QUALITATIVE CHEMICAL ANALYSIS Qualitative Analysis A qualitative characteristic
















- Slides: 17

QUALITATIVE CHEMICAL ANALYSIS

Qualitative Analysis • A qualitative characteristic is a description of something that does not involve numbers or units of measurement. • We will try to identify a substances using characteristic such as colour and solubility.

Ion Colour • The colour of substances can sometimes be used to identify ions or compounds within the substance. • See page 665

CHEMISTRY CSI • You can also use the solubility rules to determine the presence of certain ions in a solution. • Mix your sample that contains the suspected ion with a solution that contains an ion that will form a precipitate with your suspect ion. • Take advantage of the net ionic equation.

EXAMPLE 1 • Lets say you suspect your water sample has acetate in it. What could you do? • If you combine your water sample with a solution that contains silver ions (Ag+) or mercury ions (Hg+) a precipitate will form. • silver acetate/mercury acetate will form which is insoluble – meaning it will form a precipitate. Hg+(aq) + C 2 H 3 O 2 -(aq) Hg. C 2 H 3 O 2 (s) Ag+(aq) + C 2 H 3 O 2 -(aq) Ag. C 2 H 3 O 2 (s)

THE BIG PROBLEM • The major issue that has to be dealt with is that most solutions or samples contain more than one type of ion so chemists must design test procedures to identify and remove any suspected ions one at a time… • You need to find a solution that will react (form a precipitate) with only one of the ions at a time.

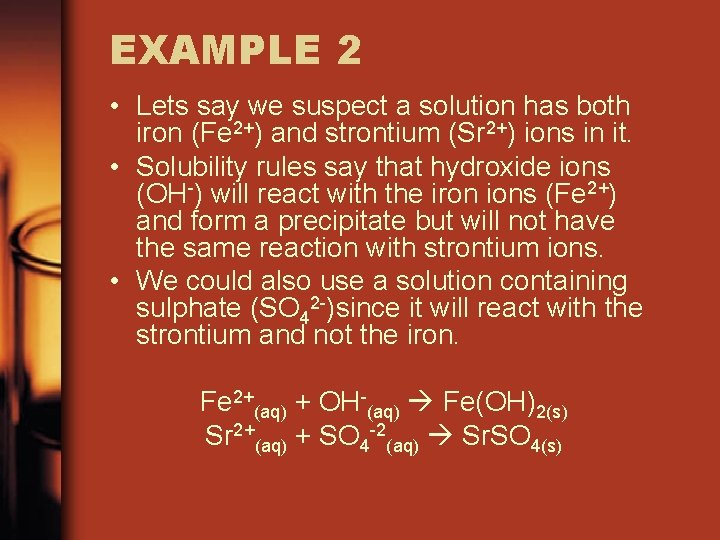
EXAMPLE 2 • Lets say we suspect a solution has both iron (Fe 2+) and strontium (Sr 2+) ions in it. • Solubility rules say that hydroxide ions (OH-) will react with the iron ions (Fe 2+) and form a precipitate but will not have the same reaction with strontium ions. • We could also use a solution containing sulphate (SO 42 -)since it will react with the strontium and not the iron. Fe 2+(aq) + OH-(aq) Fe(OH)2(s) Sr 2+(aq) + SO 4 -2(aq) Sr. SO 4(s)

EXAMPLE 2…Part 2 • To remove the compound from the solution we will filter the solution with the precipitate. The solid compound will be trapped by the filter paper. • The solution passing through the filter paper is called the filtrate and will contain the unreacted ions. We keep this for further testing.

EXAMPLE 2…Part 3 • Now we have the filtrate with the unreacted ions in it…how do we test for the ions? • As before use the appropriate ion to cause precipitation.

Practice Problem • How would you determine if a solution contains ions of silver Ag 1+ (aq) and/or ions of zinc, Zn 2+ (aq) ? • Chloride Cl 1 - and sulphate SO 42 - ions precipitate silver but not zinc. • Add a source of either ion, if a precipitate forms the solution contained silver ions. If no precipitate forms, silver ions were not present. Ag 1+(aq) + Cl 1 -(aq)→ Ag. Cl(s) • Filter out the solid if a positive test.

Practice Problem Cont. • Find an ion that will precipitate out Zn 2+(aq) • Any ion from the bottom of the solubility table will precipitate out Zn 2+(aq) ie. hydroxide ions OH 1 -(aq) • Add a source of the ion and check for precipitation. ppt = ion present, no ppt = ion not present. Zn 2+(aq) + 2 OH 1 -(aq) → Zn(OH)2(s)

Practice • Devise a method to determine if a solution contains the following ions. – strontium and/or manganese 2+ – copper+1 and/or iron 3+ – hydroxide and/or acetate

Home Work • Read section 9. 3 • Page 441 #1 -3, 5, 7, 8

Flame Test For Cations • The flame test is a procedure used in chemistry to detect the presence of certain metal ions, based on each element's characteristic emission spectrum. The color of flames in general also depends on temperature. • The flame test is fast and easy to perform, and does not require special. However, the range of detected elements is small, and the test relies on the subjective experience of the experimenter rather than any objective measurements.

Flame Tests • The test involves introducing a sample of the element or compound to a hot, non-luminous (blue) bunsen flame, and observing the color that results. Samples are usually held on a nichrome wire cleaned with hydrochloric acid to remove traces of previous analytes.

Flame Tests Potassium - Purple Sodium - Yellow Barium - Green Sodium is a common component or contaminant in many compounds and its spectrum tends to dominate over others. Thus the color yellow overpowers the true color. The test flame is often viewed through cobalt blue glass to filter out the yellow of sodium and allow for easier viewing of other metal ions.

Flame Colours • As Arsenic - Blue • B Boron - Bright Green • *Ba Barium - Apple Green • Ca Calcium - Brick Red • Cs Cesium - Pale Violet • Cu(I) Copper(I) - Blue • Cu(II) Copper(II) (nonhalide) - Green • *Cu(II) Copper(II) (halide) -Blue-Green • *Fe Iron - Gold • In Indium - Blue • *K Potassium - Lilac • Li Lithium – Carmine Red Mg Magnesium - Brilliant white Mn(II) Manganese(II) – Yellowish green Mo Molybdenum - Yellowish green *Na Sodium - Intense Yellow P Phosphorus - Pale bluish green Pb Lead - Pale green Rb Rubidium - Pale violet Sb Antimony - Pale green Se Selenium - Azure blue Sr Strontium - Crimson Red Te Tellurium - Pale green Tl Thallium - Pure green Zn Zinc - Bluish Green