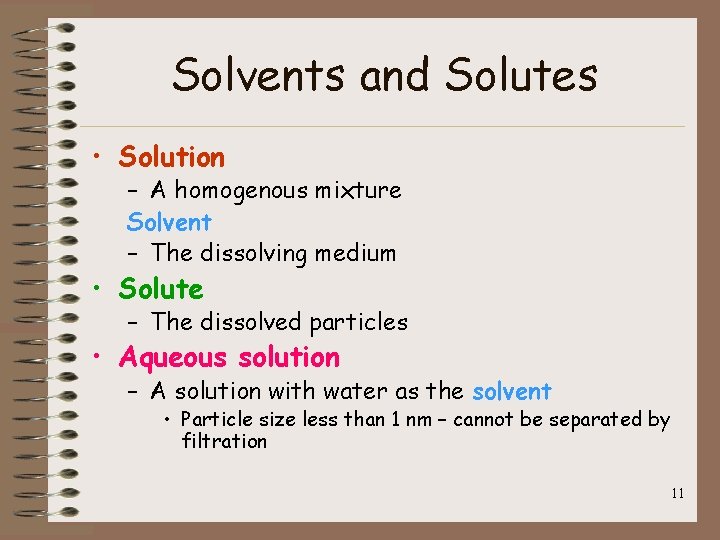
Solvents and Solutes • Solution – A homogenous mixture Solvent – The dissolving medium • Solute – The dissolved particles • Aqueous solution – A solution with water as the solvent • Particle size less than 1 nm – cannot be separated by filtration 11

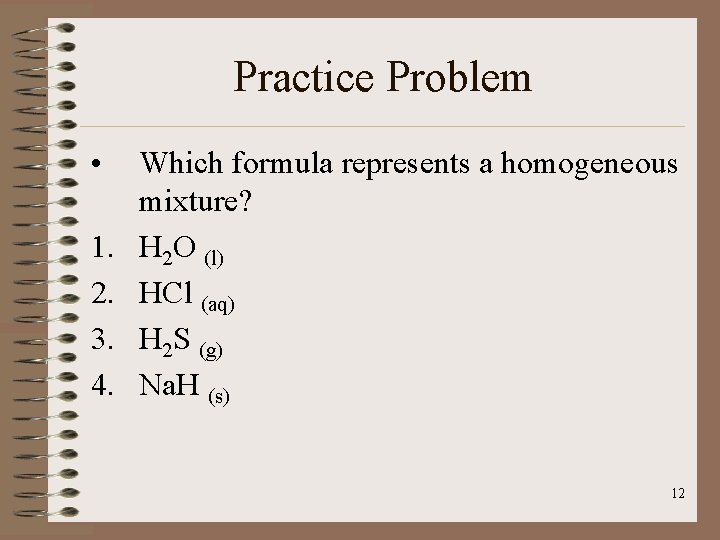
Practice Problem • 1. 2. 3. 4. Which formula represents a homogeneous mixture? H 2 O (l) HCl (aq) H 2 S (g) Na. H (s) 12

Parts of a Solution • Solute – A solute is the dissolved substance in a solution • Salt in salt water • Sugar in soda drinks • Solvent – A solvent is the dissolving medium in a solution • Water in salt water • Water in soda 13

Concentrated vs. Dilute 14

Aqueous Solutions • Water dissolves ionic compounds and polar covalent molecules best – The rule is: likes dissolve likes • Polar dissolves polar • Nonpolar dissolves nonpolar – Because Oil is nonpolar » Oil and water do not mix » Salt is ionic – dissolves in water 15

