Figure 4 1cis and trans1 2 Dimethylcyclopropane and


- Slides: 82

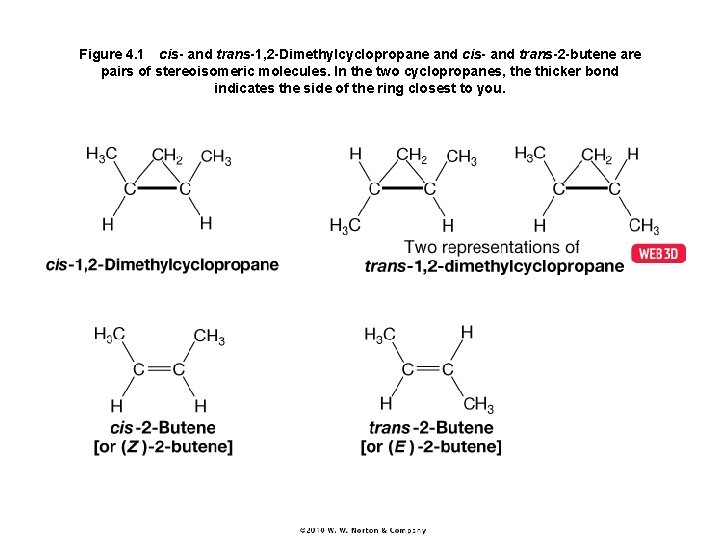
Figure 4. 1 cis- and trans-1, 2 -Dimethylcyclopropane and cis- and trans-2 -butene are pairs of stereoisomeric molecules. In the two cyclopropanes, the thicker bond indicates the side of the ring closest to you.

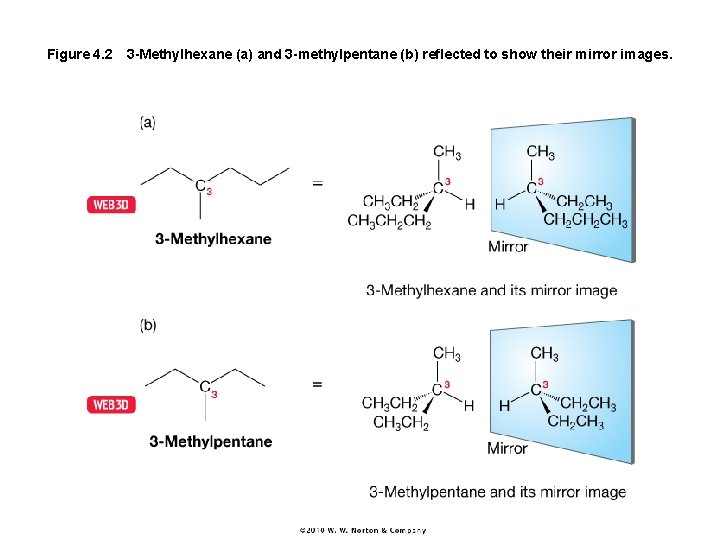
Figure 4. 2 3 -Methylhexane (a) and 3 -methylpentane (b) reflected to show their mirror images.

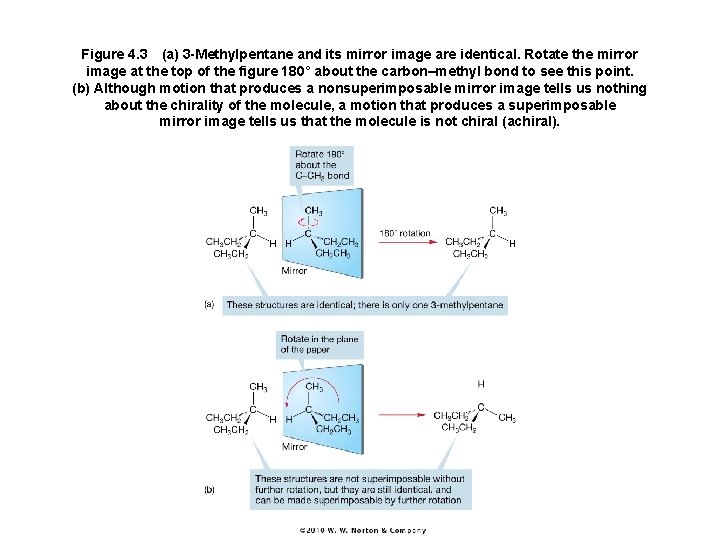
Figure 4. 3 (a) 3 -Methylpentane and its mirror image are identical. Rotate the mirror image at the top of the figure 180° about the carbon–methyl bond to see this point. (b) Although motion that produces a nonsuperimposable mirror image tells us nothing about the chirality of the molecule, a motion that produces a superimposable mirror image tells us that the molecule is not chiral (achiral).

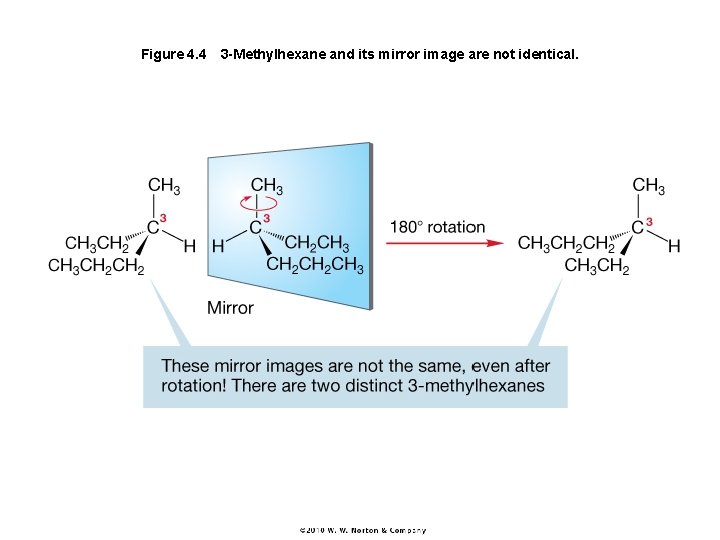
Figure 4. 4 3 -Methylhexane and its mirror image are not identical.

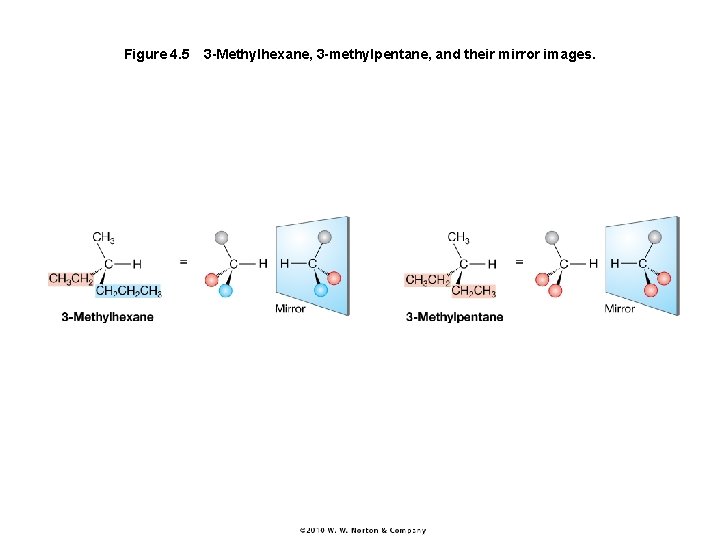
Figure 4. 5 3 -Methylhexane, 3 -methylpentane, and their mirror images.

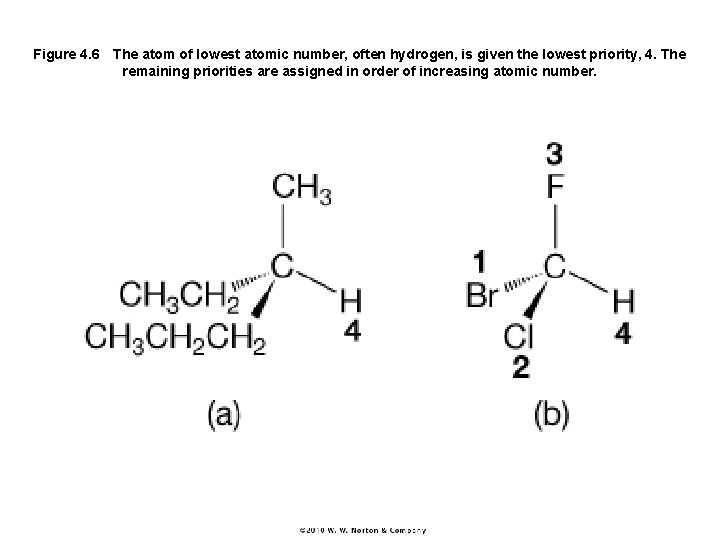
Figure 4. 6 The atom of lowest atomic number, often hydrogen, is given the lowest priority, 4. The remaining priorities are assigned in order of increasing atomic number.

