Chirality in amorphous and crystalline materials experimental aspects














![Scope: The dopants Small molecules, hydrophilic or hydrophobic: Sudan III Biologicals: D-Tryptophan Complexes: [Rh] Scope: The dopants Small molecules, hydrophilic or hydrophobic: Sudan III Biologicals: D-Tryptophan Complexes: [Rh]](https://slidetodoc.com/presentation_image/570d431b16dafa039b2c948c91de9fca/image-41.jpg)









- Slides: 69

Chirality in amorphous and crystalline materials - experimental aspects David Avnir Institute of Chemistry, The Hebrew University Summer School on Chirality Mainz, August, 15 -17, 2011, sponsored by

Main general questions to be addressed: #How is it possible to induce chirality in a material? # How is it possible to extract chiral activity from a material? Our main road: Si. O 2 -based amorphous materials and crystalline metals

Amorphous silica

How is it possible to induce chirality in a material? The classical approach: Attach covalently a chiral molecule to the surface of the (porous) material Often, a silylating reaction

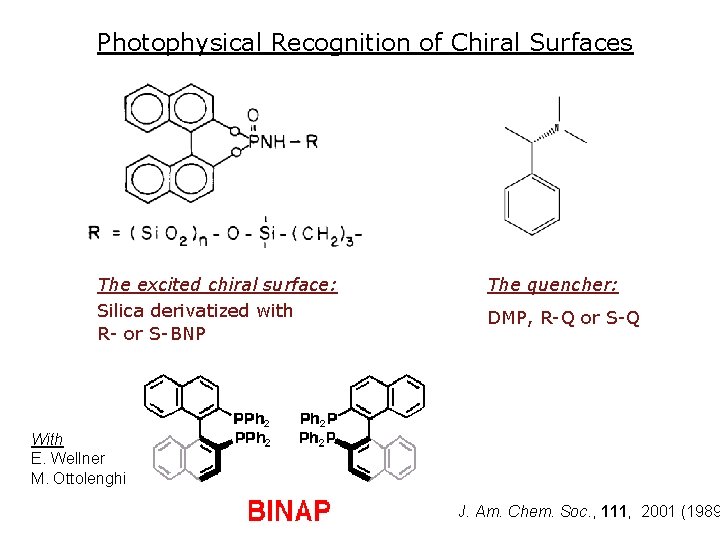
Photophysical Recognition of Chiral Surfaces The excited chiral surface: Silica derivatized with R- or S-BNP The quencher: DMP, R-Q or S-Q With E. Wellner M. Ottolenghi J. Am. Chem. Soc. , 111, 2001 (1989

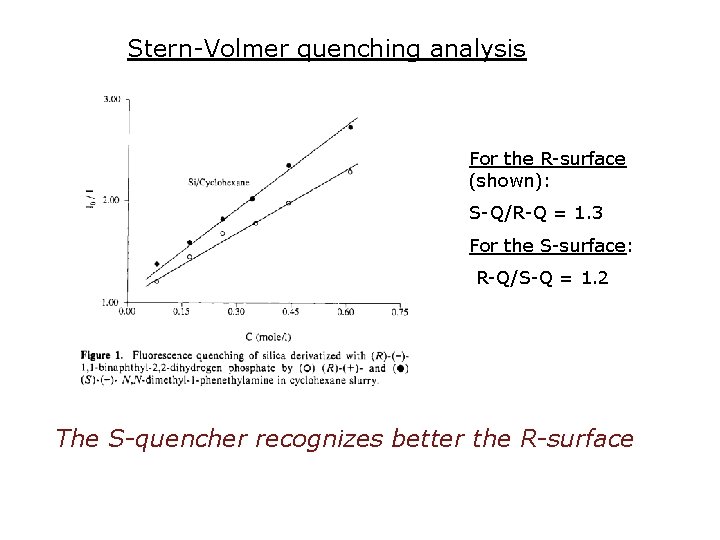
Stern-Volmer quenching analysis For the R-surface (shown): S-Q/R-Q = 1. 3 For the S-surface: R-Q/S-Q = 1. 2 The S-quencher recognizes better the R-surface

The second, newer approach Dope the material with a chiral molecule

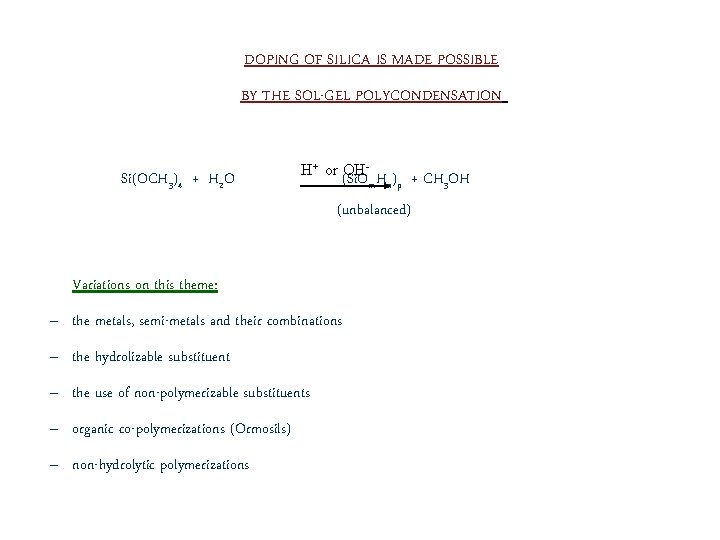
DOPING OF SILICA IS MADE POSSIBLE BY THE SOL-GEL POLYCONDENSATION Si(OCH 3)4 + H 2 O H+ or (Si. O OH- m. Hn)p (unbalanced) Variations on this theme: – the metals, semi-metals and their combinations – the hydrolizable substituent – the use of non-polymerizable substituents – organic co-polymerizations (Ormosils) – non-hydrolytic polymerizations + CH 3 OH

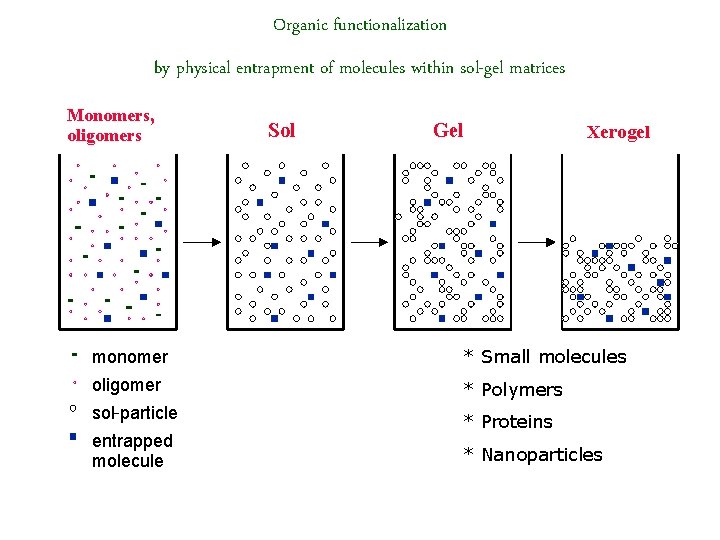
Organic functionalization by physical entrapment of molecules within sol-gel matrices Monomers, oligomers Sol Gel Xerogel monomer * Small molecules oligomer * Polymers sol-particle * Proteins entrapped molecule * Nanoparticles


