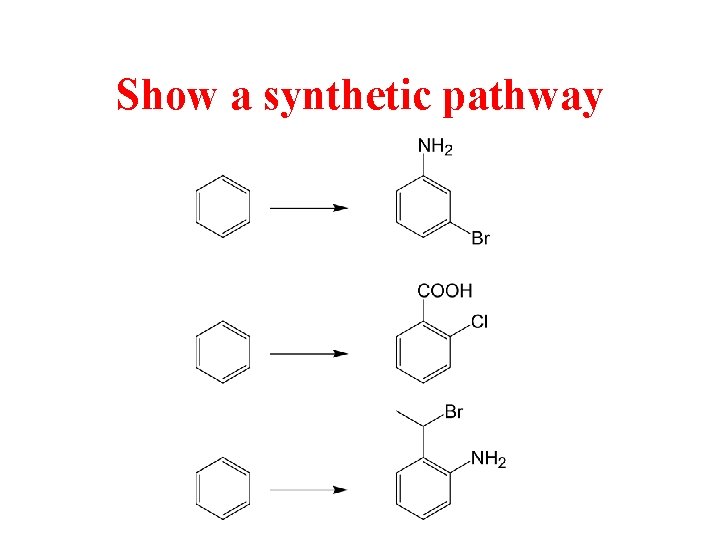
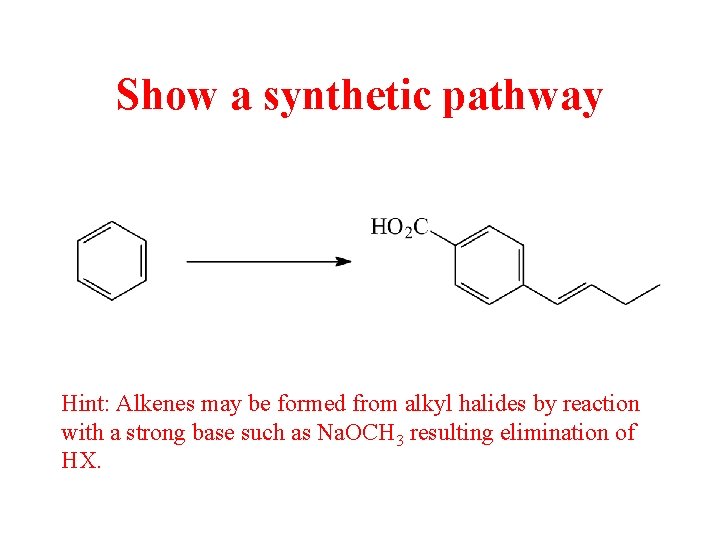
Show a synthetic pathway Show a synthetic pathway


![Specific Rotation, [α] = α / cl a = observed rotation c = concentration Specific Rotation, [α] = α / cl a = observed rotation c = concentration](https://slidetodoc.com/presentation_image/6531e429409510bbcdc0275845de6712/image-13.jpg)
![Specific Rotations of some Common Organic Compounds Compound [a] # * centers Penicillin V Specific Rotations of some Common Organic Compounds Compound [a] # * centers Penicillin V](https://slidetodoc.com/presentation_image/6531e429409510bbcdc0275845de6712/image-14.jpg)


















- Slides: 63

Show a synthetic pathway

Show a synthetic pathway Hint: Alkenes may be formed from alkyl halides by reaction with a strong base such as Na. OCH 3 resulting elimination of HX.


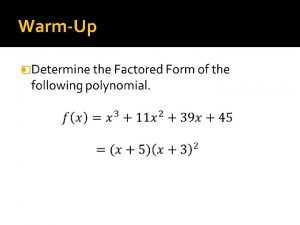
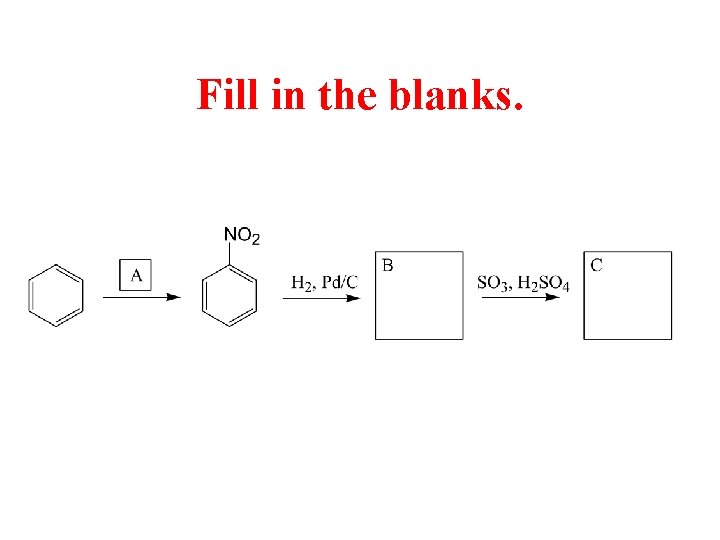
Fill in the blanks.

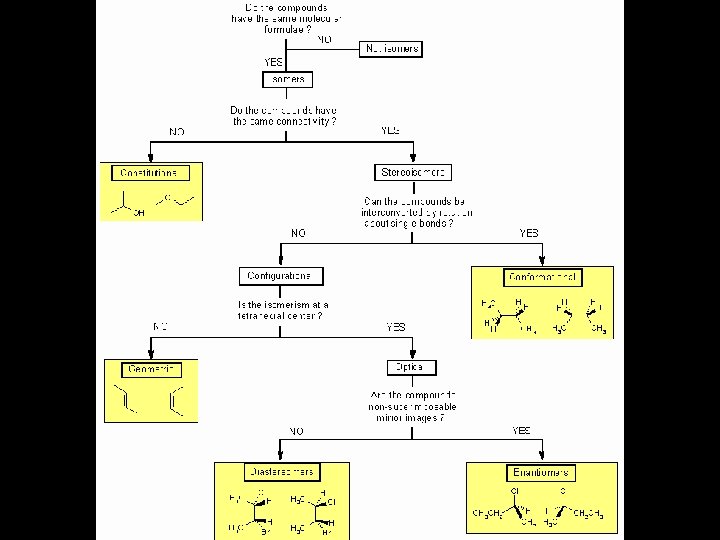
Stereochemistry refers to the 3 -dimensional properties and reactions of molecules. It has its own language and terms that need to be learned in order to fully communicate and understand the concepts.