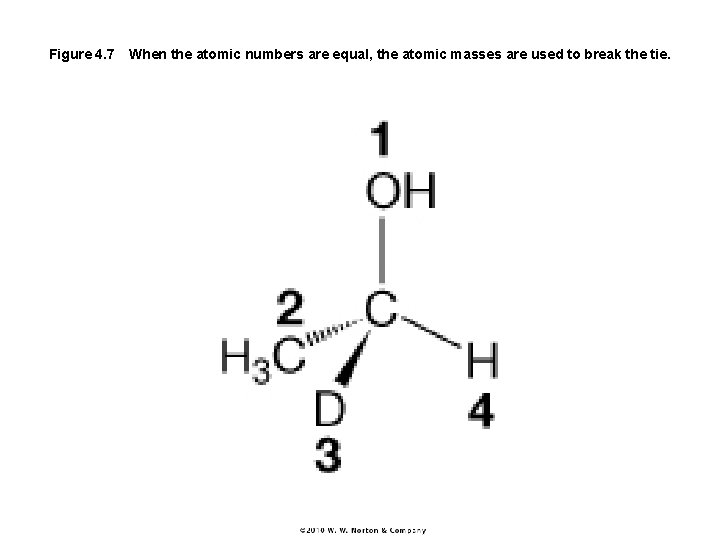
Figure 4. 7 When the atomic numbers are equal, the atomic masses are used to break the tie.

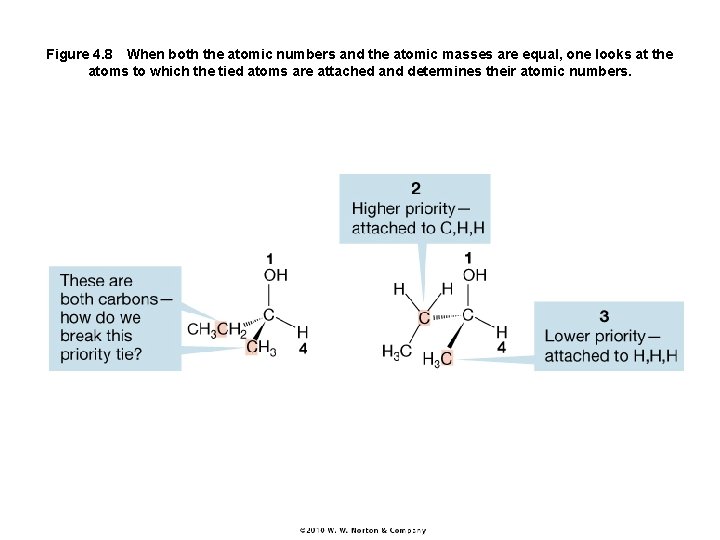
Figure 4. 8 When both the atomic numbers and the atomic masses are equal, one looks at the atoms to which the tied atoms are attached and determines their atomic numbers.

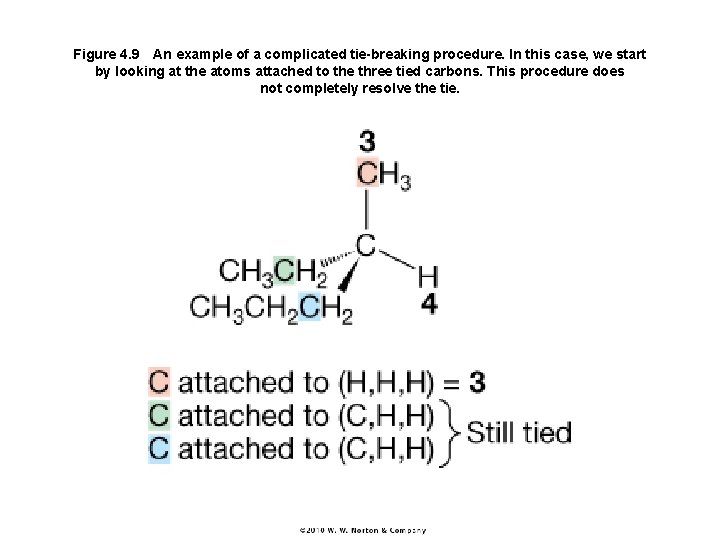
Figure 4. 9 An example of a complicated tie-breaking procedure. In this case, we start by looking at the atoms attached to the three tied carbons. This procedure does not completely resolve the tie.

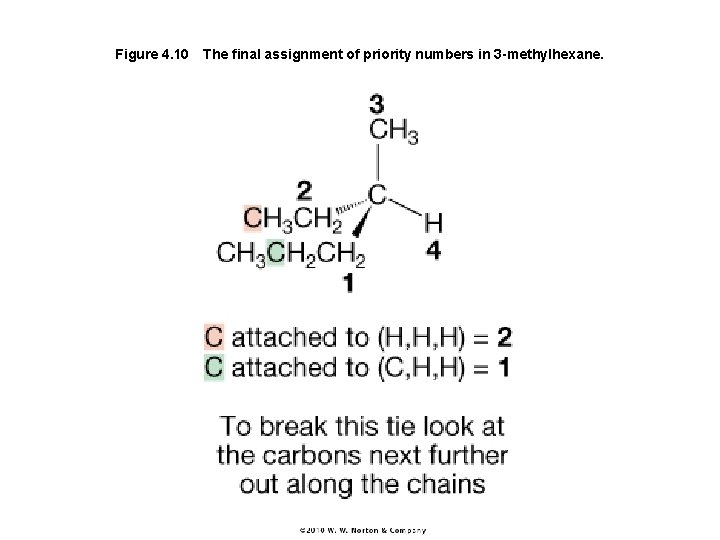
Figure 4. 10 The final assignment of priority numbers in 3 -methylhexane.

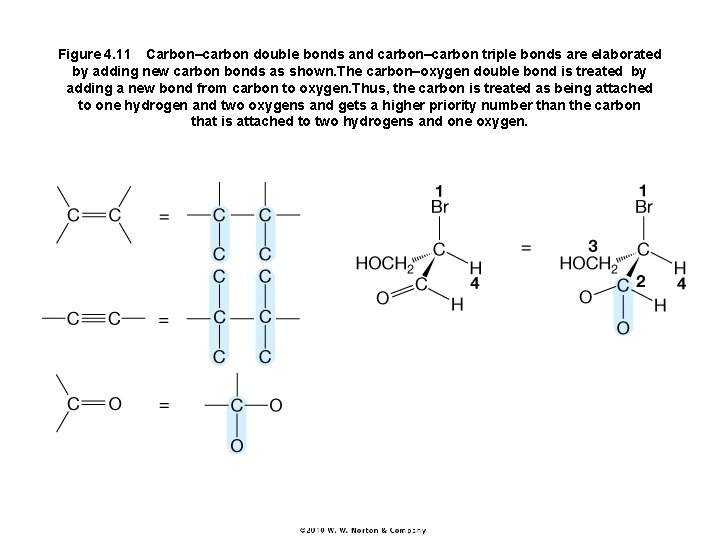
Figure 4. 11 Carbon–carbon double bonds and carbon–carbon triple bonds are elaborated by adding new carbon bonds as shown. The carbon–oxygen double bond is treated by adding a new bond from carbon to oxygen. Thus, the carbon is treated as being attached to one hydrogen and two oxygens and gets a higher priority number than the carbon that is attached to two hydrogens and one oxygen.