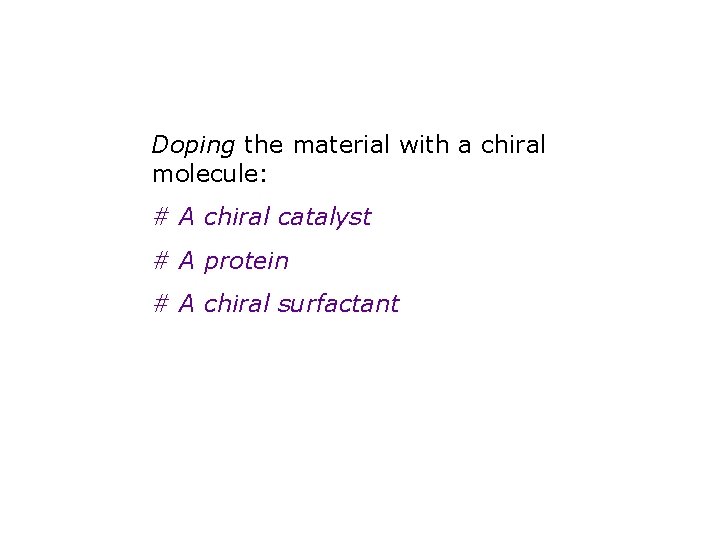
Doping the material with a chiral molecule: # A chiral catalyst # A protein # A chiral surfactant

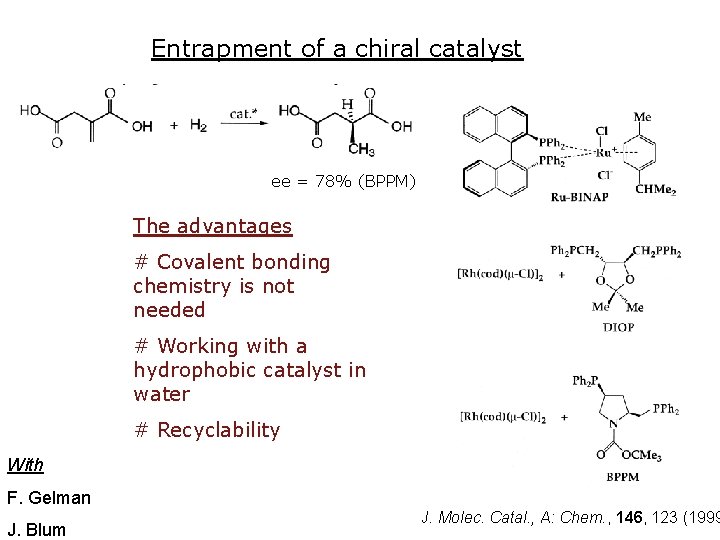
Entrapment of a chiral catalyst ee = 78% (BPPM) The advantages # Covalent bonding chemistry is not needed # Working with a hydrophobic catalyst in water # Recyclability With F. Gelman J. Blum J. Molec. Catal. , A: Chem. , 146, 123 (1999

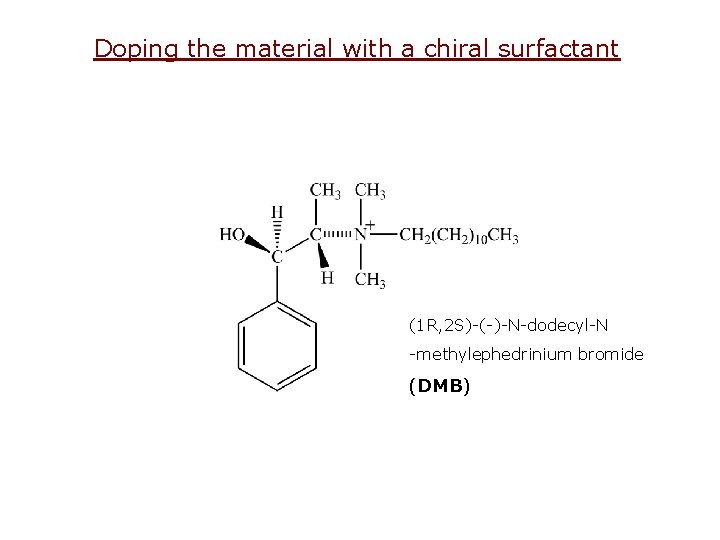
Doping the material with a chiral surfactant (1 R, 2 S)-(-)-N-dodecyl-N -methylephedrinium bromide (DMB)

The experiment: Inducing Circular Dichroism in Congo-Red Within Silica Sol The chiral inducer: DMB The achiral probe: CR

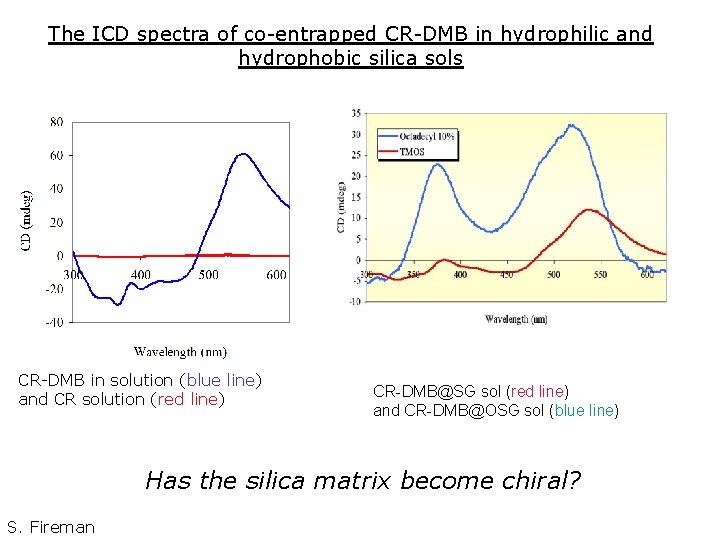
The ICD spectra of co-entrapped CR-DMB in hydrophilic and hydrophobic silica sols CR-DMB in solution (blue line) and CR solution (red line) CR-DMB@SG sol (red line) and CR-DMB@OSG sol (blue line) Has the silica matrix become chiral? S. Fireman

Second experiment with doped surfactant: NMR detection of diastereomeric interactions within phenylated-silica sols and gels S-BINAP 1 R, 2 S-DMB The possible interactions: DMB/S-BINAP With S. Fireman S. Marx DMB/R-BINAP


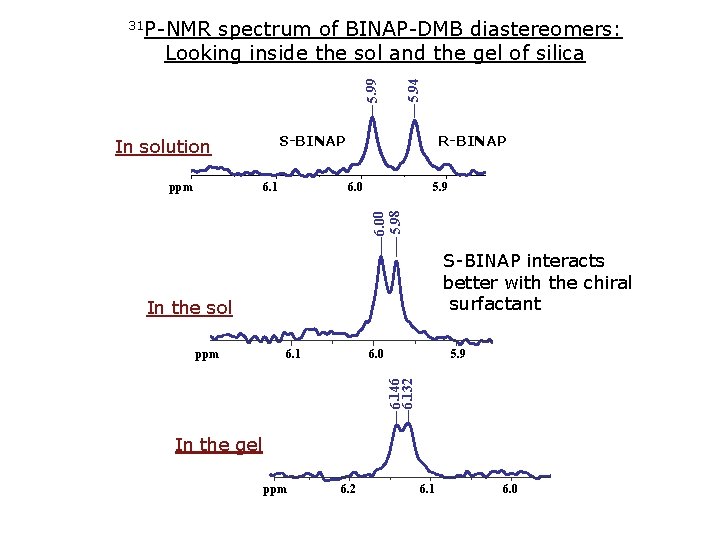
spectrum of BINAP-DMB diastereomers: Looking inside the sol and the gel of silica 5. 99 5. 94 31 P-NMR S-BINAP In solution ppm 6. 1 6. 0 5. 9 5. 8 6. 00 5. 98 ppm R-BINAP S-BINAP interacts better with the chiral surfactant In the sol ppm 6. 1 6. 0 5. 9 5. 8 6. 146 6. 132 ppm In the gel ppm 6. 2 6. 1 6. 0 5. 9 5. 8