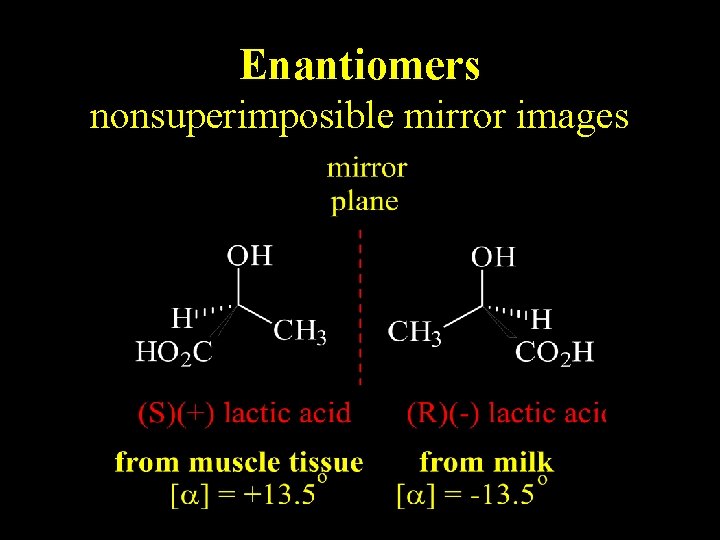
Definitions • Stereoisomers – compounds with the same connectivity, different arrangement in space • Enantiomers – stereoisomers that are nonsuperimposible mirror images; only properties that differ are direction (+ or -) of optical rotation • Diastereomers – stereoisomers that are not mirror images; different compounds with different physical properties

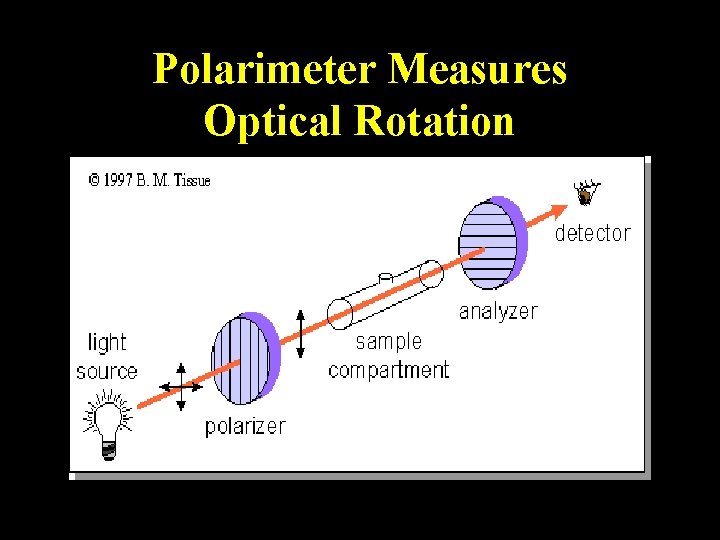
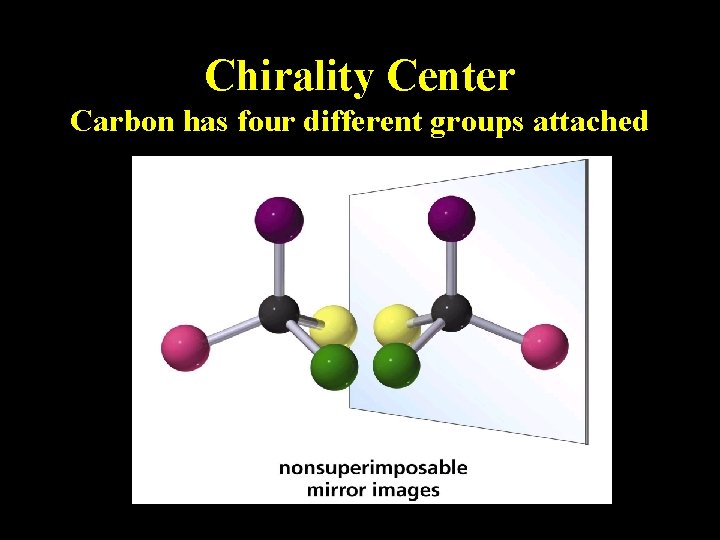
More Definitions • Asymmetric center – sp 3 carbon with 4 different groups attached • Optical activity – the ability to rotate the plane of plane –polarized light • Chiral compound – a compound that is optically active (an achiral compound will not rotate light) • Polarimeter – device that measures the optical rotation of the chiral compound

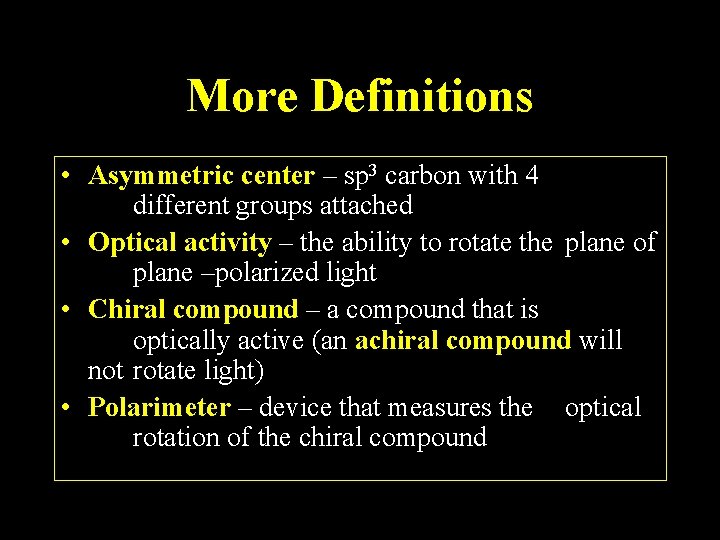
Plane-Polarized Light

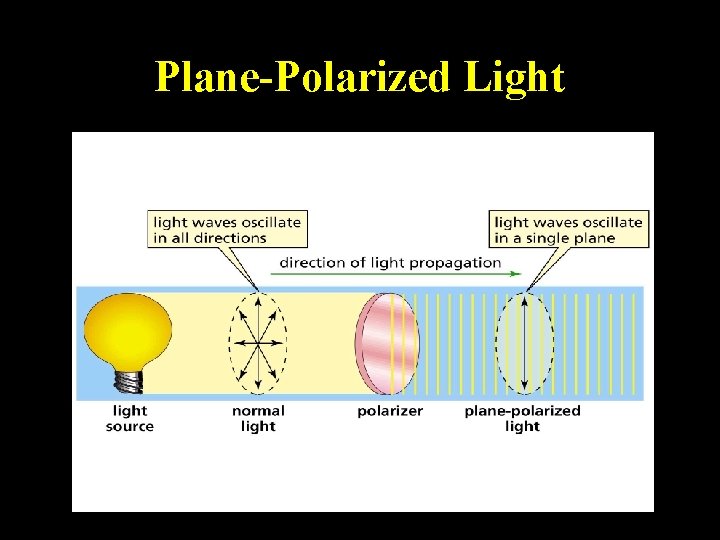
Plane-Polarized Light through an Achiral Compound

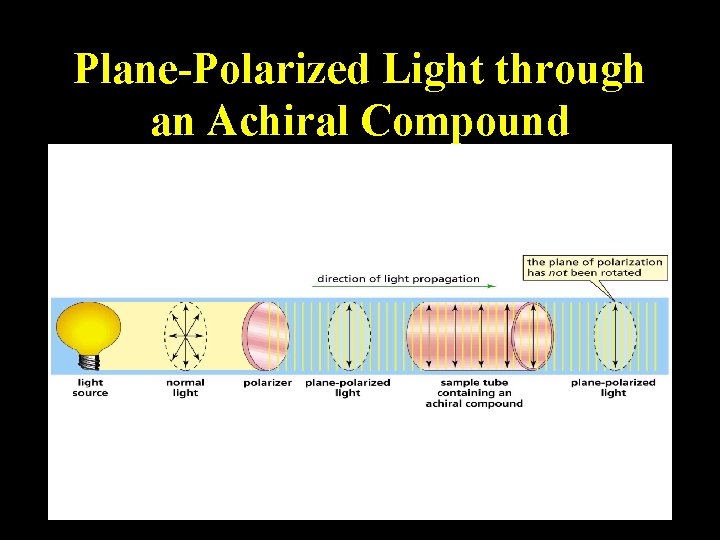
Plane-Polarized Light through a Chiral Compound