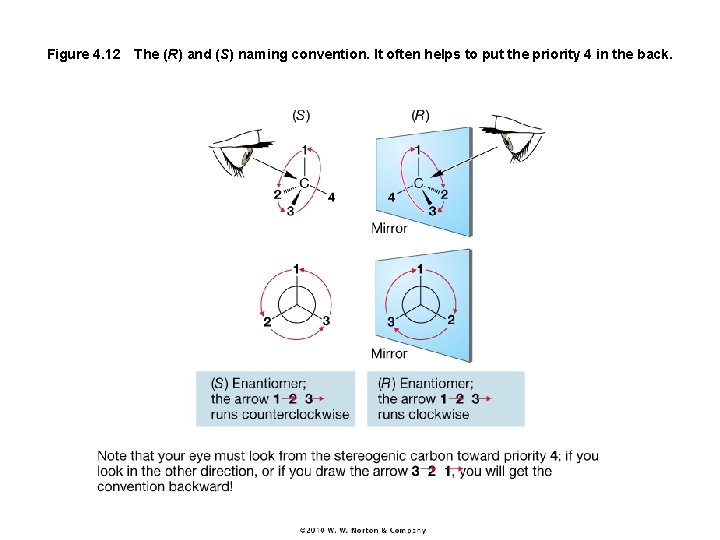
Figure 4. 12 The (R) and (S) naming convention. It often helps to put the priority 4 in the back.

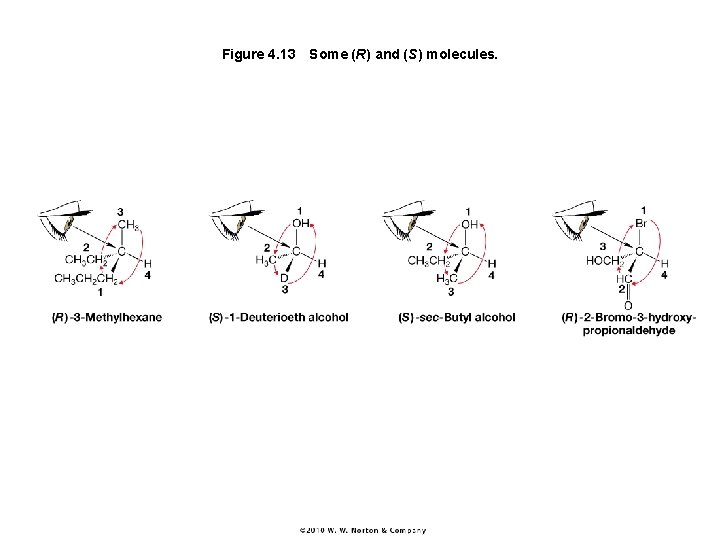
Figure 4. 13 Some (R) and (S) molecules.

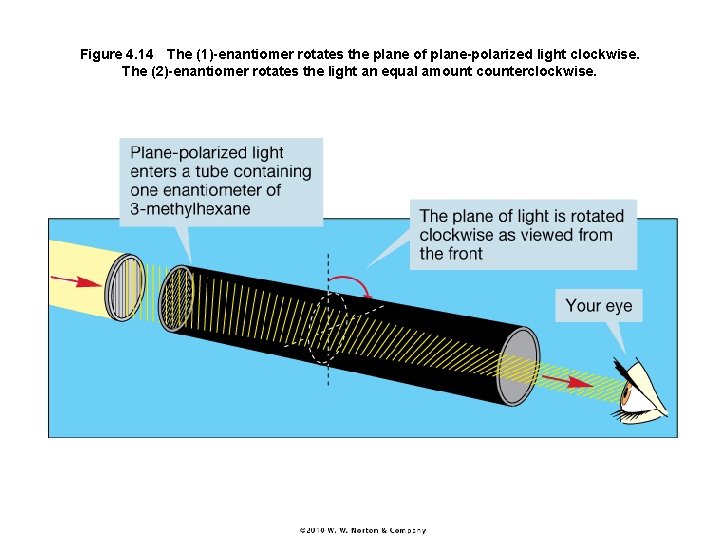
Figure 4. 14 The (1)-enantiomer rotates the plane of plane-polarized light clockwise. The (2)-enantiomer rotates the light an equal amount counterclockwise.

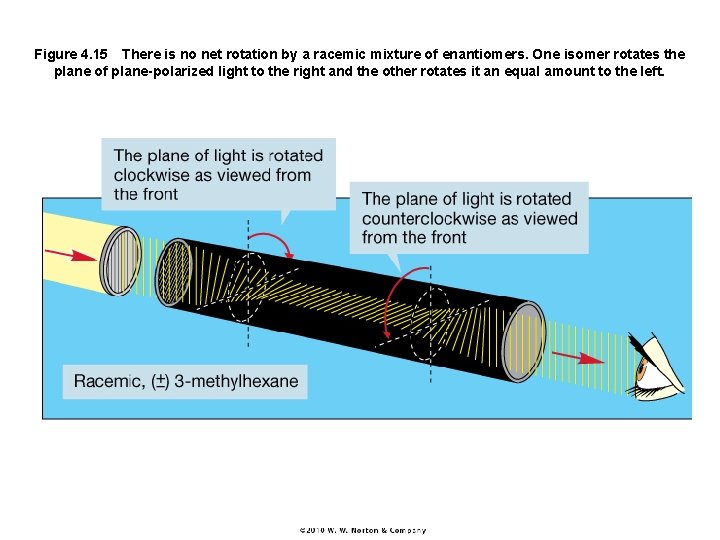
Figure 4. 15 There is no net rotation by a racemic mixture of enantiomers. One isomer rotates the plane of plane-polarized light to the right and the other rotates it an equal amount to the left.

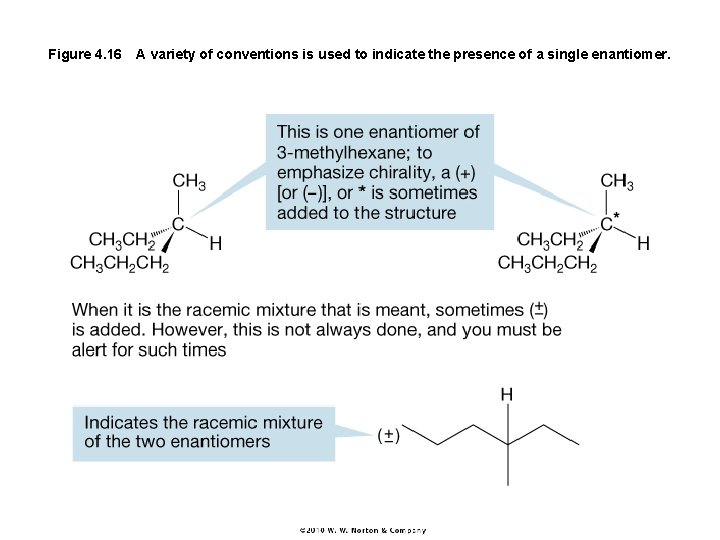
Figure 4. 16 A variety of conventions is used to indicate the presence of a single enantiomer.

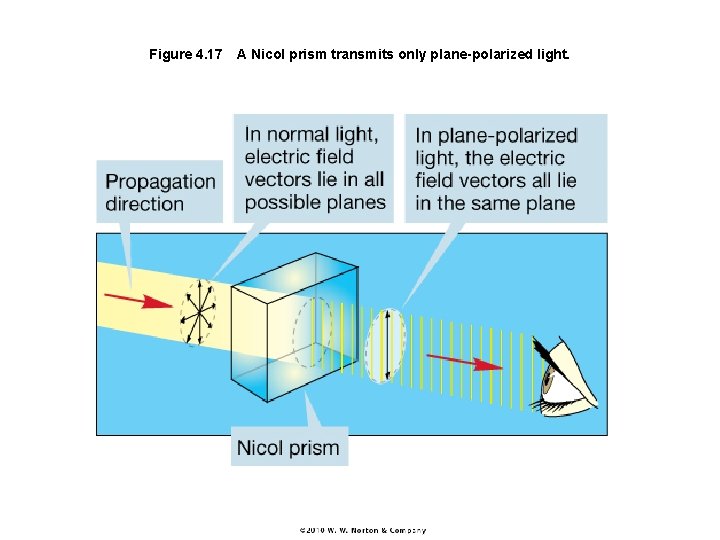
Figure 4. 17 A Nicol prism transmits only plane-polarized light.