What have we seen so far? # Covalent attachment of a chiral molecule # Physical entrapment of a chiral dopant Is it possible to induce structural chirality in a material? Make a hole which is chiral imprint the material; make a chiral silicate skeleton Dickey, 50’s

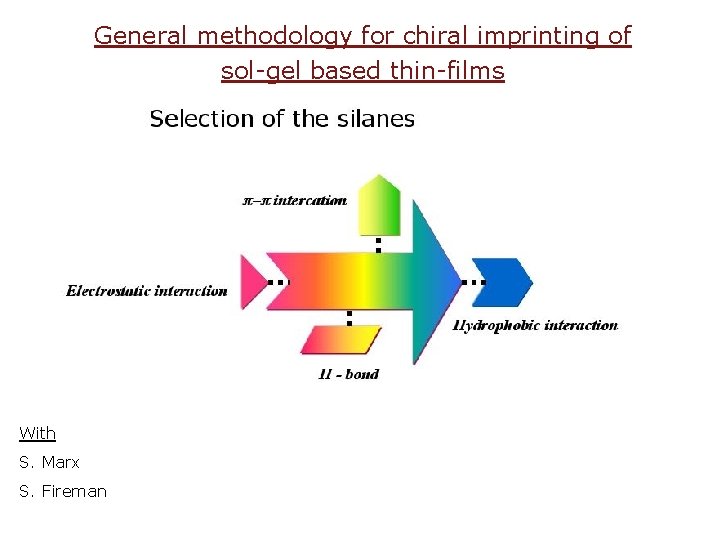
General methodology for chiral imprinting of sol-gel based thin-films With S. Marx S. Fireman

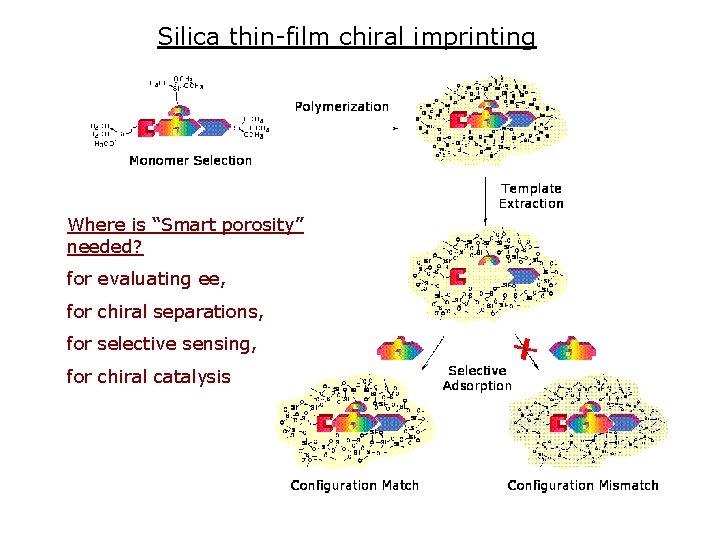
Silica thin-film chiral imprinting Where is “Smart porosity” needed? for evaluating ee, for chiral separations, for selective sensing, for chiral catalysis

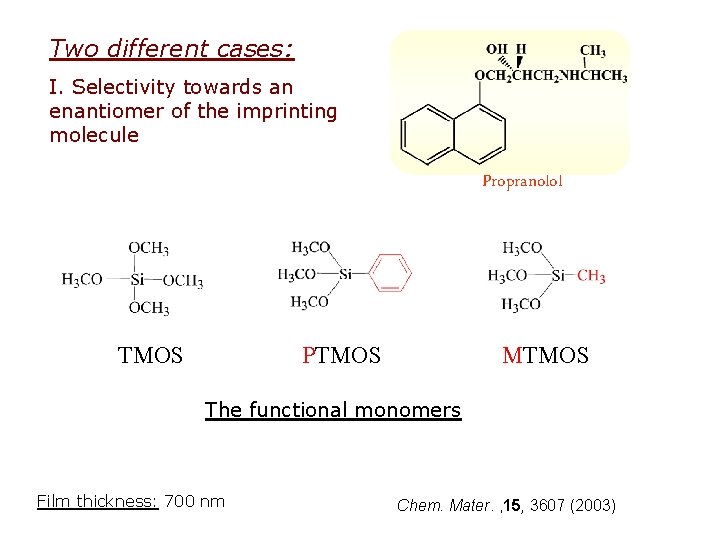
Two different cases: I. Selectivity towards an enantiomer of the imprinting molecule Propranolol TMOS PTMOS MTMOS The functional monomers Film thickness: 700 nm Chem. Mater. , 15, 3607 (2003)

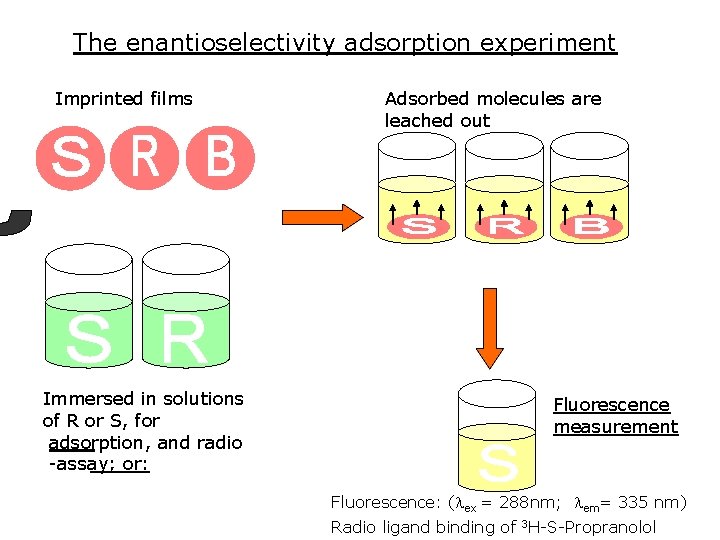
The enantioselectivity adsorption experiment Imprinted films Immersed in solutions of R or S, for adsorption, and radio -assay; or: Adsorbed molecules are leached out Fluorescence measurement Fluorescence: (lex = 288 nm; lem= 335 nm) Radio ligand binding of 3 H-S-Propranolol

Enantioselectivity towards Propranolol enantiomers

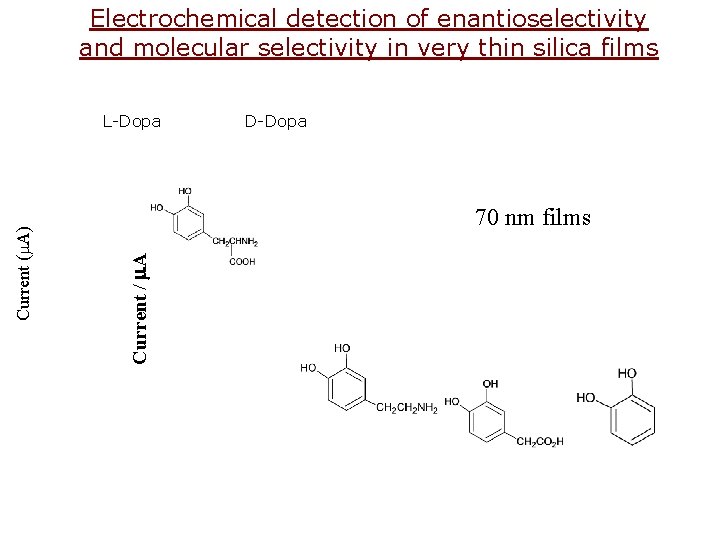
Electrochemical detection of enantioselectivity and molecular selectivity in very thin silica films D-Dopa 70 nm films Current / m. A Current (m. A) L-Dopa