Polarimeter Measures Optical Rotation
![Specific Rotation α α cl a observed rotation c concentration Specific Rotation, [α] = α / cl a = observed rotation c = concentration](https://slidetodoc.com/presentation_image/6531e429409510bbcdc0275845de6712/image-13.jpg)
Specific Rotation, [α] = α / cl a = observed rotation c = concentration in g/m. L l = length of tube in dm Dextrorotary designated as d or (+), clockwise rotation Levorotary designated as l or (-), counterclockwise rotation
![Specific Rotations of some Common Organic Compounds Compound a centers Penicillin V Specific Rotations of some Common Organic Compounds Compound [a] # * centers Penicillin V](https://slidetodoc.com/presentation_image/6531e429409510bbcdc0275845de6712/image-14.jpg)
Specific Rotations of some Common Organic Compounds Compound [a] # * centers Penicillin V +233. 0 3 Sucrose +66. 5 10 Camphor +44. 3 2 MSG +25. 5 1 Cholesterol -31. 3 8 Morphine -132. 0 5

Chirality Center Carbon has four different groups attached

Enantiomers nonsuperimposible mirror images

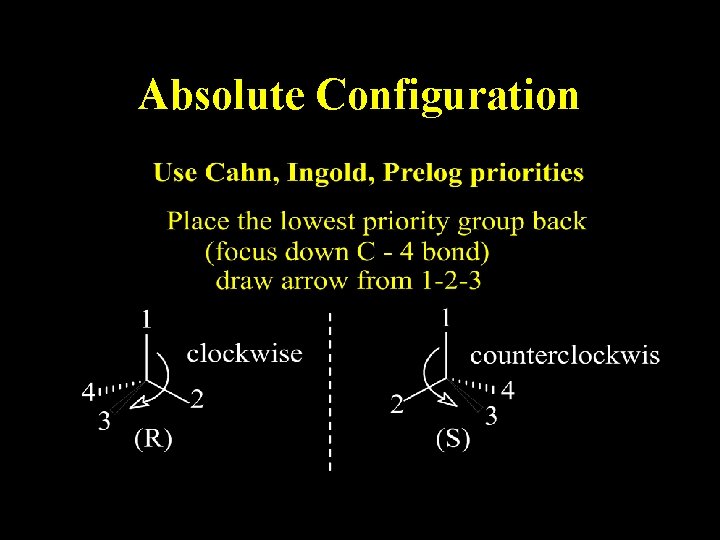
Absolute Configuration

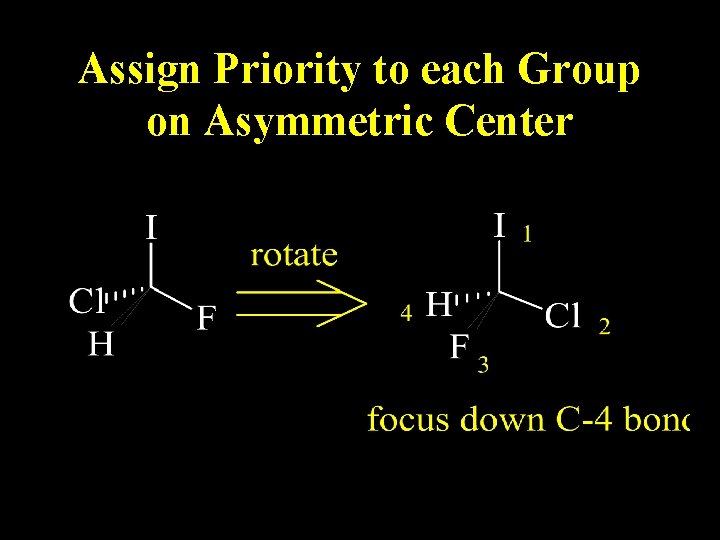
Assign Priority to each Group on Asymmetric Center

Lactic Acid

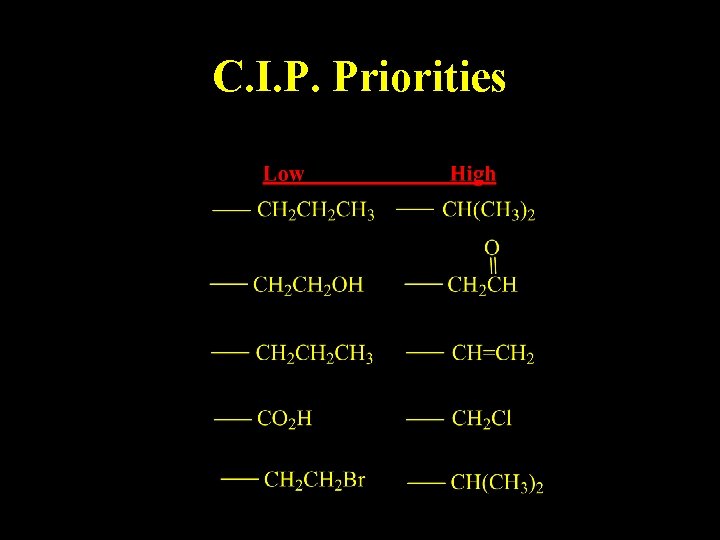
C. I. P. Priorities

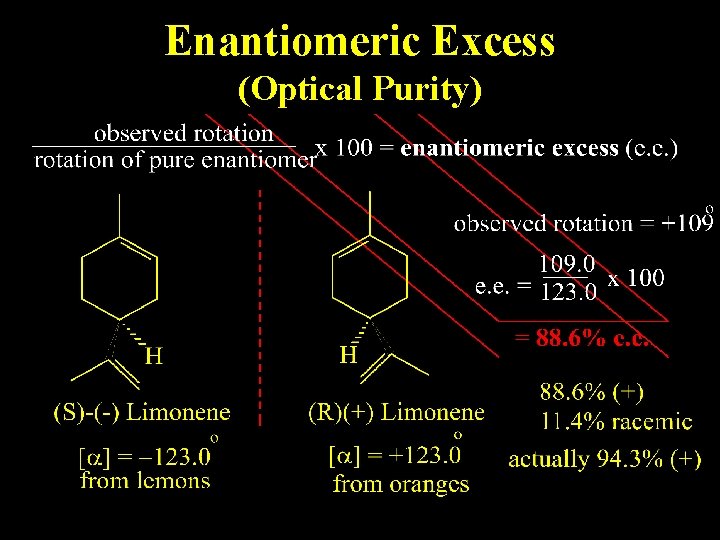
Enantiomeric Excess (Optical Purity)