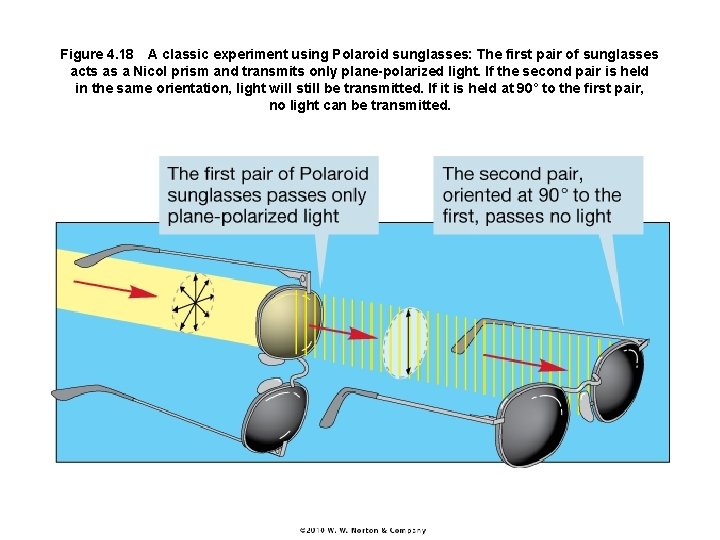
Figure 4. 18 A classic experiment using Polaroid sunglasses: The first pair of sunglasses acts as a Nicol prism and transmits only plane-polarized light. If the second pair is held in the same orientation, light will still be transmitted. If it is held at 90° to the first pair, no light can be transmitted.

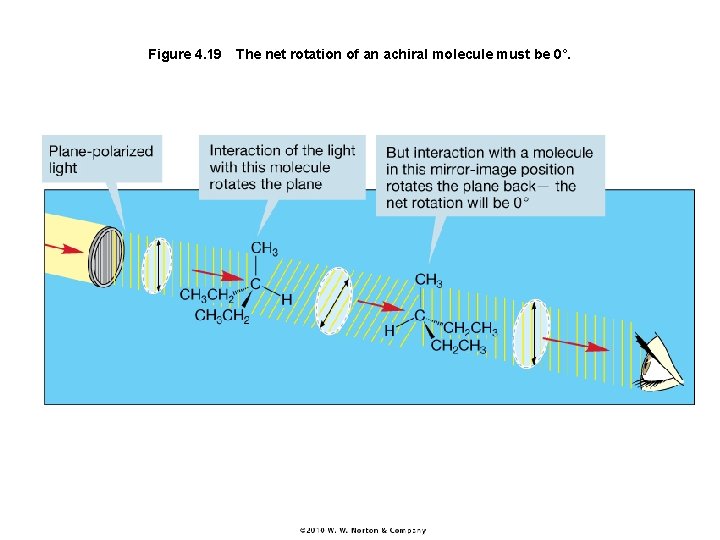
Figure 4. 19 The net rotation of an achiral molecule must be 0°.

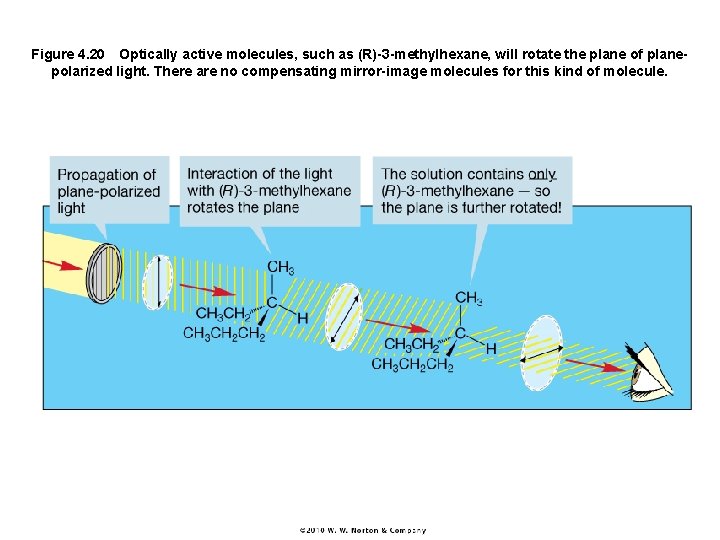
Figure 4. 20 Optically active molecules, such as (R)-3 -methylhexane, will rotate the plane of planepolarized light. There are no compensating mirror-image molecules for this kind of molecule.

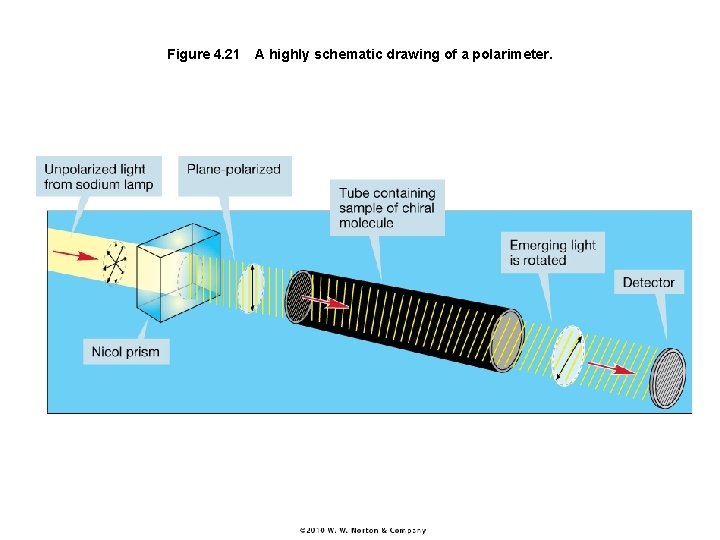
Figure 4. 21 A highly schematic drawing of a polarimeter.

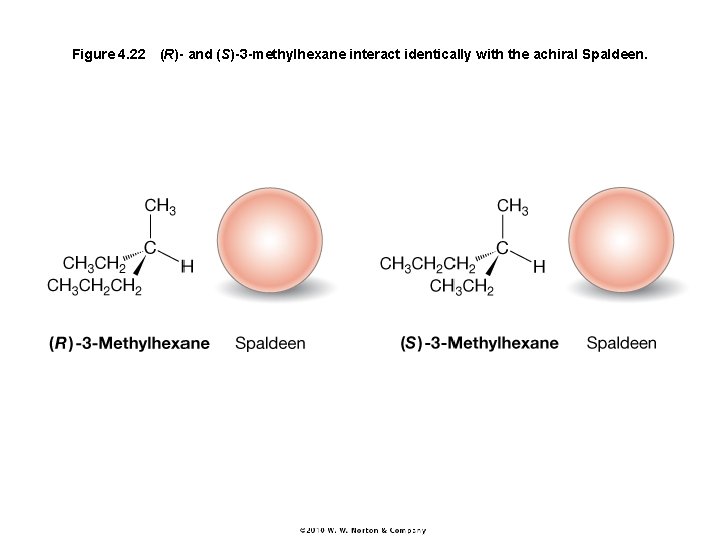
Figure 4. 22 (R)- and (S)-3 -methylhexane interact identically with the achiral Spaldeen.

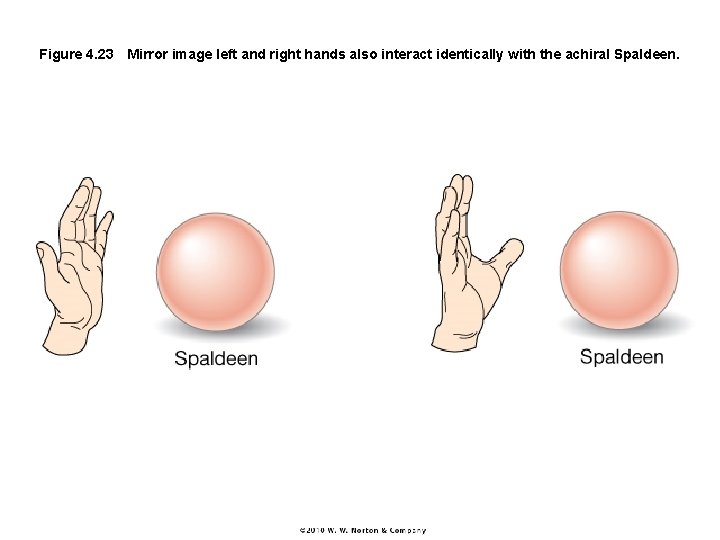
Figure 4. 23 Mirror image left and right hands also interact identically with the achiral Spaldeen.