The more general case: Enantioselectivity towards enantiomers of non-imprinting molecules Why is that important? Because a small, recyclable chiral imprinting molecules can be used and reused S. Fireman S. Marx

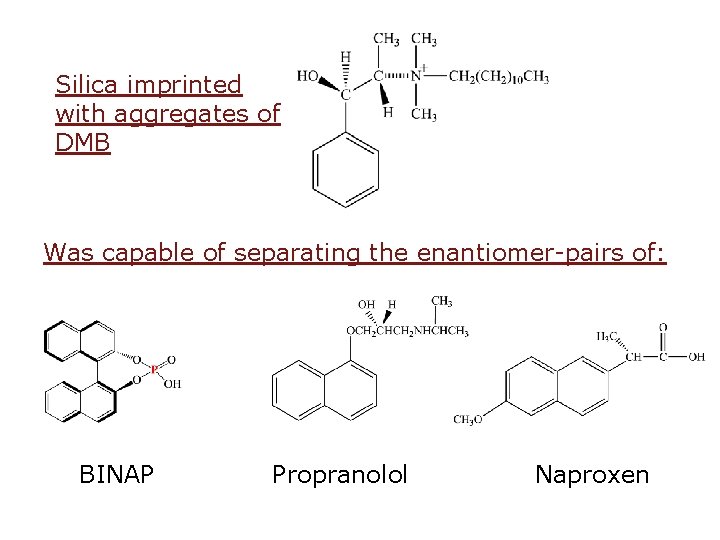
Silica imprinted with aggregates of DMB Was capable of separating the enantiomer-pairs of: BINAP Propranolol Naproxen

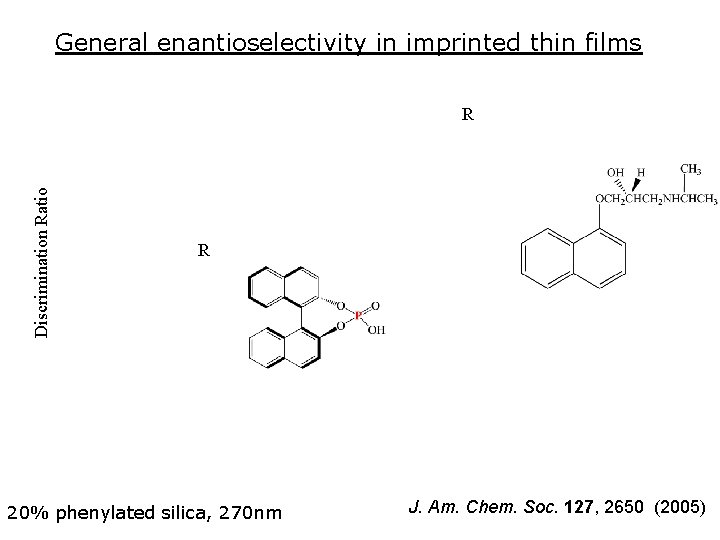
General enantioselectivity in imprinted thin films Discrimination Ratio R R 20% phenylated silica, 270 nm J. Am. Chem. Soc. 127, 2650 (2005)

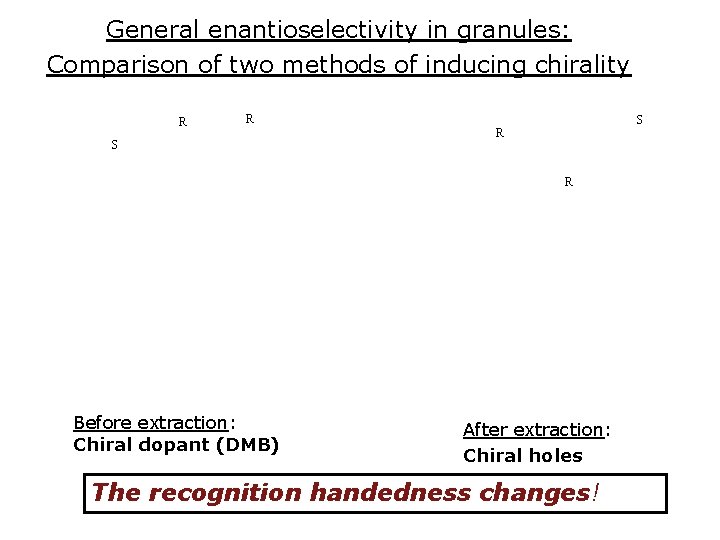
General enantioselectivity in granules: Comparison of two methods of inducing chirality R R S S R R Before extraction: Chiral dopant (DMB) After extraction: Chiral holes The recognition handedness changes!

Next: If an Si. O 2 material is made chiral by a foreign molecule which either remains there or not, then: #How are the building blocks of the material affected? #Is it possible that an Si. O 4 tetrahedron which is neighboring to the chiral event, becomes chiral itself? #Is it possible that the material becomes chiral deeper inside?

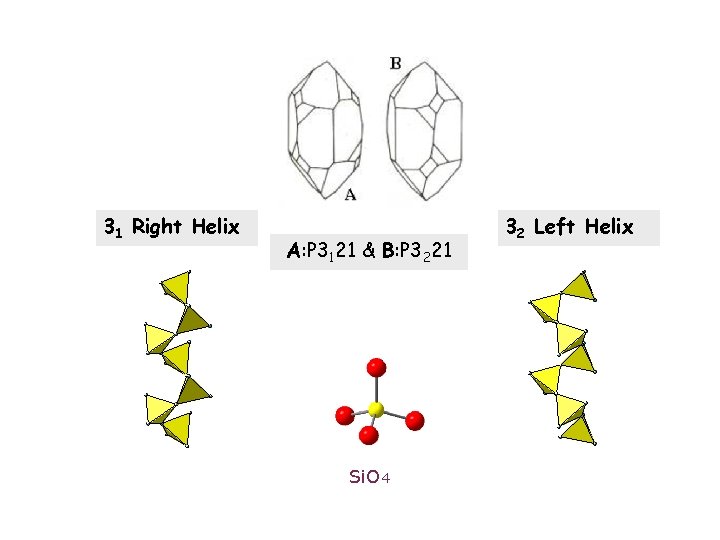
Nature has already provided an answer -Yes, it is possible! Quartz

31 Right Helix A: P 3121 & B: P 3221 Si. O 4 32 Left Helix

Silica is composed of randomly distorted Si. O 4 tetrahedra. Therefore: 1. Each of the chiral Si. O 4 tetrahedra is a single enantiomer event. # A statistically similar counter enantiomer maybe defined. 2. Silica is a racemic mixture of chiral Si. O 4 tetrahedra: # Half comprise a homochiral left-handed set, and half a right-handed set. # This is true for ANY handedness definition; but each definition will divide the set differently into two equal halves.

3. Induction of chirality by any of the methods, will enrich the chiral population of Si. O 4 tetrahedra with one type of handedness.