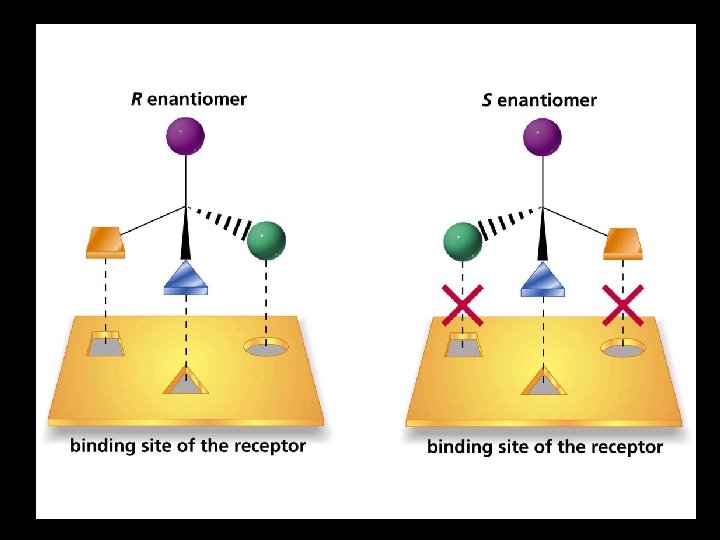
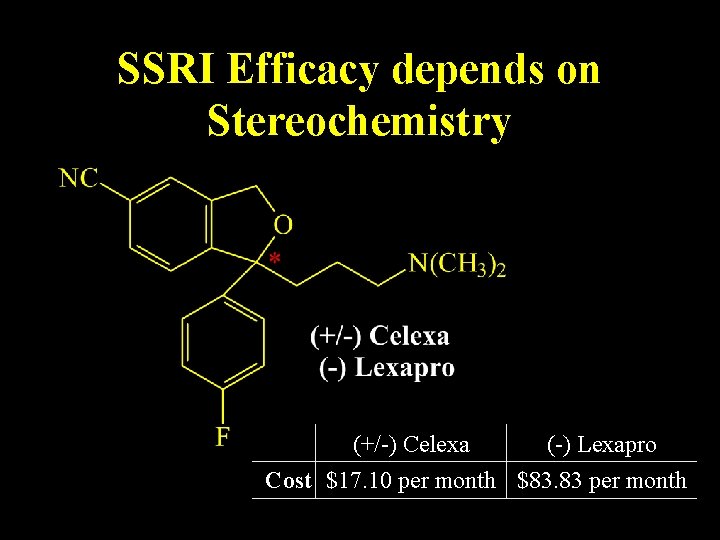
SSRI Efficacy depends on Stereochemistry (+/-) Celexa (-) Lexapro Cost $17. 10 per month $83. 83 per month

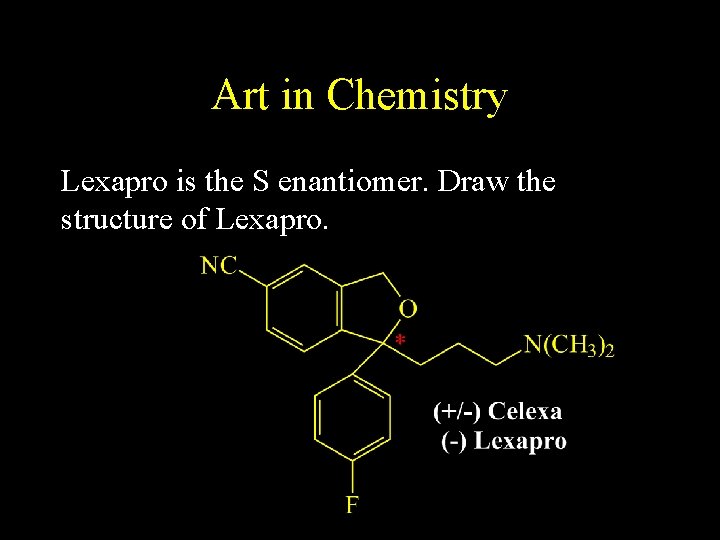
Art in Chemistry Lexapro is the S enantiomer. Draw the structure of Lexapro.

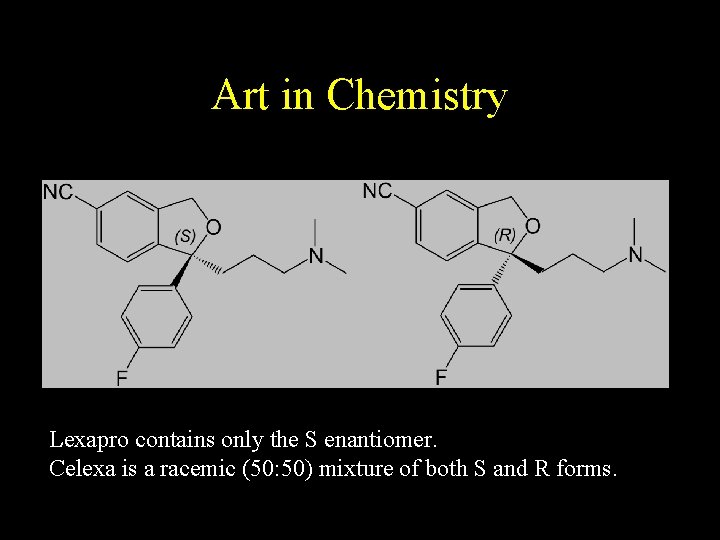
Art in Chemistry Lexapro contains only the S enantiomer. Celexa is a racemic (50: 50) mixture of both S and R forms.

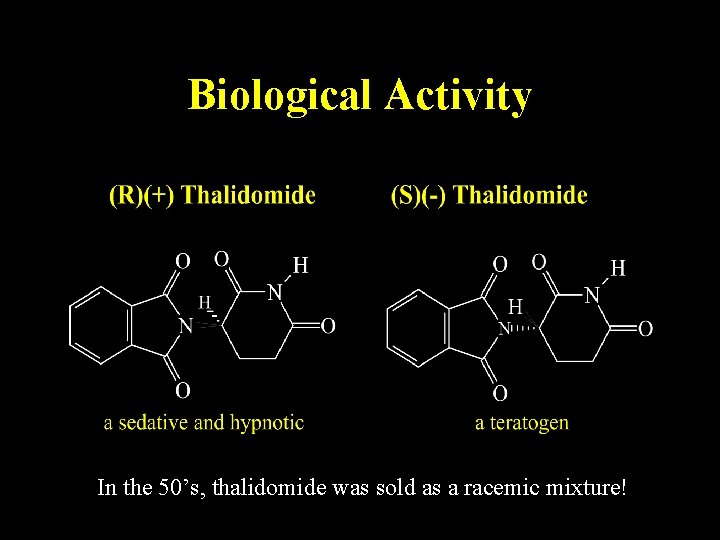
Biological Activity In the 50’s, thalidomide was sold as a racemic mixture!

Thalidomide was prescribed as a sedative and used against nausea and to alleviate morning sickness in pregnant women.

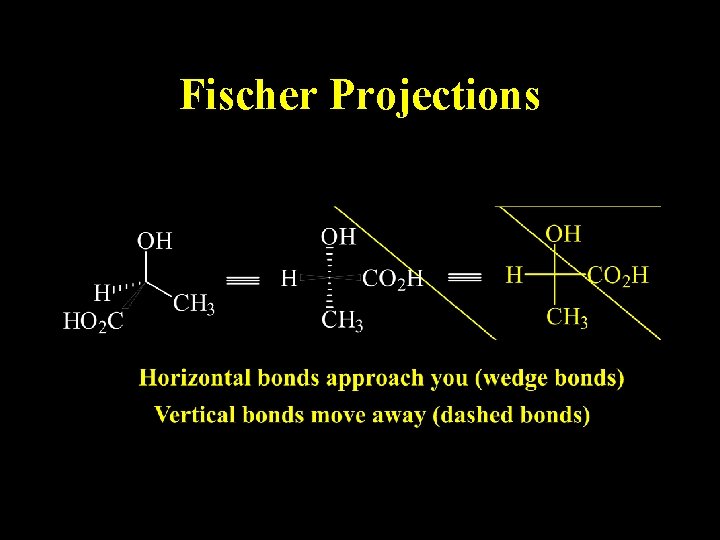
Fischer Projections

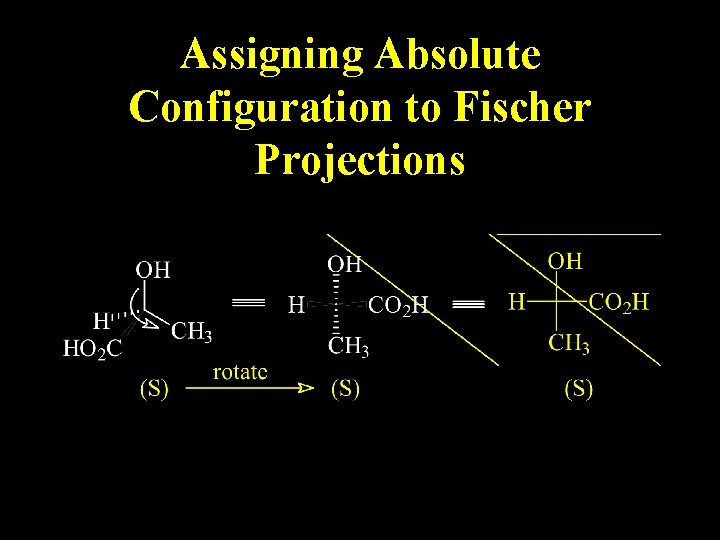
Assigning Absolute Configuration to Fischer Projections

