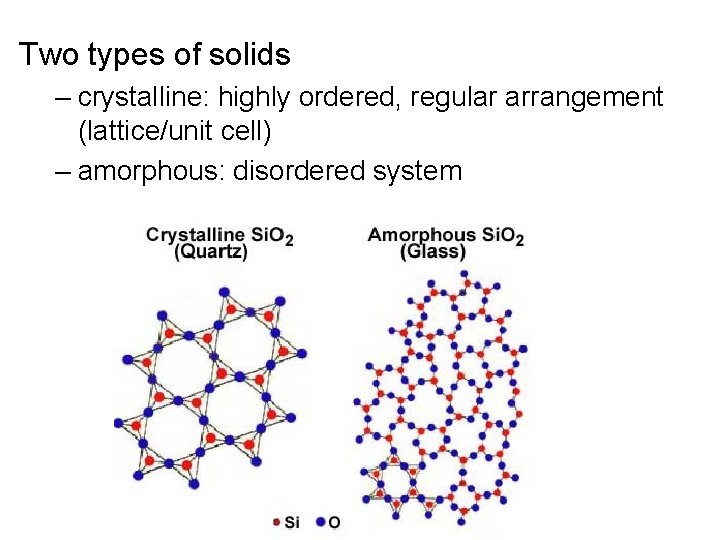
Two types of solids crystalline highly ordered regular







- Slides: 21

Two types of solids – crystalline: highly ordered, regular arrangement (lattice/unit cell) – amorphous: disordered system

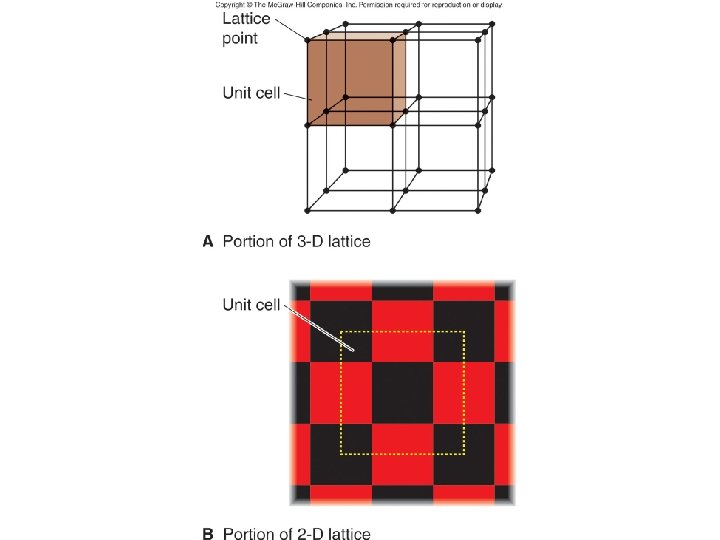
Fig. 12. 26

Components of unit cell/lattice? • At lattice points can have – ions = ionic solid – covalent molecules = molecular solid – atoms = atomic solid • 3 types depending on bonding Metallic solid Network solid Group 8 A solid (noble gas)

Molecular Solids • Molecules along lattice • Mostly covalent (either polar or np) • Intramolecular bonding is stronger than intermolecular – If np, London forces only (S 8, P 4, CO 2 (s)) – If polar, London and dipole-dipole (H 2 O) • Range of IM forces, gives wide range of physical properties (as discussed with liquids)


Ionic Solids • Ions along lattice • Stable, high mp • Packing is done in a way to minimize repulsion and maximize attractive forces – Fixed ion position – Very strong interionic forces – High lattice energies, mp – Low electrical conductivity


Atomic Solids • Atoms along lattice – Metallic solid – Network solid – Group 8 A solid (noble gas)