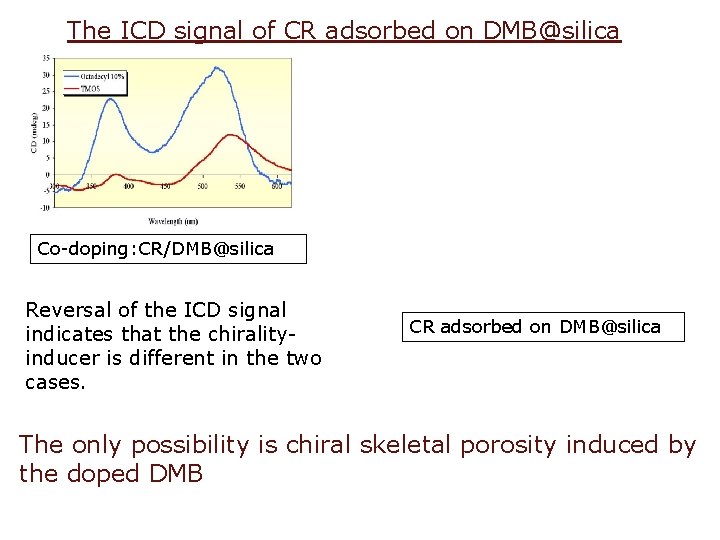
The ICD signal of CR adsorbed on DMB@silica Co-doping: CR/DMB@silica Reversal of the ICD signal indicates that the chiralityinducer is different in the two cases. CR adsorbed on DMB@silica The only possibility is chiral skeletal porosity induced by the doped DMB

Inducing chirality in metals

Motivation: Why should one dope metals with organic molecules? * Hybrid materials of metals and organics have been unknown * Most elements are metals * Metals are everywhere – any new methodology of affecting their properties is interesting * The library of organic compounds is huge; the number of metals is small * Placing a molecule in a sea of electrons may affect its properties; and the properties of the metal * Synergetic effects between the metal and the dopant may emerge

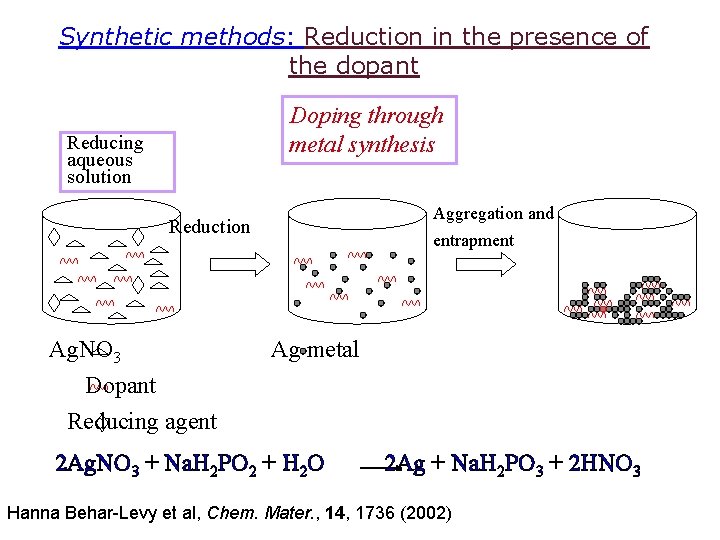
Synthetic methods: Reduction in the presence of the dopant Doping through metal synthesis Reducing aqueous solution Aggregation and Reduction Ag. NO 3 entrapment Ag metal Dopant Reducing agent 2 Ag. NO 3 + Na. H 2 PO 2 + H 2 O 2 Ag + Na. H 2 PO 3 + 2 HNO 3 Hanna Behar-Levy et al, Chem. Mater. , 14, 1736 (2002)

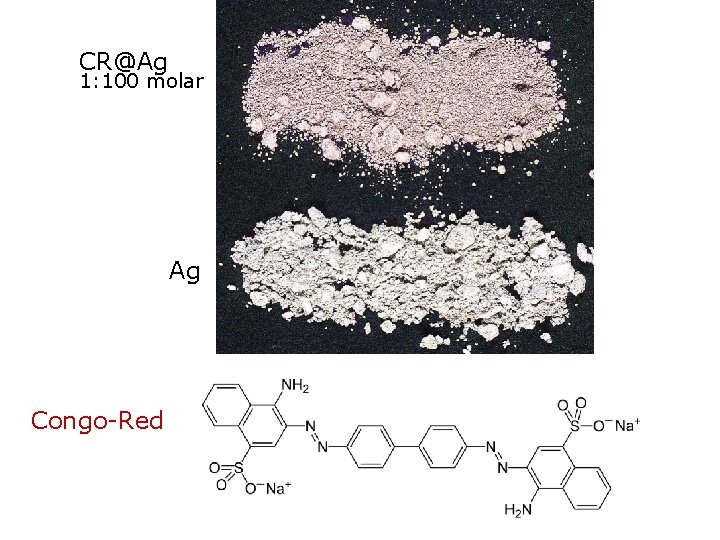
CR@Ag 1: 100 molar Ag Congo-Red

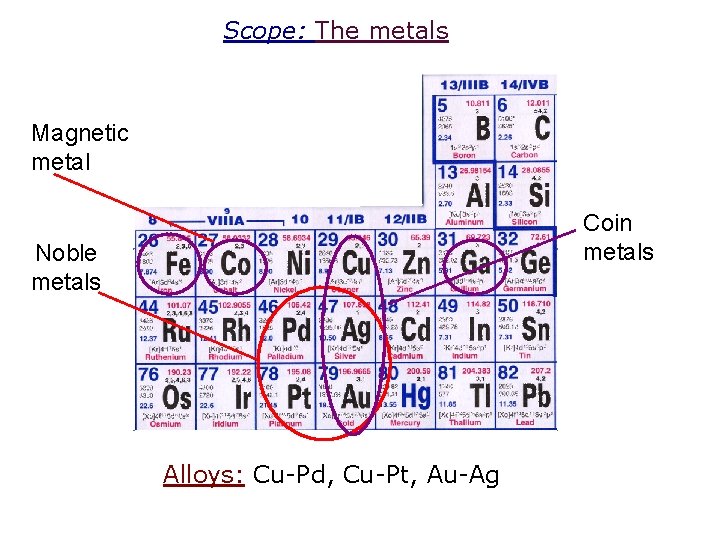
Scope: The metals Magnetic metal Coin metals Noble metals Alloys: Cu-Pd, Cu-Pt, Au-Ag
![Scope The dopants Small molecules hydrophilic or hydrophobic Sudan III Biologicals DTryptophan Complexes Rh Scope: The dopants Small molecules, hydrophilic or hydrophobic: Sudan III Biologicals: D-Tryptophan Complexes: [Rh]](https://slidetodoc.com/presentation_image/570d431b16dafa039b2c948c91de9fca/image-41.jpg)
Scope: The dopants Small molecules, hydrophilic or hydrophobic: Sudan III Biologicals: D-Tryptophan Complexes: [Rh] Inorganic compounds: H 3[P(Mo 3 O 10)4] Polymers, hydrophobic or hydrophilic: Polyacrylonitrile Proteins: Alkaline phosphatase Nanoparticles: Carbon nanofibers

The New Materials Nafion@Ag PSSA@Au CR@Co CR@Cu

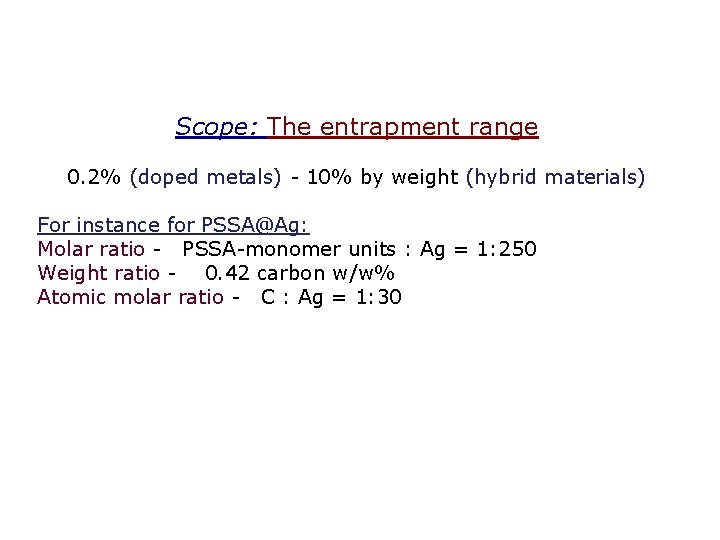
Scope: The entrapment range 0. 2% (doped metals) - 10% by weight (hybrid materials) For instance for PSSA@Ag: Molar ratio - PSSA-monomer units : Ag = 1: 250 Weight ratio - 0. 42 carbon w/w% Atomic molar ratio - C : Ag = 1: 30