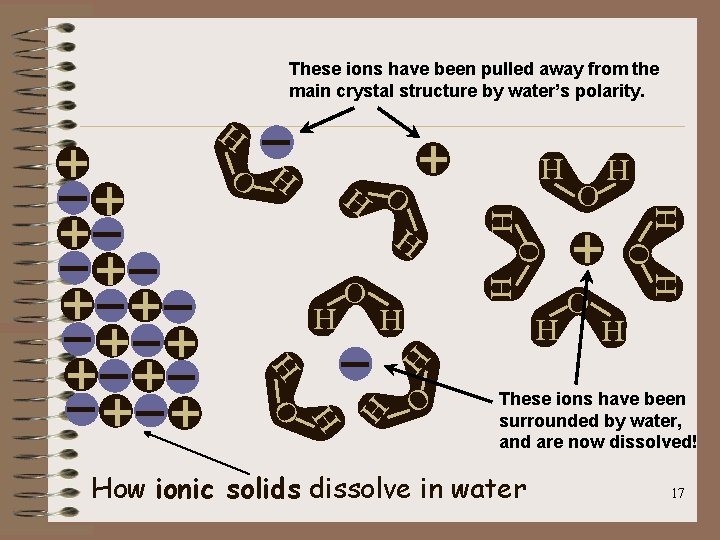
The Solution Process – Water molecule are in continuous motion – When Na. Cl is placed in water, the water molecules collide with the Na. Cl compounds • Water molecules pull at the + and – charged pieces in the ionic compound • As individual Na+ and Cl- ions break away from the crystal, the + and – charged ions become surrounded by the water molecules and thus the solid crystals disappear or dissolves • Figure 15. 7 – page 451 See REFERENCE TABLE F for compounds that will dissolve in Aqueous Solutions 16

These ions have been pulled away from the main crystal structure by water’s polarity. H O H O H H H H O O H H H O H H These ions have been surrounded by water, and are now dissolved! How ionic solids dissolve in water 17

Practice Problem • Which compound is insoluble or will not dissolve in water? 1. 2. 3. 4. KCl. O 3 Ba. SO 4 Na 2 S Ca. Cr. O 4 18

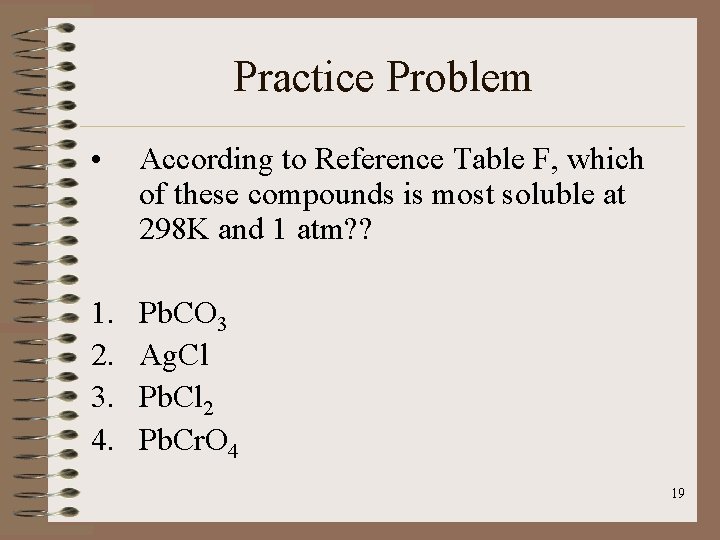
Practice Problem • According to Reference Table F, which of these compounds is most soluble at 298 K and 1 atm? ? 1. 2. 3. 4. Pb. CO 3 Ag. Cl Pb. Cl 2 Pb. Cr. O 4 19

Practice Problem • Which of the following ions when combined with Cl- forms an insoluble substance in water? 1. 2. 3. 4. Fe 2+ Mg 2+ Pb 2+ Zn 2+ 20

Practice Problem • Based on Reference Table F, which of these solutions has the lowest saturated concentration of dissolved ions? 1. 2. 3. 4. Na. Cl(aq) Mg. Cl 2 (aq) Ni. Cl 2(aq) Ag. Cl(aq) 21