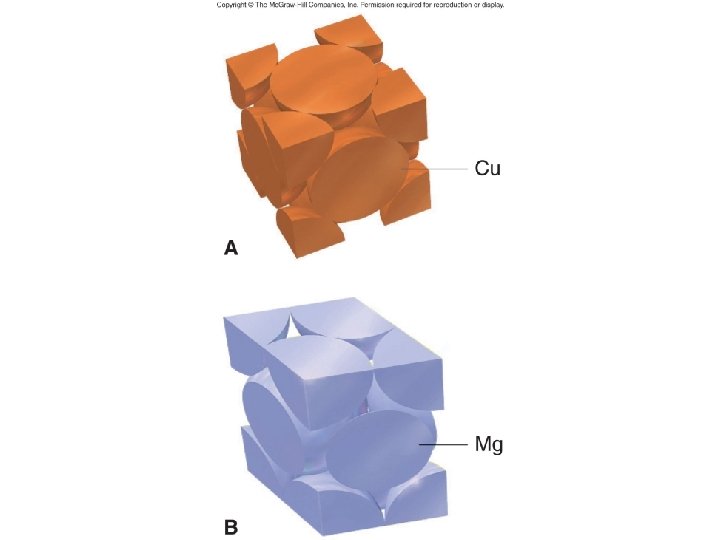
Metallic Solids • Metal along lattice • Pack to minimize empty space – packing efficiency determines things like mp and hardness • Delocalized electrons – high thermal and electrical conductivity – luster – malleability – ductile

Fig. 12. 34

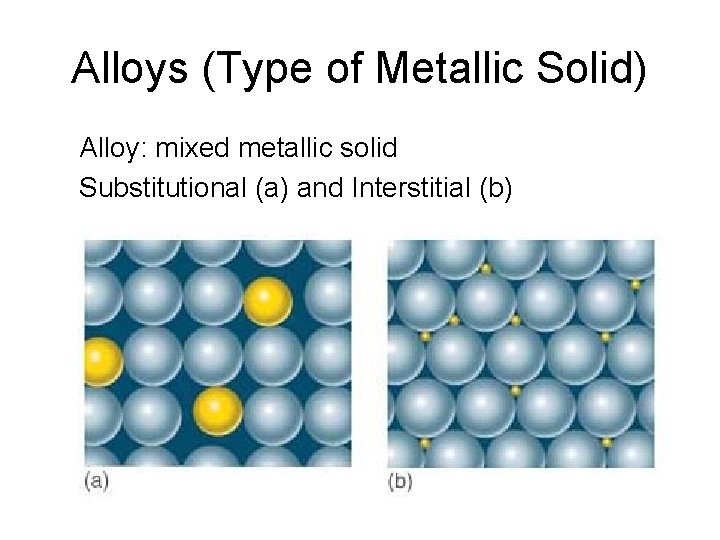
Alloys (Type of Metallic Solid) Alloy: mixed metallic solid Substitutional (a) and Interstitial (b)

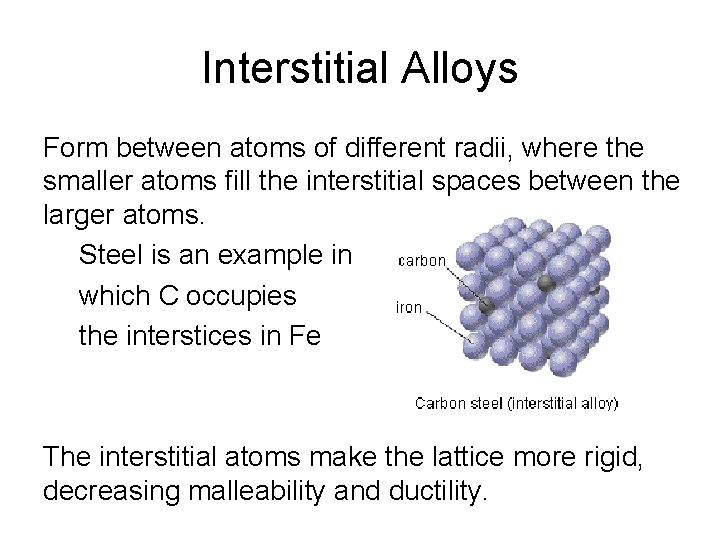
Interstitial Alloys Form between atoms of different radii, where the smaller atoms fill the interstitial spaces between the larger atoms. Steel is an example in which C occupies the interstices in Fe The interstitial atoms make the lattice more rigid, decreasing malleability and ductility.