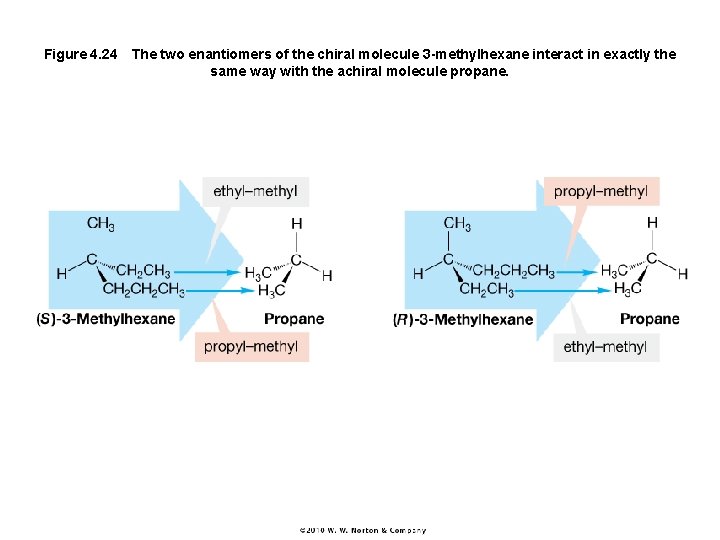
Figure 4. 24 The two enantiomers of the chiral molecule 3 -methylhexane interact in exactly the same way with the achiral molecule propane.

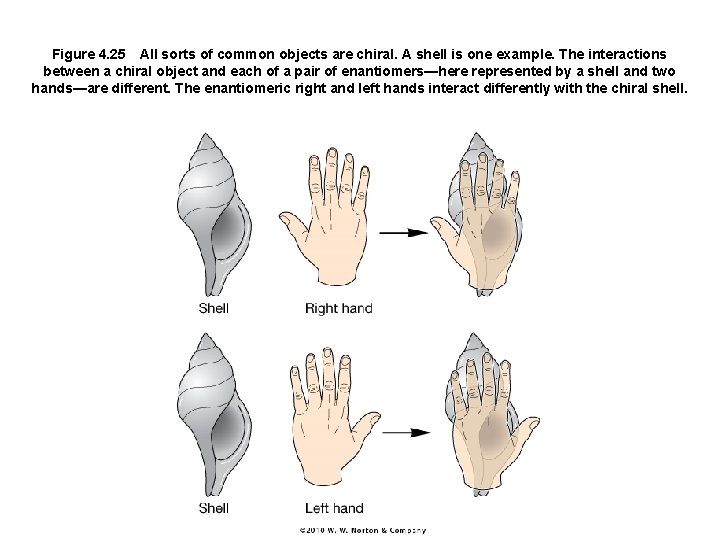
Figure 4. 25 All sorts of common objects are chiral. A shell is one example. The interactions between a chiral object and each of a pair of enantiomers—here represented by a shell and two hands—are different. The enantiomeric right and left hands interact differently with the chiral shell.

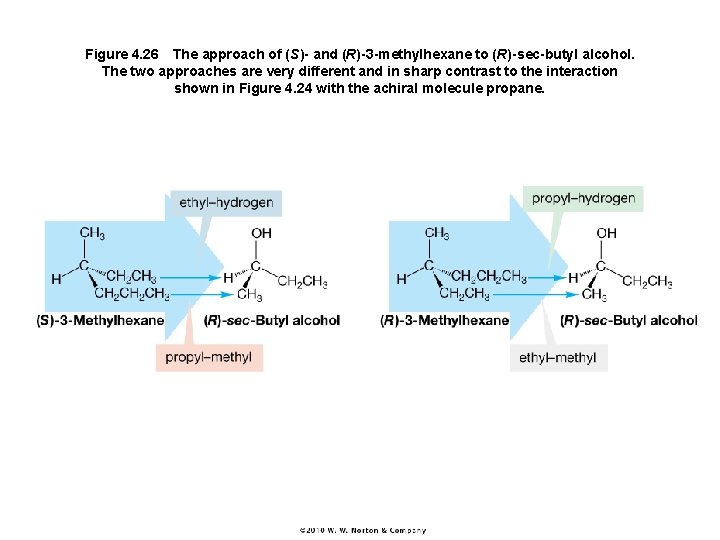
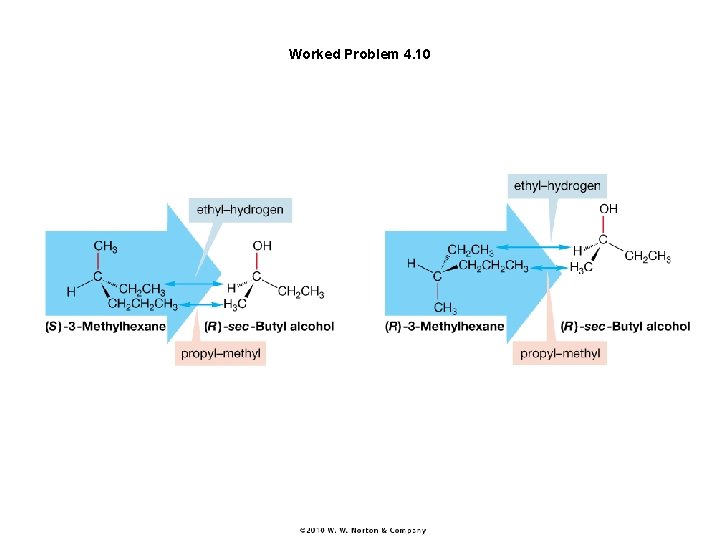
Figure 4. 26 The approach of (S)- and (R)-3 -methylhexane to (R)-sec-butyl alcohol. The two approaches are very different and in sharp contrast to the interaction shown in Figure 4. 24 with the achiral molecule propane.

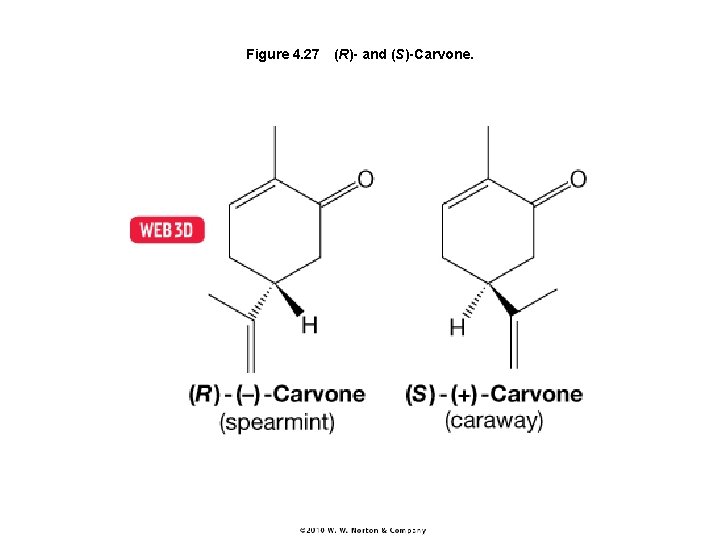
Figure 4. 27 (R)- and (S)-Carvone.

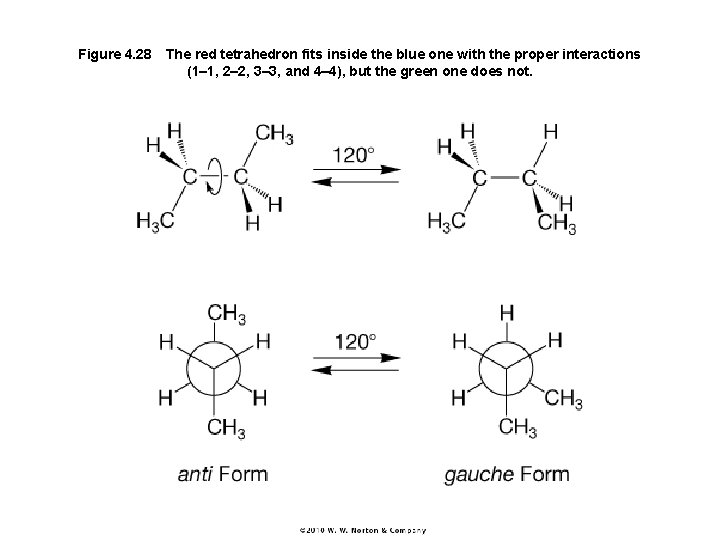
Figure 4. 28 The red tetrahedron fits inside the blue one with the proper interactions (1– 1, 2– 2, 3– 3, and 4– 4), but the green one does not.