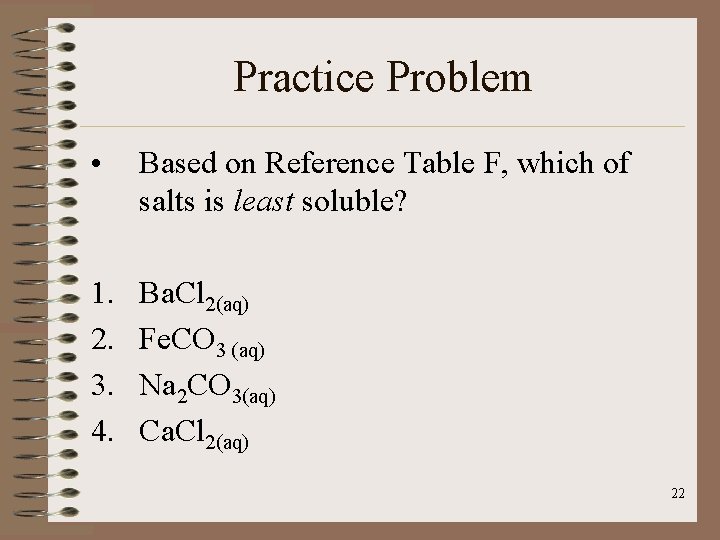
Practice Problem • Based on Reference Table F, which of salts is least soluble? 1. 2. 3. 4. Ba. Cl 2(aq) Fe. CO 3 (aq) Na 2 CO 3(aq) Ca. Cl 2(aq) 22

The Solution Process – Why do some ionic compounds dissolve and others do not? – In some ionic compounds, the attraction between ions is greater than the attraction exerted by water • Ba. SO 4 and Ca. CO 3 – Solids will dissolve ONLY if the attractive force of water molecules is stronger than the attractive force of the ionic compound • If not, the solids are insoluble • Water does not dissolve nonpolar molecules (like oil) • Water has no positive or negative site on a Nonpolar molecule to which it would be attracted. 23

Electrolytes and Nonelectrolytes – Electrolytes • Compounds that conduct an electric current in aqueous solution OR in the molten state – Conduction of electricity requires ions that are mobile and thus able to carry an electrical current – All ionic compounds are electrolytes because they dissociate into ions (also called salts) » Barium sulfate – will conduct when molten, but is insoluble in water • Nonelectrolytes • Do not conduct electricity • Most are molecular materials because they do not have ions • Polar covalent molecules such as alcohols - methanol (CH 3 OH) do not fall apart into ions when they dissolve 24

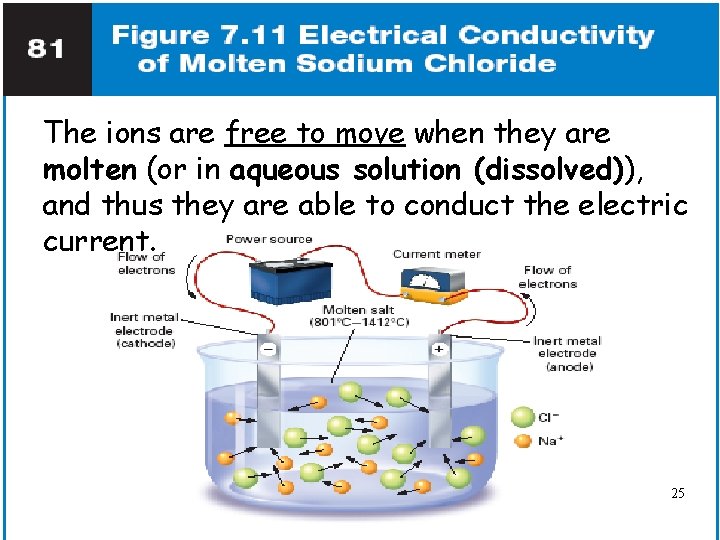
The ions are free to move when they are molten (or in aqueous solution (dissolved)), and thus they are able to conduct the electric current. 25

Chapter 16 Solutions 26

Section 16. 1 Properties of Solutions • Objectives – Identify the factors that determine the rate at which a solute dissolves – Identify the units usually used to express the solubility of a solute – Identify the factors that determine the mass of solute that will dissolve in a given mass of solvent 27