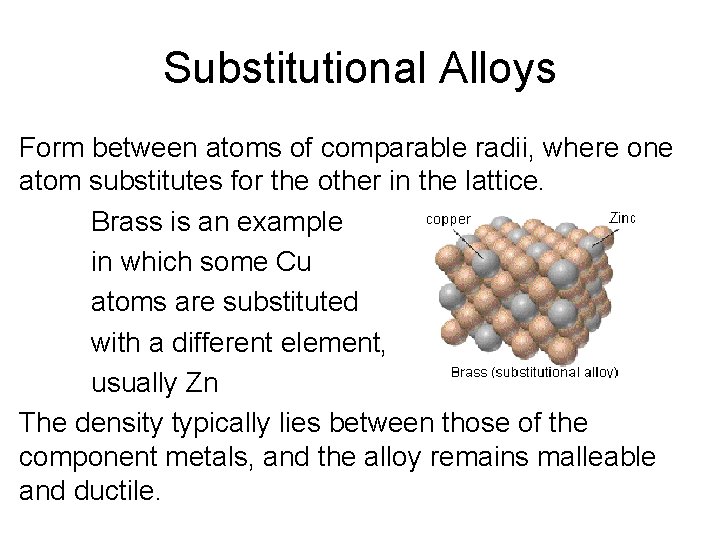
Substitutional Alloys Form between atoms of comparable radii, where one atom substitutes for the other in the lattice. Brass is an example in which some Cu atoms are substituted with a different element, usually Zn The density typically lies between those of the component metals, and the alloy remains malleable and ductile.

Network Solids • In contrast to metallic solids, they – Are brittle – Do not conduct electricity or heat • Classic Examples are solids of – C: diamond and graphite – Si: silica, Si. O 2 (quartz, sand) and silicates, Si. O 4

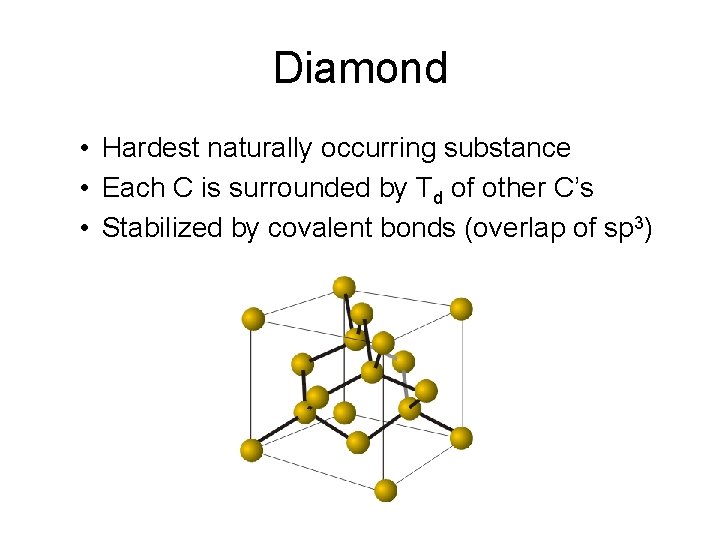
Diamond • Hardest naturally occurring substance • Each C is surrounded by Td of other C’s • Stabilized by covalent bonds (overlap of sp 3)

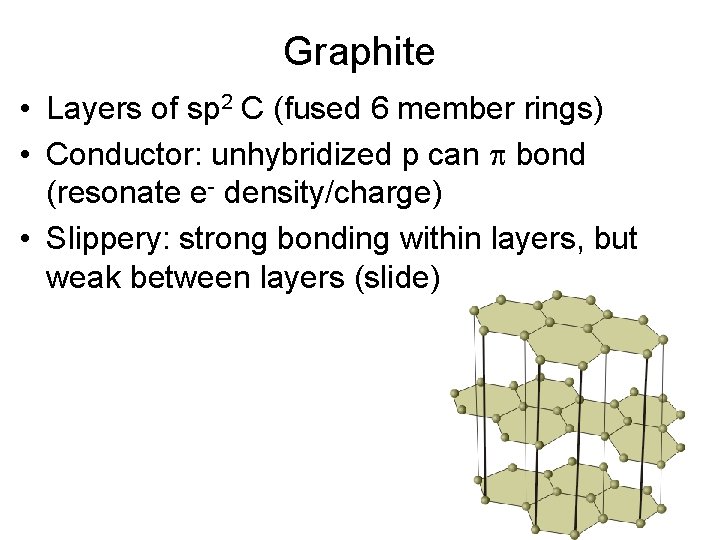
Graphite • Layers of sp 2 C (fused 6 member rings) • Conductor: unhybridized p can bond (resonate e- density/charge) • Slippery: strong bonding within layers, but weak between layers (slide)