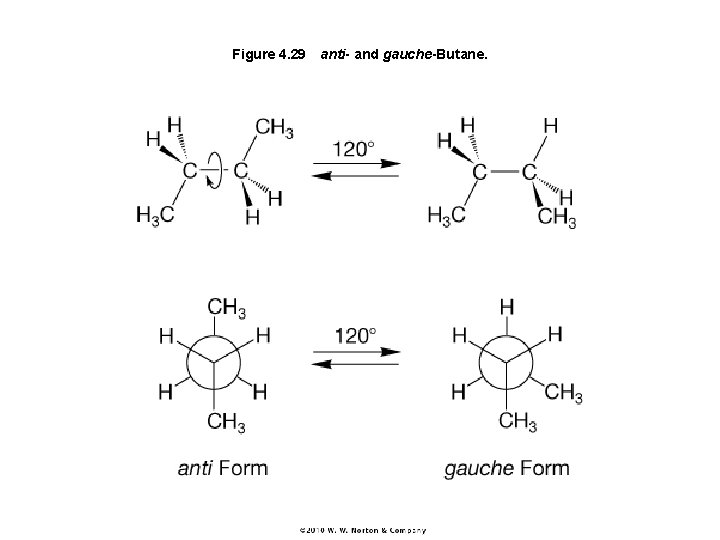
Figure 4. 29 anti- and gauche-Butane.

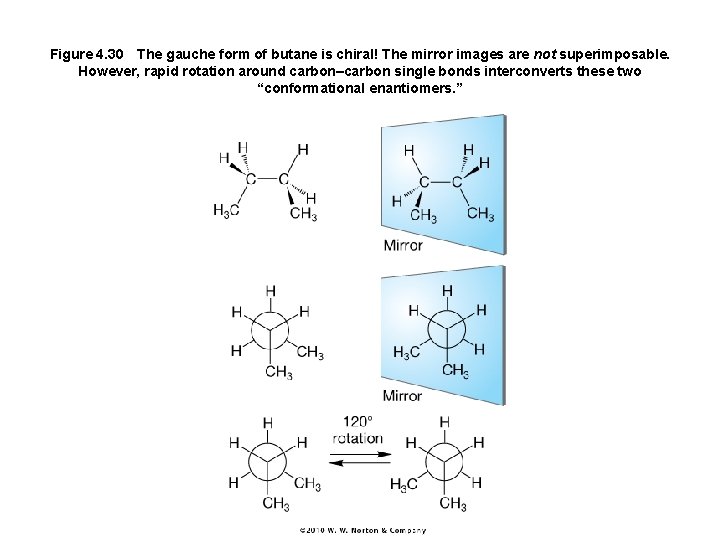
Figure 4. 30 The gauche form of butane is chiral! The mirror images are not superimposable. However, rapid rotation around carbon–carbon single bonds interconverts these two “conformational enantiomers. ”

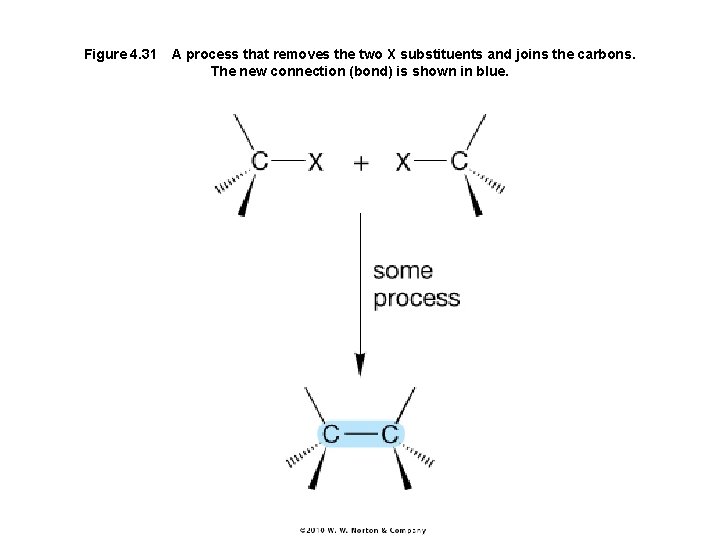
Figure 4. 31 A process that removes the two X substituents and joins the carbons. The new connection (bond) is shown in blue.

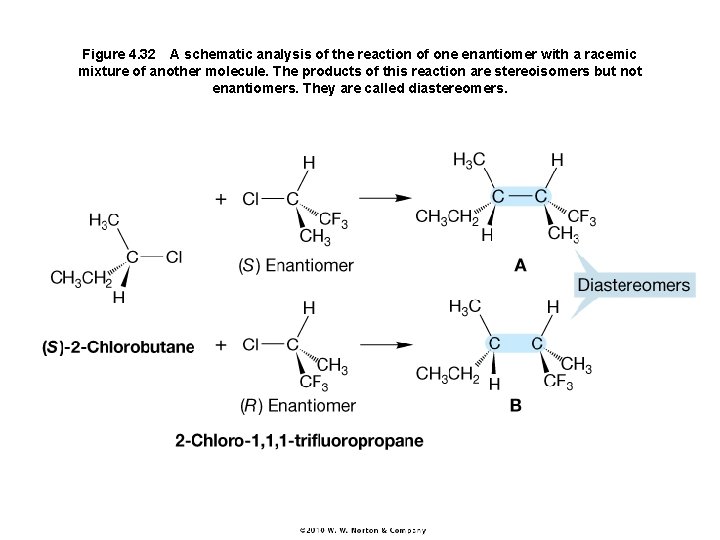
Figure 4. 32 A schematic analysis of the reaction of one enantiomer with a racemic mixture of another molecule. The products of this reaction are stereoisomers but not enantiomers. They are called diastereomers.

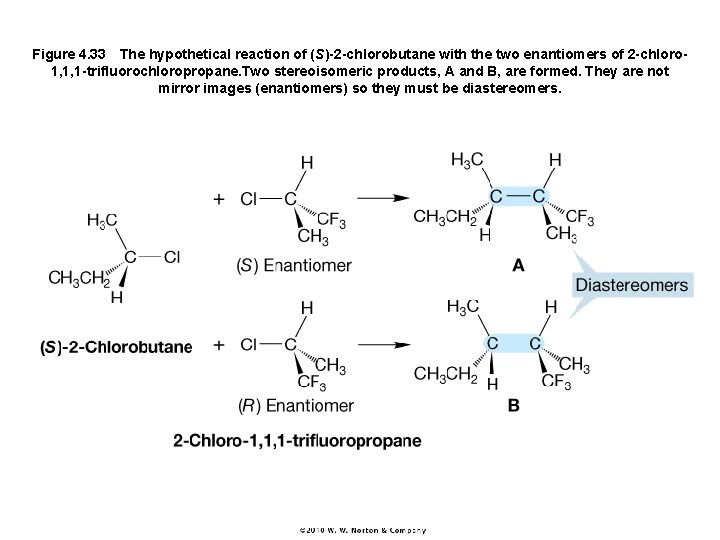
Figure 4. 33 The hypothetical reaction of (S)-2 -chlorobutane with the two enantiomers of 2 -chloro 1, 1, 1 -trifluorochloropropane. Two stereoisomeric products, A and B, are formed. They are not mirror images (enantiomers) so they must be diastereomers.

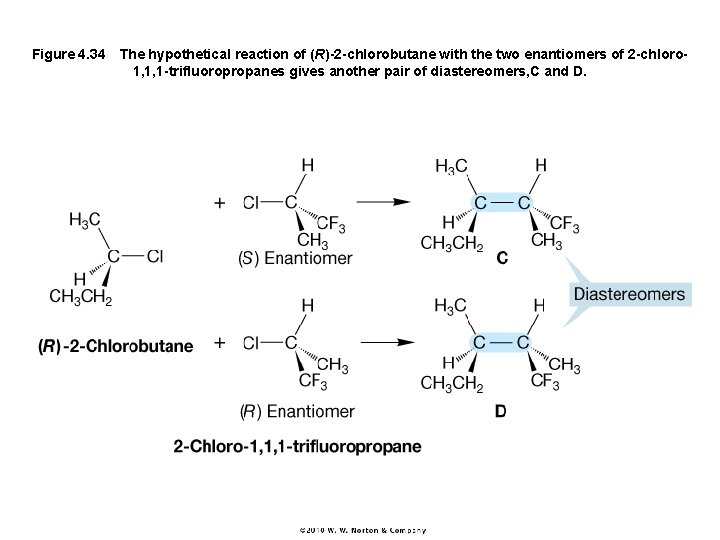
Figure 4. 34 The hypothetical reaction of (R)-2 -chlorobutane with the two enantiomers of 2 -chloro 1, 1, 1 -trifluoropropanes gives another pair of diastereomers, C and D.