Solution Formation • – Remember: Solutions are homogenous mixtures • Three factors determine the rate of solution formation: 1. Stirring – agitation 2. Surface area of the dissolving particles 3. Temperature 28

Solution Formation • In order to dissolve, the solvent molecules must come in contact with the solute 1. Stirring • Moves the fresh solvent into contact with the solute 2. Surface area • • Smaller pieces increase the amount of surface area of the solute The more surface area of the solute that is exposed the faster the rate of dissolving 29

Solution Formation 3. Temperature • Higher temperature makes the molecules of the solvent move around faster and contact the solute harder and more often • Speeds up dissolving – Higher temperature also usually increases the amount that will dissolve » Exception – gases 30

Solution Formation • Solids tend to dissolve best when – Heated – Stirred – Ground into smaller pieces • Gases tend to dissolve best when – The solution is cold – Pressure is high 31

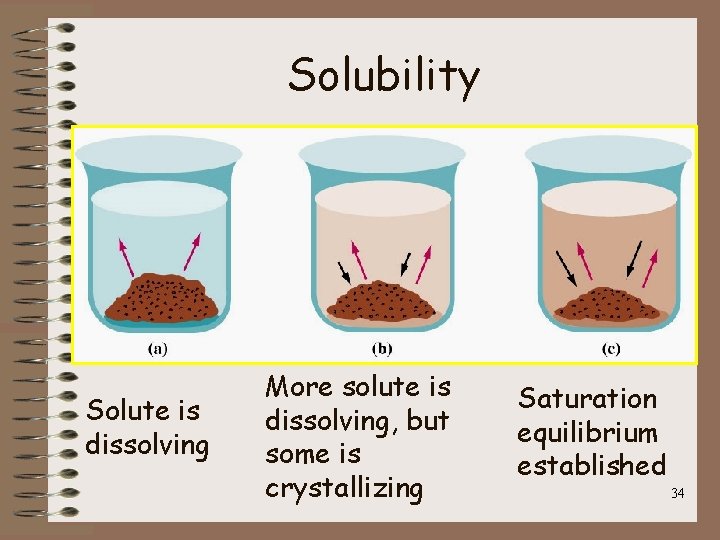
Solubility • How Much? – Solubility • The maximum amount of substance that will dissolve at a specific temperature – If the solvent is water the units can be either: g solute/ 100 g water OR g solute/ 100 ml water – Saturated Solution • Contains the maximum amount of solute dissolved at constant temperature and pressure – Na. Cl = 36. 0 g/100 g of water 32

Solubility – Unsaturated solution • Can still dissolve more solute – Example – 28. 0 g Na. Cl/100 g of water » Can add more solute until the solution is saturated » Once solution is saturated - amount of solute dissolving equals the amount of dissolved solute crystallizing (EQUILIBRIUM) 33

Solubility Solute is dissolving More solute is dissolving, but some is crystallizing Saturation equilibrium established 34

Solubility – Supersaturated • Solution that is holding more than it theoretically can hold at a given temperature – Adding a “seed crystal” will cause crystallization » Seed crystal = very small crystal of the solute » Disruption of the solution causes the crystallization » Example – rock candy » Video on Supersaturated Solution (1: 00) http: //www. youtube. com/watch? v=GTj 8 Zq 901 x. M &feature=related 35