Hierarchical structure: PSSA@Ag H. Behar-Levy, G. Shter, G. Grader, Chem. Mater. , 16, 3197 (2004)


Rhodium-complex @silver Rhodium- First taken after a few seconds

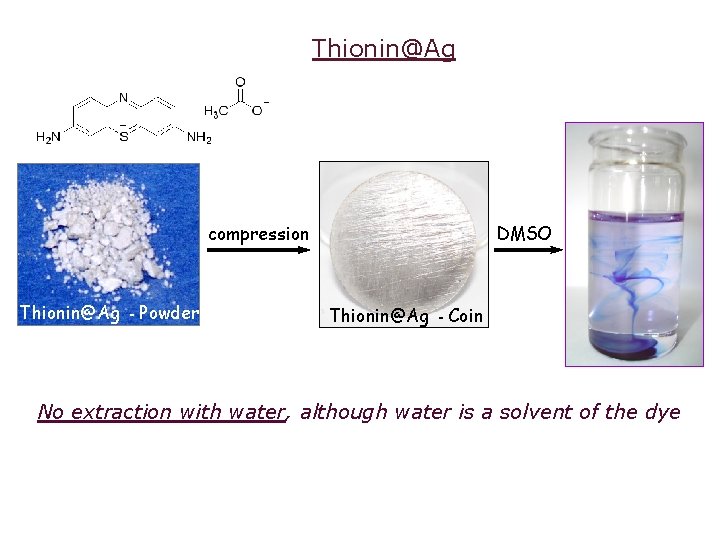
Thionin@Ag compression Thionin@Ag - Powder DMSO Thionin@Ag - Coin No extraction with water, although water is a solvent of the dye

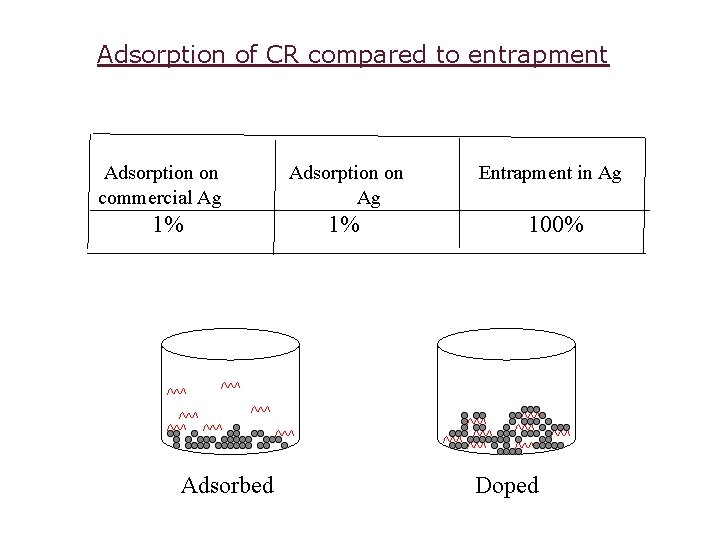
Adsorption of CR compared to entrapment Adsorption on commercial Ag 1% Adsorbed Adsorption on Ag Entrapment in Ag 1% 100% Doped

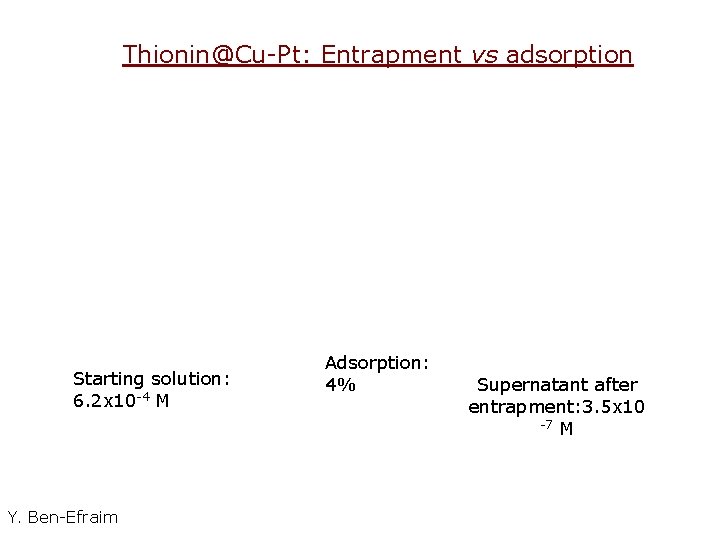
Thionin@Cu-Pt: Entrapment vs adsorption Starting solution: 6. 2 x 10 -4 M Y. Ben-Efraim Adsorption: 4% Supernatant after entrapment: 3. 5 x 10 -7 M

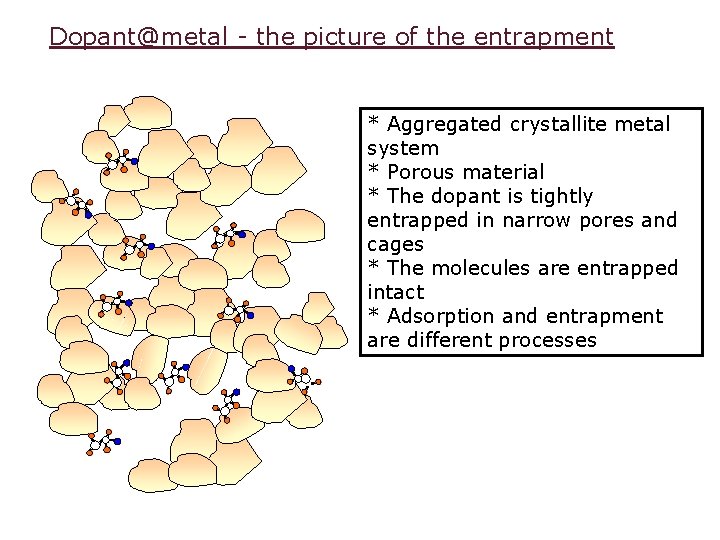
Dopant@metal - the picture of the entrapment * Aggregated crystallite metal system * Porous material * The dopant is tightly entrapped in narrow pores and cages * The molecules are entrapped intact * Adsorption and entrapment are different processes

Scope: Properties and functionalities *Affecting the metal properties - conductivity *Affecting the reactivity characteristics – “acidic metal” *Affecting the metal structure – chiral metals *Affecting the catalytic properties of the metal *Using a metal as a support for heterogeneous catalysis *Bioapplication: Synergism in antibacterial activity *Bioapplication: Enzyme entrapment within metals *Corrosion prevention *New concept in batteries

Chlorhexidine digluconate@Ag Thermal gravimetric analysis Racheli Ben-Knaz, Rami Pedahzur, Adv. Funct. Mater. , 20, 2324 (2010)

Enzymatic activity of acid-phospatase@gold Michaelis-Menten doseresponse kinetics is obeyed Km = 9. 3 m. M (free enzyme: 1. 25 m. M ) Racheli Ben-Knaz, Biomaterials, 30 126 (2009)

What is chiral doping doing to the metal? Is it inducing chirality in it?