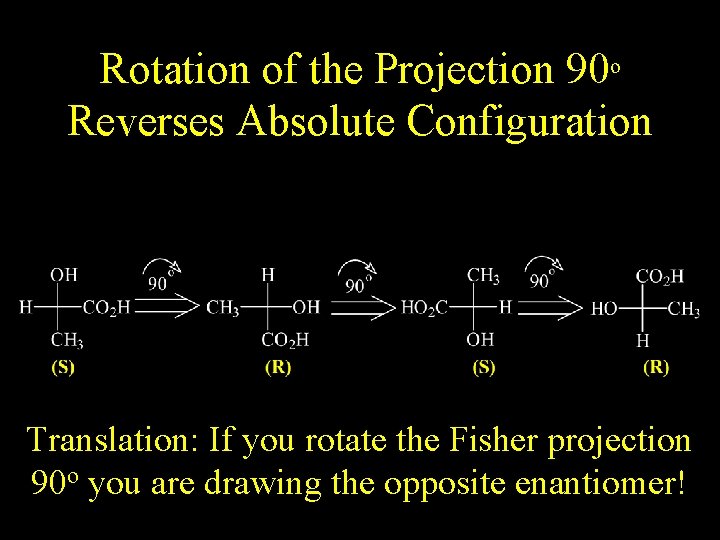
Rotation of the Projection 90 o Reverses Absolute Configuration Translation: If you rotate the Fisher projection 90 o you are drawing the opposite enantiomer!

Determine the stereochemistry at all chiral centers.


1 (7) Should be R, R, S, R from C-1; 5 th chiral center not included.

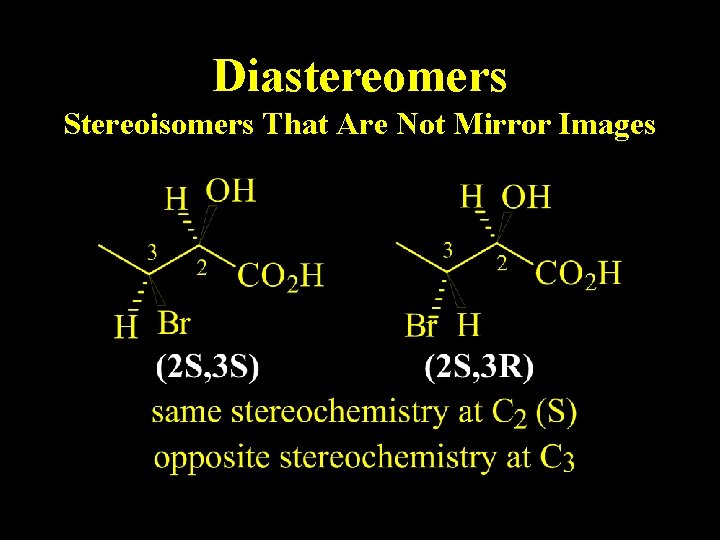
Diastereomers Stereoisomers That Are Not Mirror Images

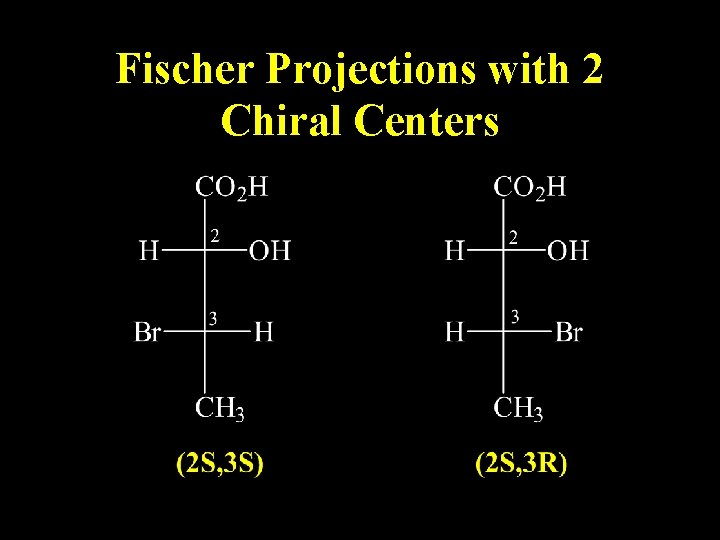
Fischer Projections with 2 Chiral Centers