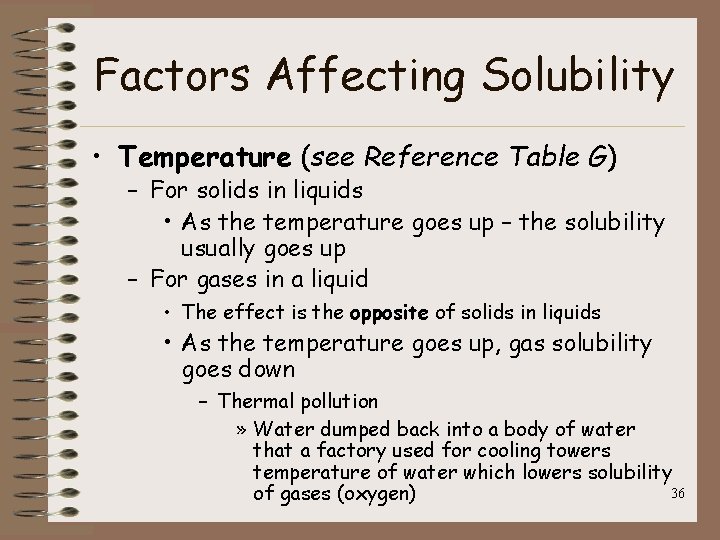
Factors Affecting Solubility • Temperature (see Reference Table G) – For solids in liquids • As the temperature goes up – the solubility usually goes up – For gases in a liquid • The effect is the opposite of solids in liquids • As the temperature goes up, gas solubility goes down – Thermal pollution » Water dumped back into a body of water that a factory used for cooling towers temperature of water which lowers solubility 36 of gases (oxygen)

Practice Problem • 1. 2. 3. 4. If 60 g of KNO 3 (s) is dissolved in 100 ml of water at 50 o. C, the solution will be: Saturated Unsaturated Supersaturated Dilute 37

Practice Problem • 1. 2. 3. 4. According to your Reference Tables, which substance forms an unsaturated solution when 80 grams of the substance is dissolved in 100 grams of H 2) at 10 OC? Na. NO 3 KI KNO 3 Na. Cl 38

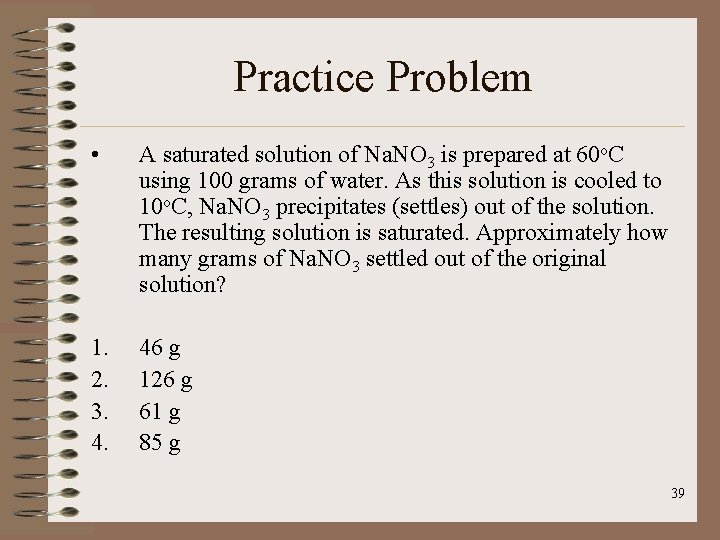
Practice Problem • A saturated solution of Na. NO 3 is prepared at 60 o. C using 100 grams of water. As this solution is cooled to 10 o. C, Na. NO 3 precipitates (settles) out of the solution. The resulting solution is saturated. Approximately how many grams of Na. NO 3 settled out of the original solution? 1. 2. 3. 4. 46 g 126 g 61 g 85 g 39

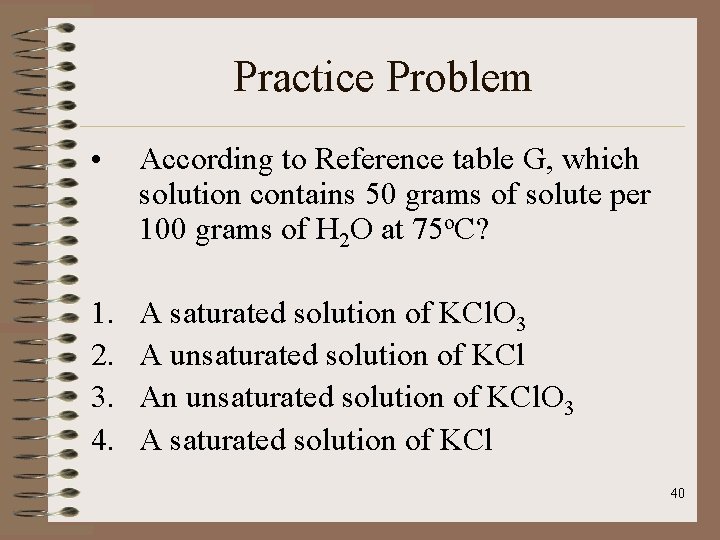
Practice Problem • According to Reference table G, which solution contains 50 grams of solute per 100 grams of H 2 O at 75 o. C? 1. 2. 3. 4. A saturated solution of KCl. O 3 A unsaturated solution of KCl An unsaturated solution of KCl. O 3 A saturated solution of KCl 40