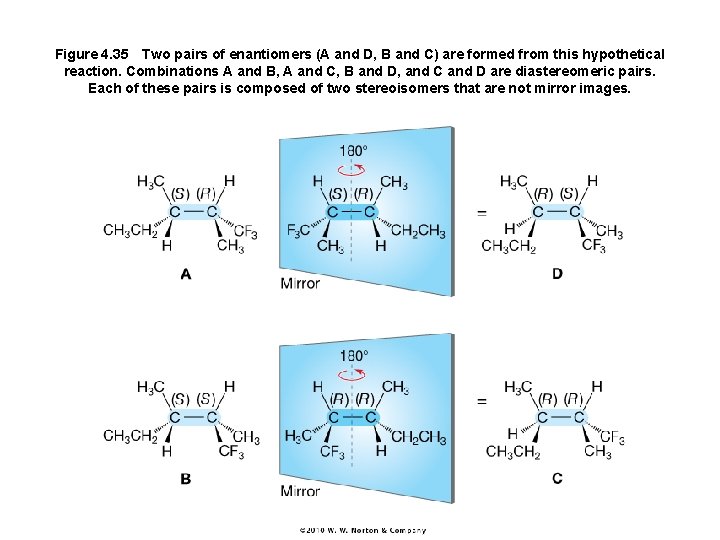
Figure 4. 35 Two pairs of enantiomers (A and D, B and C) are formed from this hypothetical reaction. Combinations A and B, A and C, B and D, and C and D are diastereomeric pairs. Each of these pairs is composed of two stereoisomers that are not mirror images.

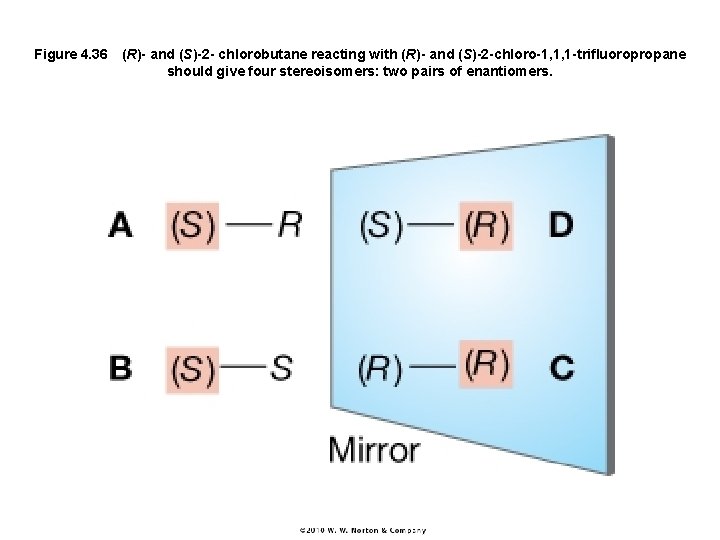
Figure 4. 36 (R)- and (S)-2 - chlorobutane reacting with (R)- and (S)-2 -chloro-1, 1, 1 -trifluoropropane should give four stereoisomers: two pairs of enantiomers.

Figure 4. 37 The eight possible stereoisomers for a molecule containing three stereogenic atoms.

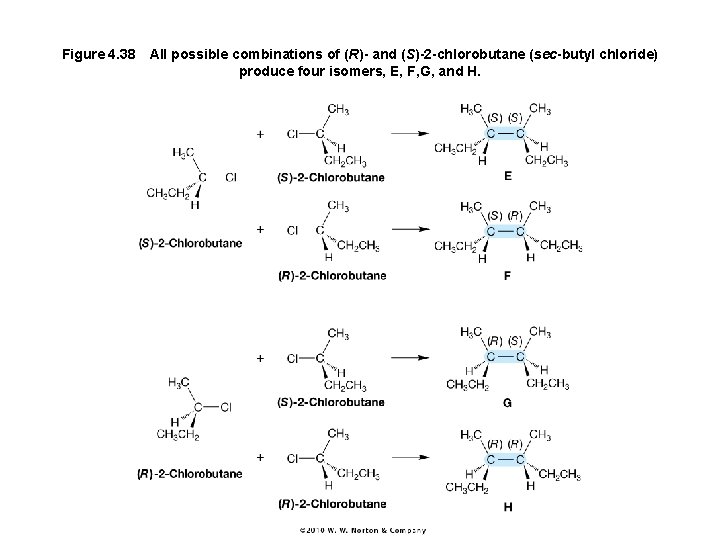
Figure 4. 38 All possible combinations of (R)- and (S)-2 -chlorobutane (sec-butyl chloride) produce four isomers, E, F, G, and H.

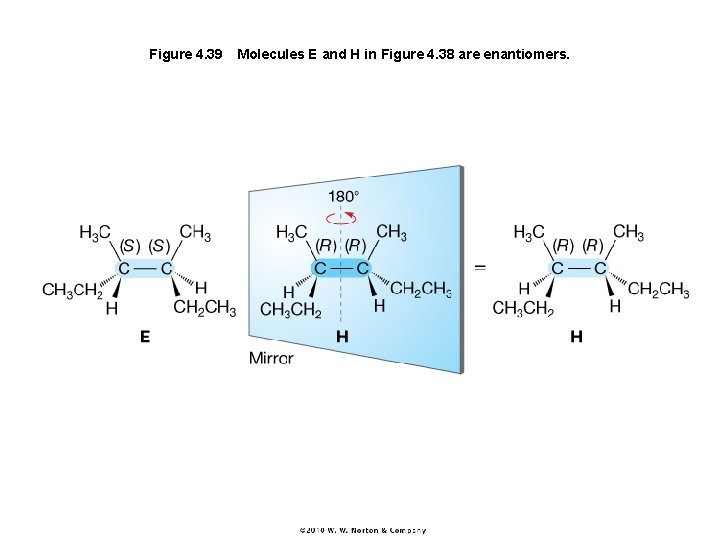
Figure 4. 39 Molecules E and H in Figure 4. 38 are enantiomers.

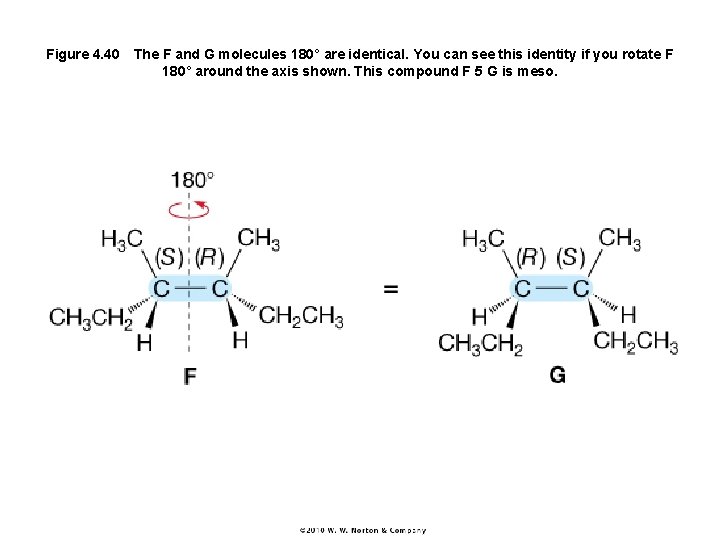
Figure 4. 40 The F and G molecules 180° are identical. You can see this identity if you rotate F 180° around the axis shown. This compound F 5 G is meso.