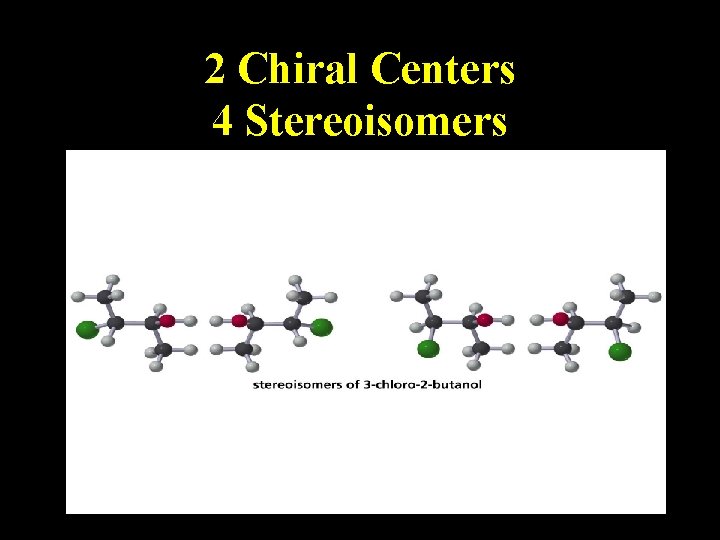
2 Chiral Centers 4 Stereoisomers

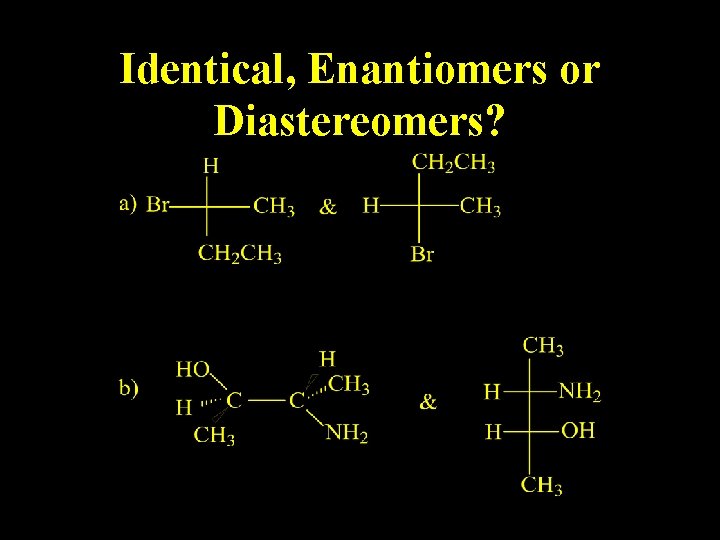
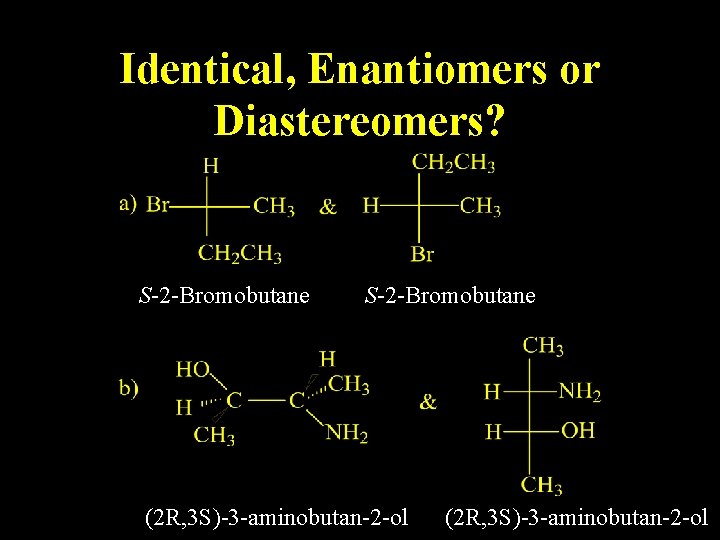
Identical, Enantiomers or Diastereomers?

Identical, Enantiomers or Diastereomers? S-2 -Bromobutane (2 R, 3 S)-3 -aminobutan-2 -ol

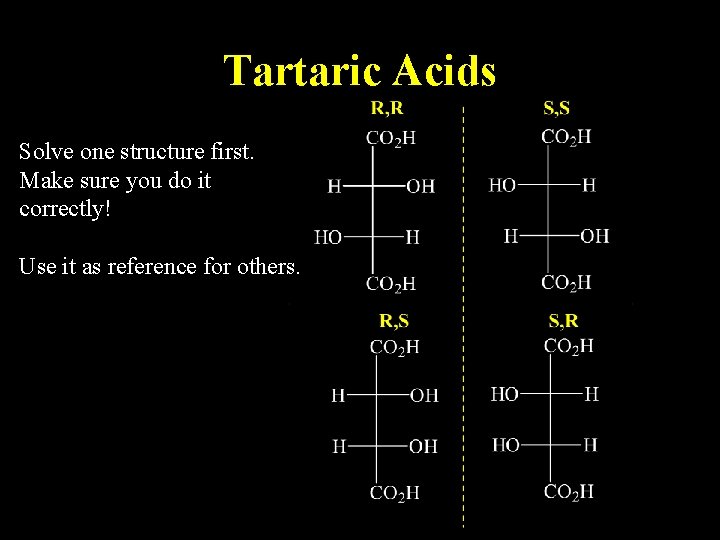
Tartaric Acids Solve one structure first. Make sure you do it correctly! Use it as reference for others.

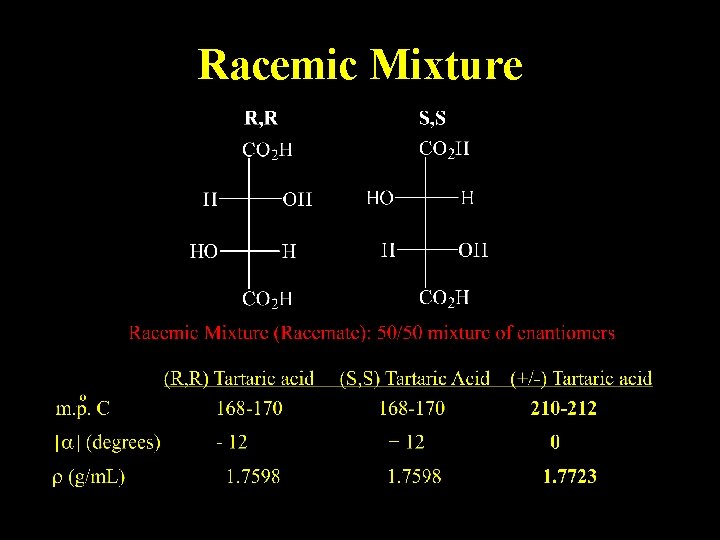
Racemic Mixture

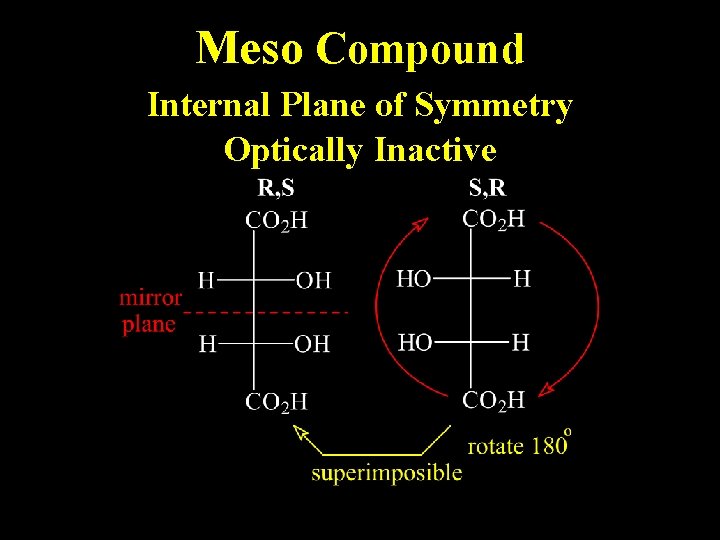
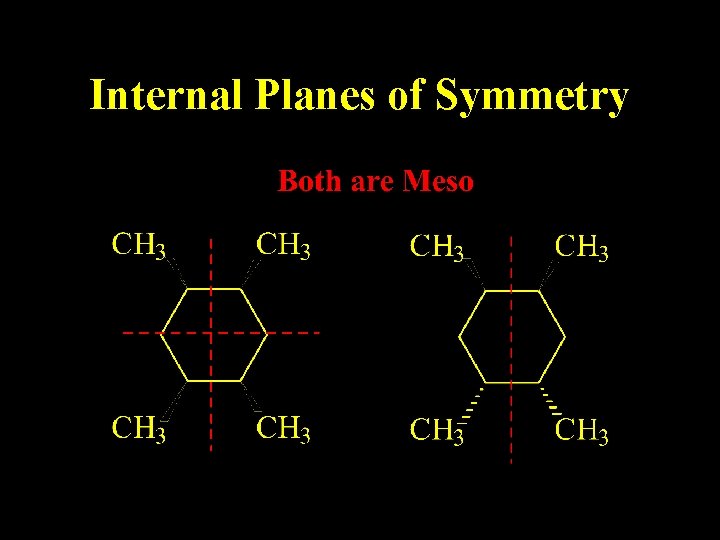
Meso Compound Internal Plane of Symmetry Optically Inactive