Factors Affecting Solubility • Pressure – Change in pressure has LITTLE effect on solubility of solids and liquids – HOWEVER – STRONGLY influences solubility of gases • Gas solubility increases as pressure of the gas above the solution increases – Example – Soda bottle – removing the lid releases the pressure 41

Practice Problem • A capped bottle of cola contains CO 2 (g) under high pressure. When the cap is removed, how does pressure affect the solubility of the dissolved CO 2 (g)? 42

Section 16. 2 Concentrations of Solution • Objectives: – Solve problems involving the molarity of a solution – Describe the effect of dilution on the total moles of solute in solution – Define percent by volume and percent by mass solutions 43

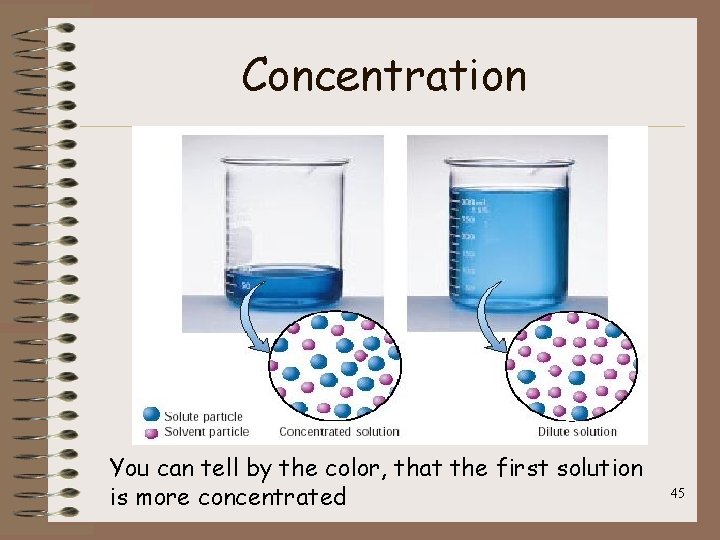
Concentration • Concentrations is a measure of the amount of solute dissolved in a given quantity of solvent – A concentrated solution has a large amount of solute – A dilute solution has a small amount of solute • These are QUALITATIVE descriptions 44

Concentration You can tell by the color, that the first solution is more concentrated 45

Concentrations Affect on Boiling & Freezing Points • • • More concentrated solution will (Boiling Point Elevation) Salt water boils above 100 o. C More concentrated solution will (Freezing Point Depression). increase the boiling point lower the freezing point – Salt water freezes below 0 o. C How much affected depends on the amount of solute and the NUMBER OF DISSOLVED IONS !!!!! – MORE IONS IN A SOLUTE THAN THE GREATER CHANGE IN FREEZING & BOILING 46

- Page 488 Ca. Cl 2 will have three particles in Glucose will only have Na. Cl will have two solution for each one particle in solution particles in solution for each one particle it starts with. 47

Practice Problem • Compared to the freezing point of 1. 0 M KCl(aq) at standard pressure, the freezing point of 1. 0 M Ca. Cl 2 (aq) at standard pressure is 1. Lower 2. Higher 3. The same 48

Practice Problem • When Ethylene glycol (an antifreeze) is added to water, the boiling point of the water 1. 2. 3. 4. Increases, and the freezing point decreases Decreases, and the freezing point increases Decreases, and the freezing point decreases Increases, and the freezing point increases 49