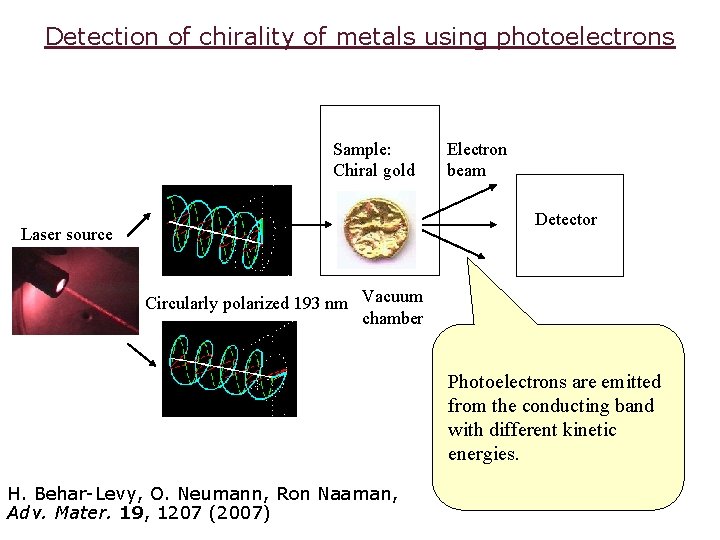
Detection of chirality of metals using photoelectrons Sample: Chiral gold Electron beam Detector Laser source Circularly polarized 193 nm Vacuum chamber Photoelectrons are emitted from the conducting band with different kinetic energies. H. Behar-Levy, O. Neumann, Ron Naaman, Adv. Mater. 19, 1207 (2007)

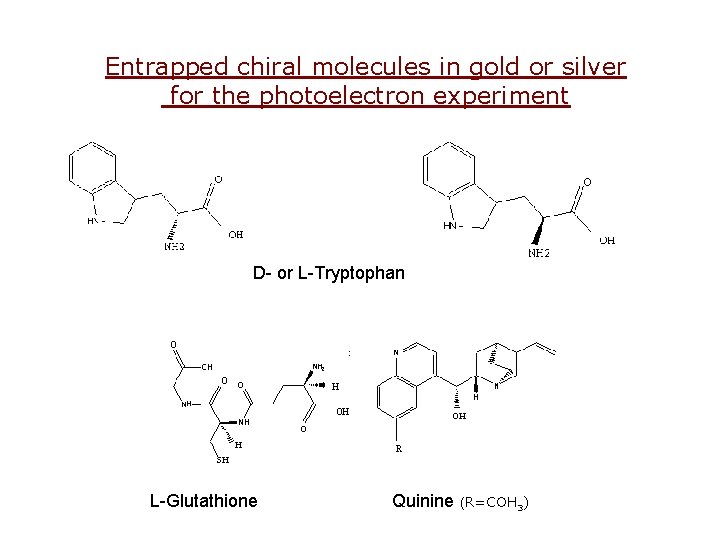
Entrapped chiral molecules in gold or silver for the photoelectron experiment D- or L-Tryptophan L-Glutathione Quinine (R=COH 3)

Blank: Scattering from undoped Au

Scattering from gold doped with L-quinine

Two enantiomers of gold Reversal of scattering behavior by switching between the enantiomers of tryptophan Silver was made chiral too!

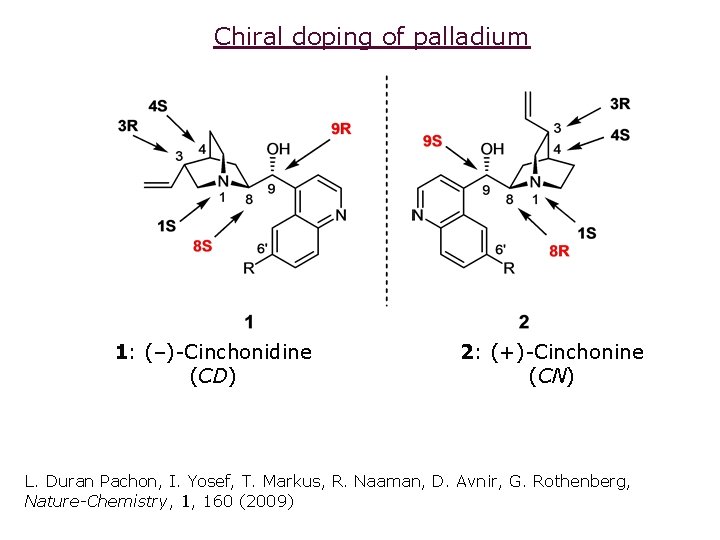
Chiral doping of palladium 1: (–)-Cinchonidine (CD) 2: (+)-Cinchonine (CN) L. Duran Pachon, I. Yosef, T. Markus, R. Naaman, D. Avnir, G. Rothenberg, Nature-Chemistry, 1, 160 (2009)

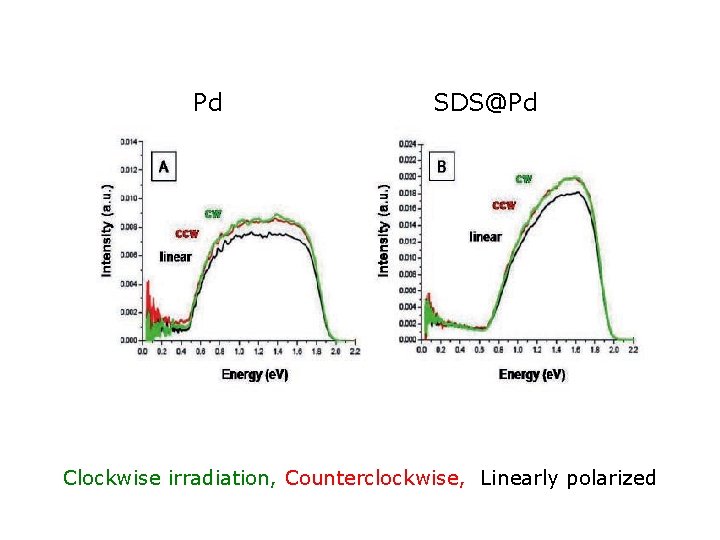
Pd SDS@Pd Clockwise irradiation, Counterclockwise, Linearly polarized

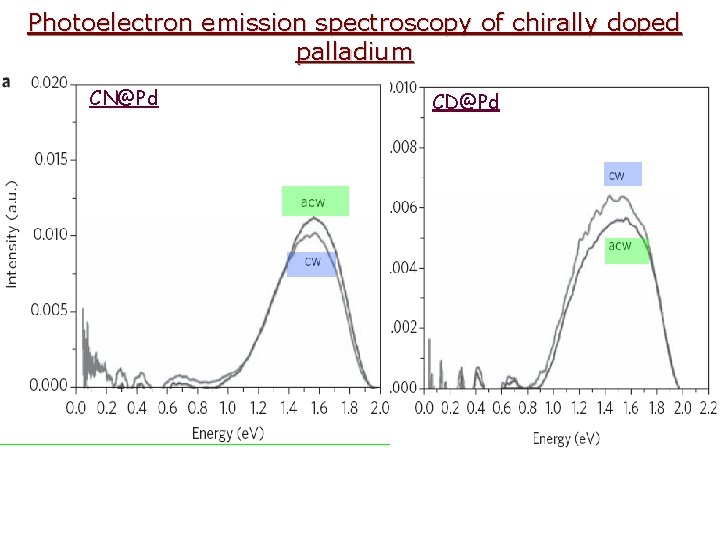
Photoelectron emission spectroscopy of chirally doped palladium CN@Pd CD@Pd

What is chiral in the metal? # The chiral dopant affects the metal molecular orbitals, distorting them chirally # The geometry of the metal pore around the doped molecule is chiral These are two different chiral entities!