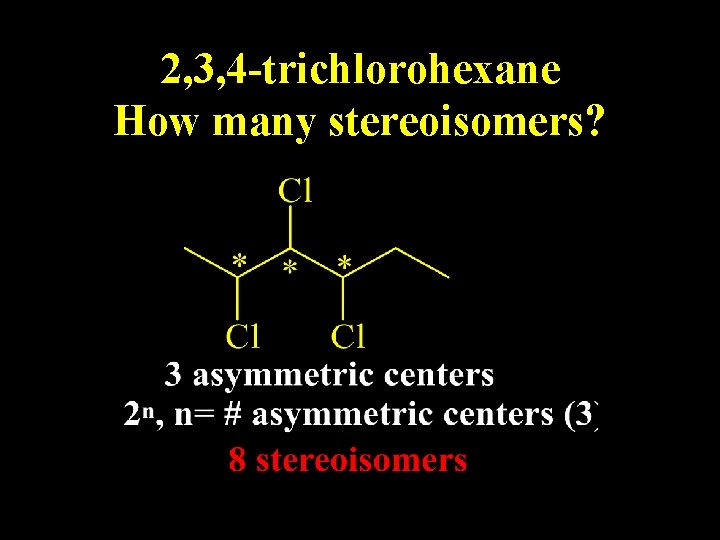
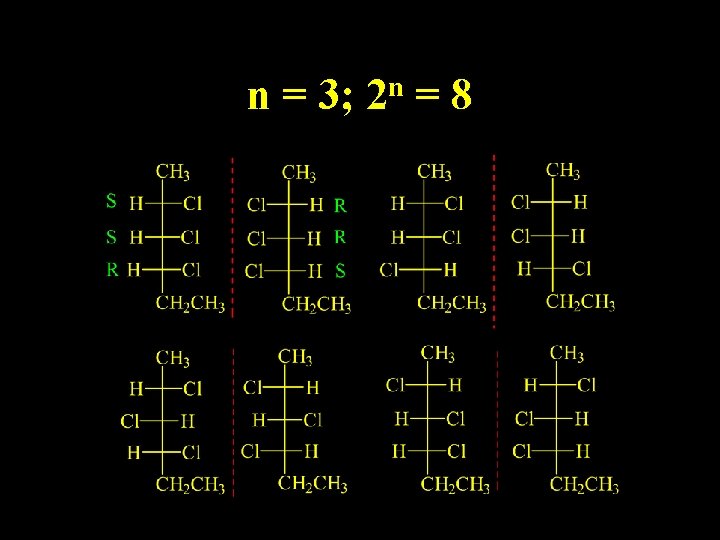
2, 3, 4 -trichlorohexane How many stereoisomers?

n = 3; n 2 =8

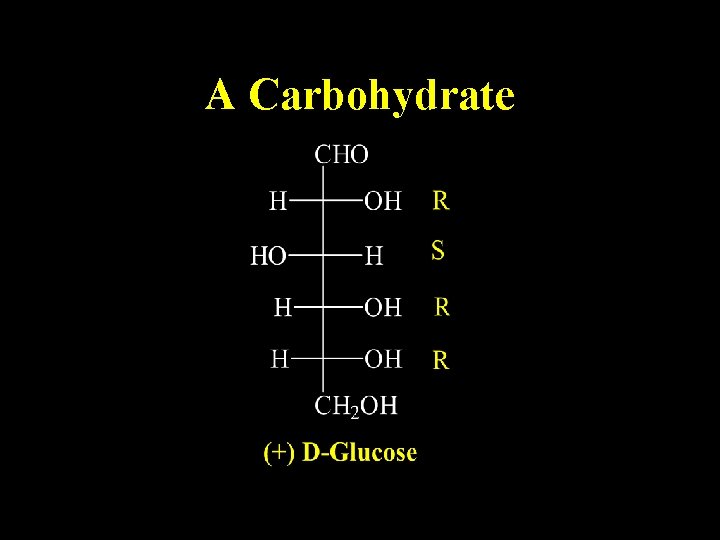
A Carbohydrate

Internal Planes of Symmetry

Asymmetric Centers on Rings

Reactions that Generate Chirality Centers Hydrogenation, syn

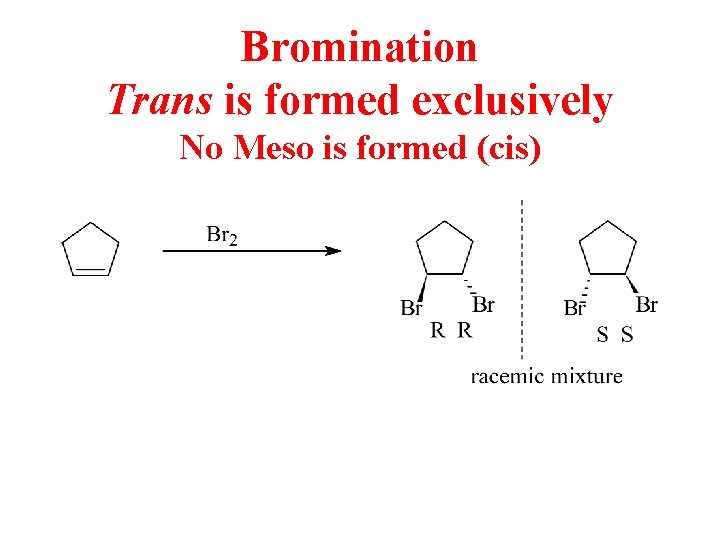
Bromination Trans is formed exclusively No Meso is formed (cis)