Practice Problem • Which ratio of solute to solvent could be used to prepare a solution with the highest boiling point? 1. 2. 3. 4. 1 g of Na. Cl dissolved per 100 g of water 1 g of Na. Cl dissolved per 1000 g of water 1 g of C 12 H 22 O 11 dissolved per 100 g of water 50

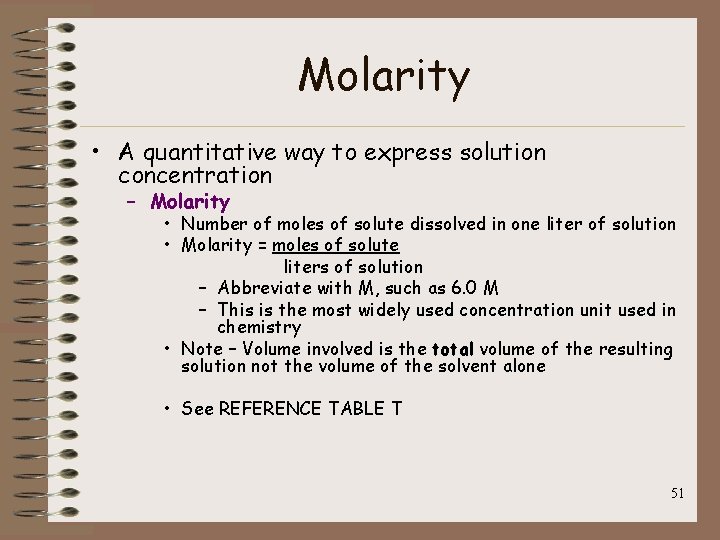
Molarity • A quantitative way to express solution concentration – Molarity • Number of moles of solute dissolved in one liter of solution • Molarity = moles of solute liters of solution – Abbreviate with M, such as 6. 0 M – This is the most widely used concentration unit used in chemistry • Note – Volume involved is the total volume of the resulting solution not the volume of the solvent alone • See REFERENCE TABLE T 51

Practice Problem Household laundry bleach is a dilute solution of sodium hypochlorite (Na. Cl. O). How many moles of solute are present in 1. 5 L of 0. 7 M Na. Cl. O? 52

Practice Problem • Intravenous (IV) saline solutions are often administered to patients in the hospital. One saline solution contains 0. 90 g Na. Cl in exactly 100 m. L of solution. What is the molarity of the solution? 53

Practice Problem • 1. 2. 3. 4. What is the total number of grams of Na. I (s) needed to make 1. 0 Liter of 0. 010 M solution? 1. 5 g 0. 0015 g 0. 15 g 54

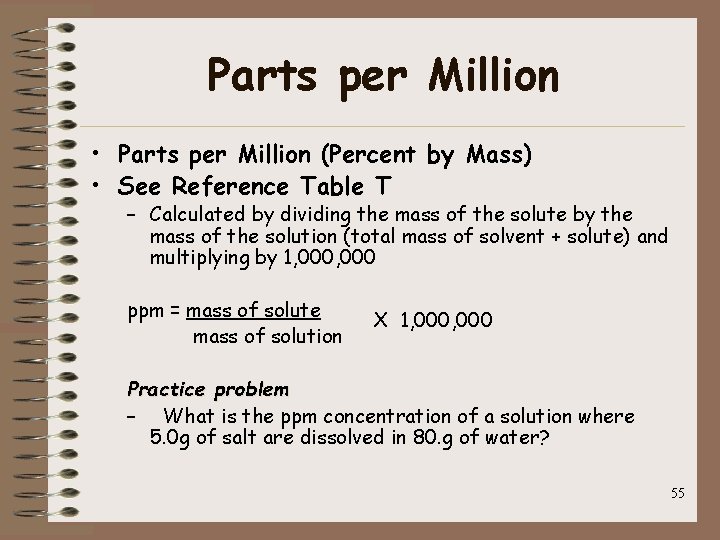
Parts per Million • Parts per Million (Percent by Mass) • See Reference Table T – Calculated by dividing the mass of the solute by the mass of the solution (total mass of solvent + solute) and multiplying by 1, 000 ppm = mass of solute mass of solution X 1, 000 Practice problem – What is the ppm concentration of a solution where 5. 0 g of salt are dissolved in 80. g of water? 55