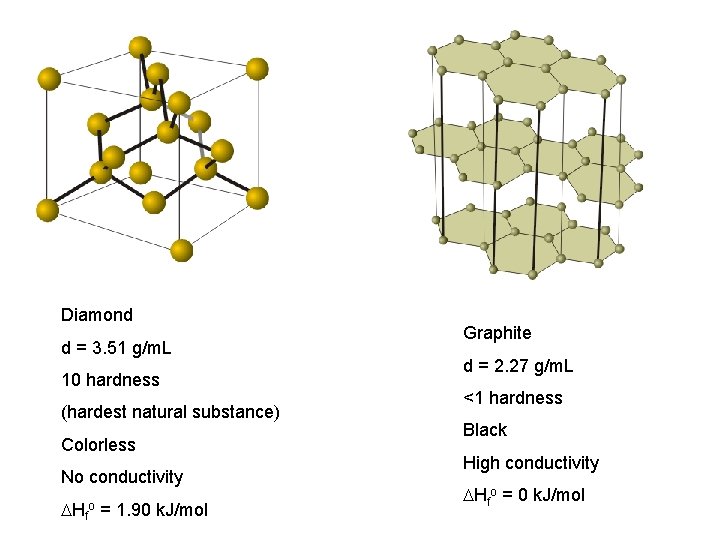
Diamond d = 3. 51 g/m. L 10 hardness (hardest natural substance) Colorless No conductivity Hfo = 1. 90 k. J/mol Graphite d = 2. 27 g/m. L <1 hardness Black High conductivity Hfo = 0 k. J/mol

Conductors and Semiconductors • Solids that have delocalized electrons – Electrons that are free to move between HOMO (valence band) and LUMO (conductance band) • Examples include: – Metallic solids and alloys – Some network solids like graphite – Note: ionic solids are not conductive because the ions are in fixed positions. Only conduct when melted or dissolved in water (ions can move freely) – Molecular solids tend to be nonconductive


Doping • Can dope a semiconductor to make it more conductive • n-type semiconductor: increase #val e– Ex. dope Si with P: extra e- from P enters conductance band lowers E gap • p-type semiconductor: decrease #val e– Ex. dope Si with Ga: Ga has 1 less e-, some of the orbitals in valence band are empty, creates a positive site. Si e- can migrate to these sites

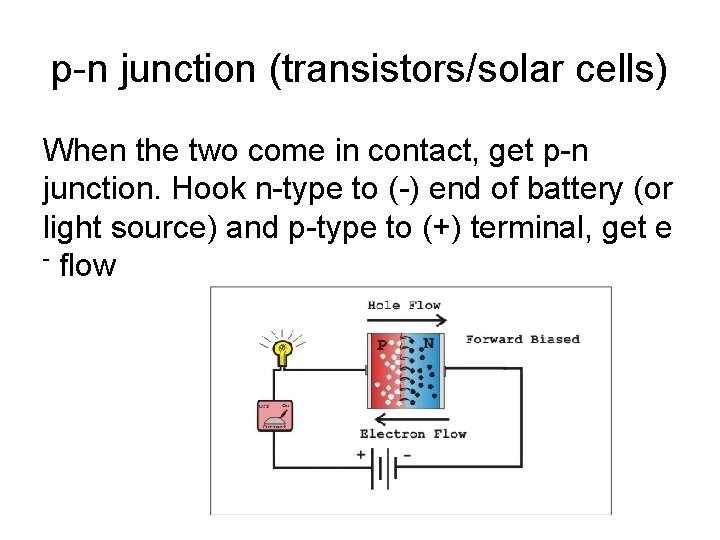
p-n junction (transistors/solar cells) When the two come in contact, get p-n junction. Hook n-type to (-) end of battery (or light source) and p-type to (+) terminal, get e - flow