Bromonium Ion is Opened Equally from Both Sides

trans alkene + anti addition = MESO

cis Alkene + anti addition = racemic mixture

Brominations Often Generate Asymmetric Centers

Asymmetric Center is Generated Racemic Mixture Formed

Asymmetric Induction

Preparation of (L)-Dopa for Treatment of Parkinson’s

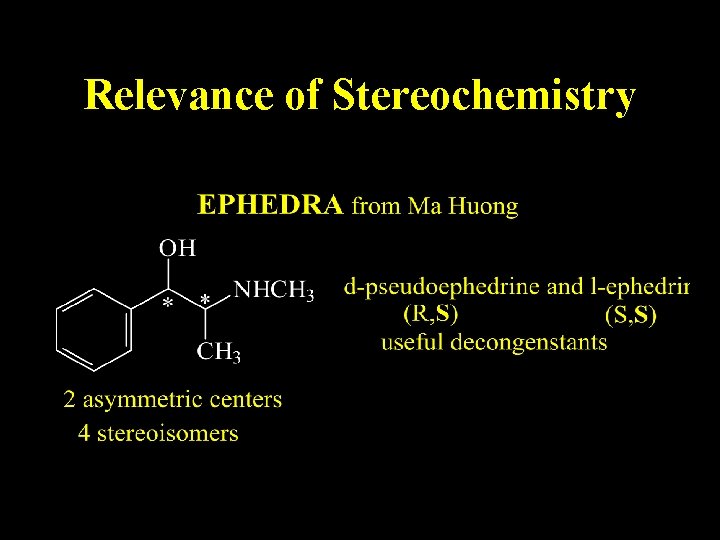
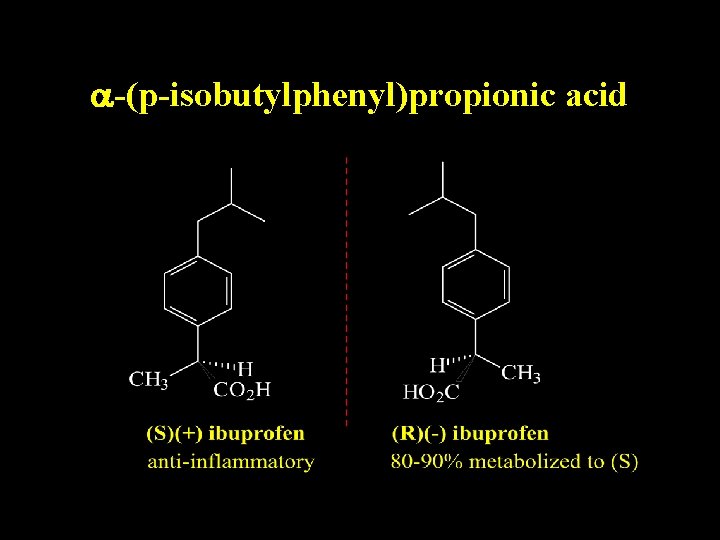
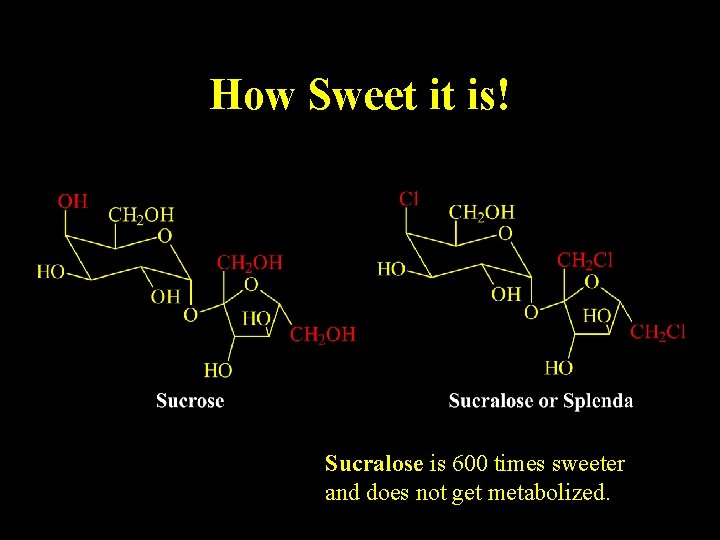
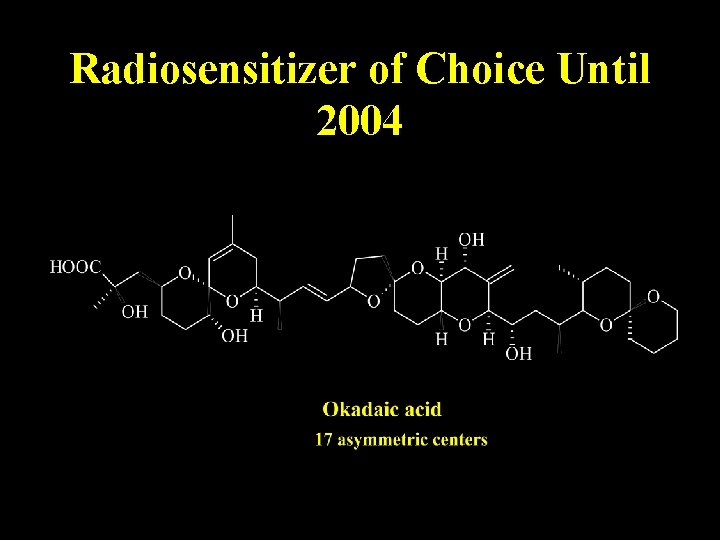
Relevance of Stereochemistry

One-step synthesis

a-(p-isobutylphenyl)propionic acid

How Sweet it is! Sucralose is 600 times sweeter and does not get metabolized.

Sildenafil (Viagra) and Caffeine

Radiosensitizer of Choice Until 2004

How is each Cpd related to X?

Identical: D, G Enantiomers: A, C Diastereomers: B, E, F, H, I
 Pubic hair under microscope
Pubic hair under microscope Synthetic division
Synthetic division Synthetic division fraction
Synthetic division fraction The magician touched the child with the wand structure tree
The magician touched the child with the wand structure tree Synthetic division method
Synthetic division method Canadian oil trusts
Canadian oil trusts Terylene is made from
Terylene is made from What are fibres
What are fibres Sifat bahan yang tepat untuk membuat jas hujan
Sifat bahan yang tepat untuk membuat jas hujan Synthetic language
Synthetic language Synthetic camera model in computer graphics
Synthetic camera model in computer graphics Posteriori knowledge
Posteriori knowledge Synthetic cdo
Synthetic cdo Interest coverage ratio damodaran
Interest coverage ratio damodaran Rational root theory
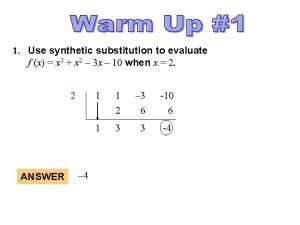
Rational root theory Evaluate using synthetic substitution
Evaluate using synthetic substitution The first
The first Synthetic bible study method
Synthetic bible study method What is fiber evidence
What is fiber evidence Synthetic sounds used to convey information
Synthetic sounds used to convey information Analytic cubism synthetic cubism
Analytic cubism synthetic cubism Futures and forwards
Futures and forwards Graphing calculator
Graphing calculator Analytic phonics
Analytic phonics Damodaran interest coverage ratio
Damodaran interest coverage ratio Disadvantages of suppositories
Disadvantages of suppositories José ruiz y blasco
José ruiz y blasco Principles of synthetic intelligence
Principles of synthetic intelligence Synthetic a priori judgments
Synthetic a priori judgments Synthetic language
Synthetic language Synthetic organic polymers
Synthetic organic polymers Synthetic substitution definition
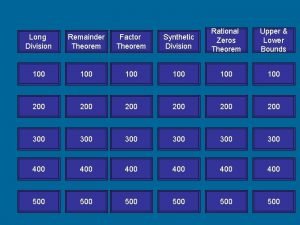
Synthetic substitution definition Remainder theorem
Remainder theorem Semi synthetic emulsifying agent
Semi synthetic emulsifying agent Substitution division
Substitution division Synthetic wave
Synthetic wave Synthetic option
Synthetic option The most common type of plant fiber is
The most common type of plant fiber is Synthetic audio
Synthetic audio Hair evidence
Hair evidence Vásárlás synthetic cocaine
Vásárlás synthetic cocaine Factor theorem synthetic division
Factor theorem synthetic division Synthetic divison
Synthetic divison De re de dicto
De re de dicto Synthetic division steps
Synthetic division steps Rectal fluids
Rectal fluids What is a remainder theorem
What is a remainder theorem Foreign exchange forward contract
Foreign exchange forward contract Synthetic buffalo hides
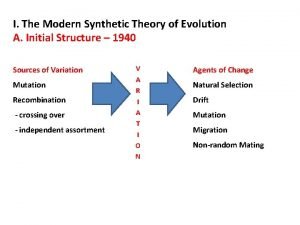
Synthetic buffalo hides Modern synthetic theory of evolution notes
Modern synthetic theory of evolution notes Poly cyclopentene repeat unit
Poly cyclopentene repeat unit Expanded synthetic division
Expanded synthetic division Ruby crystal
Ruby crystal Synthetic vs analytic phonics
Synthetic vs analytic phonics Deena pierott
Deena pierott Synthetic data
Synthetic data Synthetic division worksheet doc
Synthetic division worksheet doc Synthetic fibe
Synthetic fibe Synthetic aperture radar tutorial
Synthetic aperture radar tutorial Rheolube 363
Rheolube 363 Father of analytical geometry
Father of analytical geometry Synthetic function of liver
Synthetic function of liver Synthetic wave
Synthetic wave Dr sathish rajasekaran
Dr sathish rajasekaran