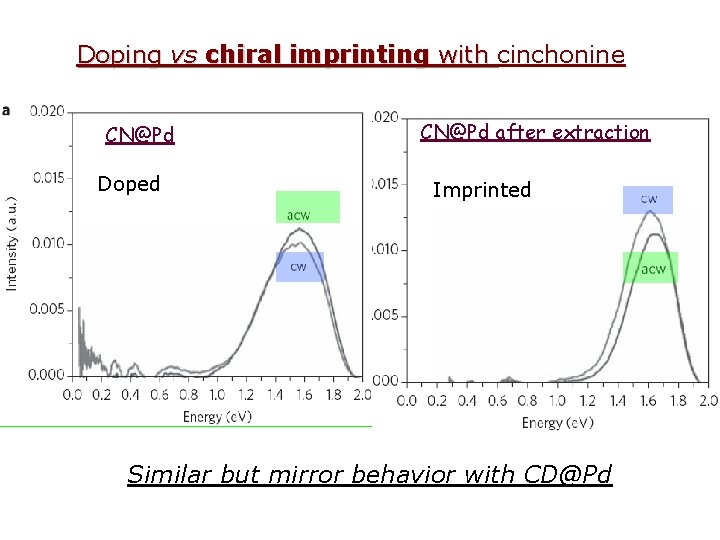
Doping vs chiral imprinting with cinchonine CN@Pd Doped CN@Pd after extraction Imprinted Similar but mirror behavior with CD@Pd

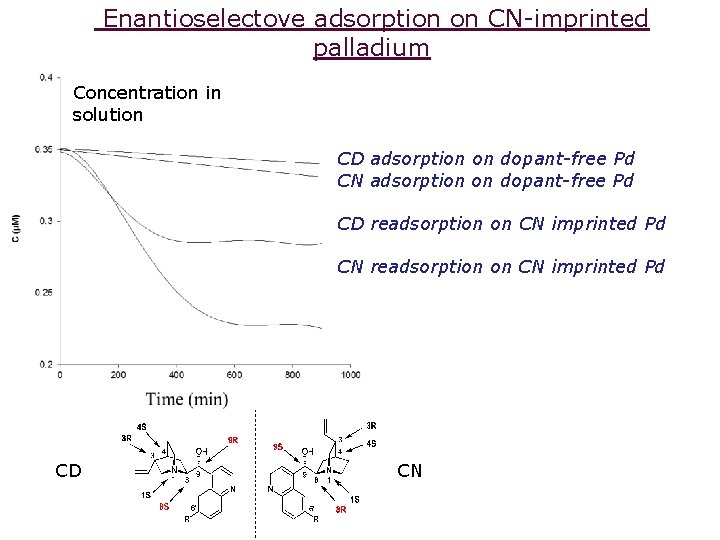
Enantioselectove adsorption on CN-imprinted palladium Concentration in solution CD adsorption on dopant-free Pd CN adsorption on dopant-free Pd CD readsorption on CN imprinted Pd CN readsorption on CN imprinted Pd CD CN

Chiral catalysis in the context of metals

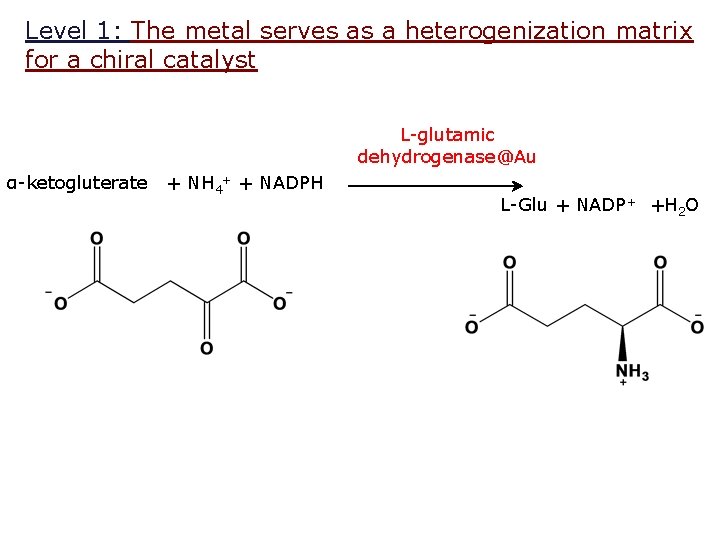
Level 1: The metal serves as a heterogenization matrix for a chiral catalyst L-glutamic dehydrogenase@Au α-ketogluterate + NH 4+ + NADPH L-Glu + NADP + +H 2 O

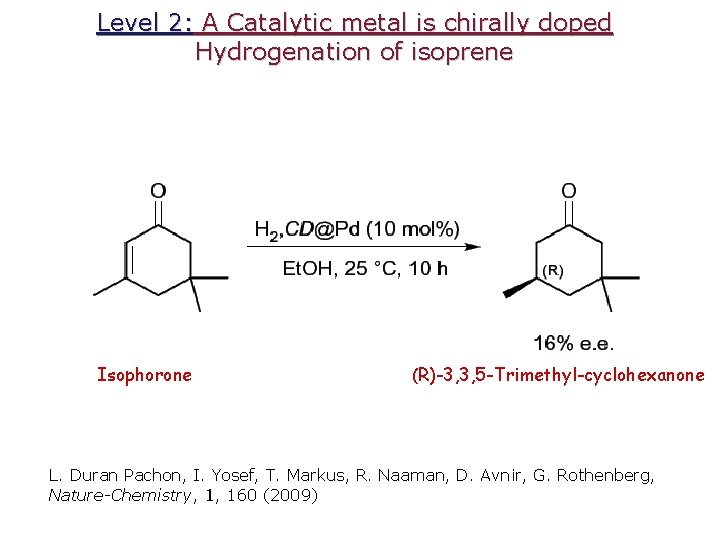
Level 2: A Catalytic metal is chirally doped Hydrogenation of isoprene Isophorone (R)-3, 3, 5 -Trimethyl-cyclohexanone L. Duran Pachon, I. Yosef, T. Markus, R. Naaman, D. Avnir, G. Rothenberg, Nature-Chemistry, 1, 160 (2009)

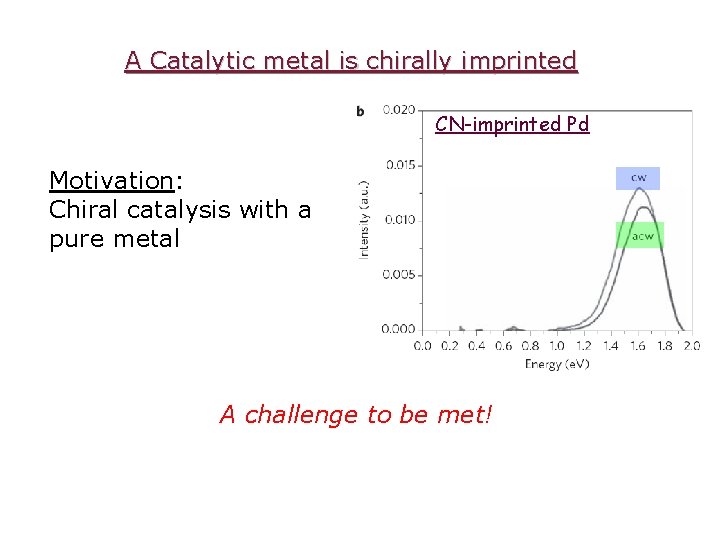
A Catalytic metal is chirally imprinted CN-imprinted Pd Motivation: Chiral catalysis with a pure metal A challenge to be met!