Practice Problem • 1. 2. 3. 4. What is the concentration of a solution, in parts per million, if 0. 02 grams of Na 3 PO 4 is dissolved in 1000 grams of water? 2 ppm 0. 02 ppm 20 ppm 56

Practice Problem • How many grams of C 6 H 12 O 6 are needed to dissolve in water to make 100 grams of a 250 ppm solution 1. 2. 3. 4. 00 x 105 g 2. 50 x 10 -4 g 4. 00 x 10 -1 g 2. 50 x 10 -2 g 57

Percent Solutions • Parts per million (ppm) – Often used when only small amounts of solute are dissolved in a solvent. – Number of milligrams of solute per liter of solvent ppm = mg L 58

Percent Solutions • Percent solutions are another way to describe the concentration of a solution – Percent of solute in the solvent • Percent solutions can be expressed by – Volume – Mass • Percent means parts per 100 59

Percent Solutions • Percent by Volume – Both solute and solvent are liquids % by volume = volume of solute X 100 volume of solution Practice Problem – What is the percent by volume of ethanol (C 2 H 6 O) in the final solution when 85 m. L of ethanol is diluted to a volume of 250 m. L of water? 60

Percent Solutions • Percent by Mass – Calculated by dividing the mass of the solute by the mass of the solution (total mass of solvent + solute) and multiplying by 100% % mass = mass of solute X 100 mass of solution Practice problem – What is the percent by mass concentration of a solution where 2. 0 g of salt are dissolved in 10. 0 g of water? 61

Websites • • • Eating tea with chopsticks: http: //www. youtube. com/watch? v=7 ob. LT 4 s 2 -HA&NR=1 Greg Olsen drinking water in space: http: //www. youtube. com/watch? v=Fg 1 RMEIP 6 i 4&feature=related Water Balloon in space: http: //www. youtube. com/watch? v=2 Lwf 3 G 5 eiwo&feature=related Drinking Water in Space Video: http: //www. youtube. com/watch? v=Ta 5 zi. JJ 1 ex. M&feature=related Water Balloon in zero gravity (1: 30 sec): http: //www. youtube. com/watch? v=r 7 f. EHYk. Gxd 0&feature=related Fun in Space Video(2: 18 minutes): http: //www. youtube. com/watch? v=co. X 1 u 2_KBs. Q&feature=related Alka Selzer Bubble in Zero Gravity(4: 43 min): http: //www. youtube. com/watch? v=bg. Cocn. TTto&feature=related Apollo 11 Water experiment: http: //www. youtube. com/watch? v=A 19 o 1 Cs 4 WKw Making a water bubble(1: 10 minutes) : http: //www. youtube. com/watch? v=q. Rc. E 5 N 2 BHmg&feature=related 62

Websites • • • Video on Solution and Solvent (1: 37 minutes): http: //www. youtube. com/watch? v=hyd. UVGUbyv. U&feature=related Solution Animation(30 seconds): http: //www. youtube. com/watch? v=HMx. VEpi. M 5 Xw&feature=related Dissolving Salt (47 seconds): http: //www. youtube. com/watch? v=g. N 9 euz 9 jzwc&feature=related Water “to stick or not to stick” (7: 42 minutes): http: //www. youtube. com/watch? v=CT 4 p. URp. Xkb. Y&feature=related Water simulation Video(less than 1 minute): http: //www. youtube. com/watch? v=b. A 3 TNk. ABk 0 k&feature=related World of Chemistry – Water Video link: http: //www. learner. org/resources/series 61. html# 63

Do Now Problem 1. • 2. • 3. • What is meant by the term polarity? Polarity refers to the dipole resulting from electro-negativity differences between covalently bonded atoms What part of the water molecule has a partial negative charge? Oxygen atom What part of the water molecule has a partial positive charge? Each Hydrogen atom 64