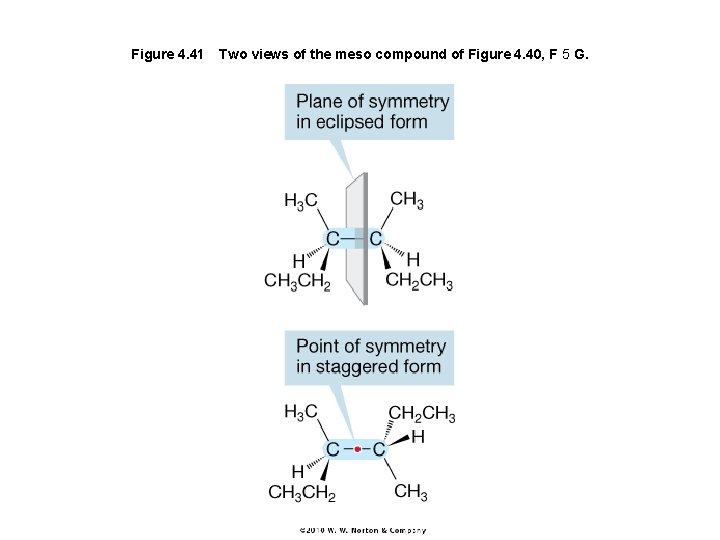
Figure 4. 41 Two views of the meso compound of Figure 4. 40, F 5 G.

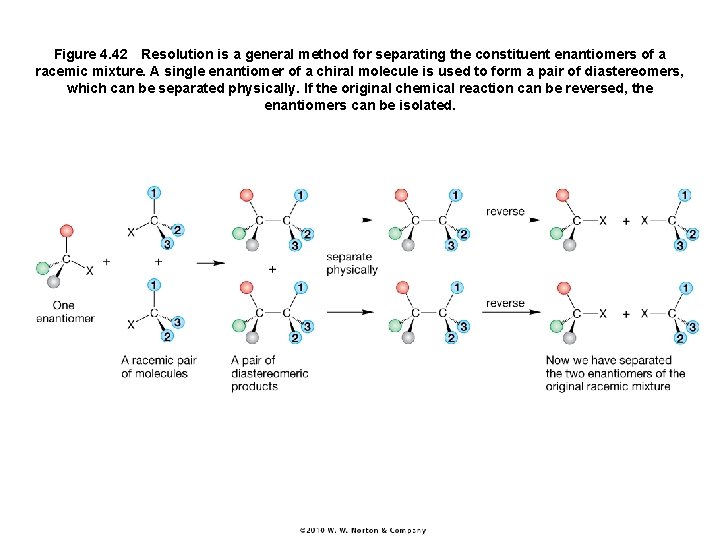
Figure 4. 42 Resolution is a general method for separating the constituent enantiomers of a racemic mixture. A single enantiomer of a chiral molecule is used to form a pair of diastereomers, which can be separated physically. If the original chemical reaction can be reversed, the enantiomers can be isolated.

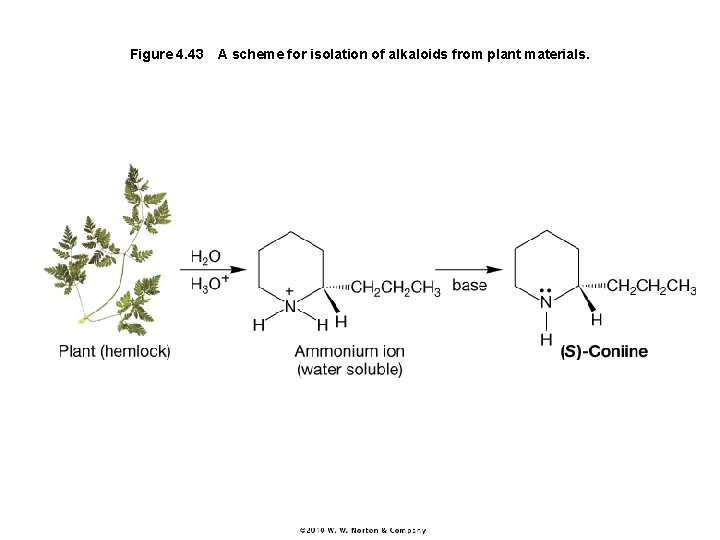
Figure 4. 43 A scheme for isolation of alkaloids from plant materials.

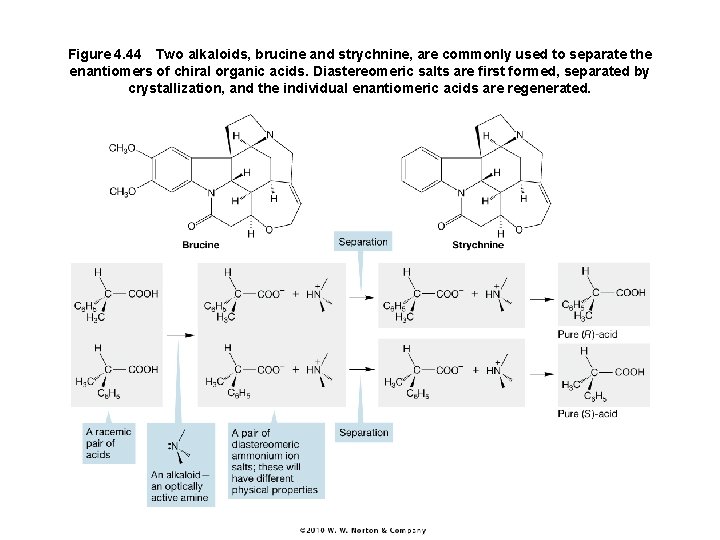
Figure 4. 44 Two alkaloids, brucine and strychnine, are commonly used to separate the enantiomers of chiral organic acids. Diastereomeric salts are first formed, separated by crystallization, and the individual enantiomeric acids are regenerated.

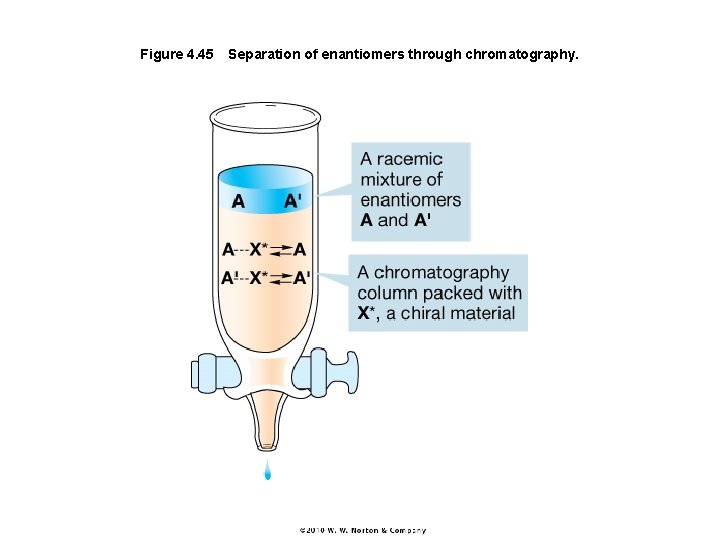
Figure 4. 45 Separation of enantiomers through chromatography.

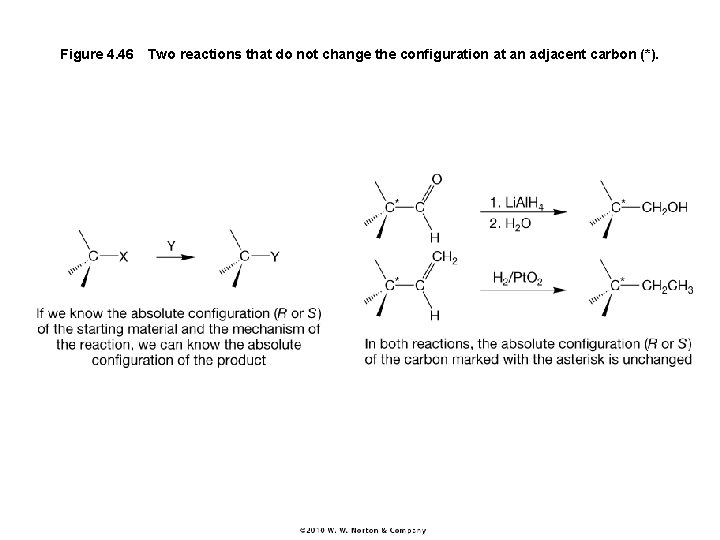
Figure 4. 46 Two reactions that do not change the configuration at an adjacent carbon (*).

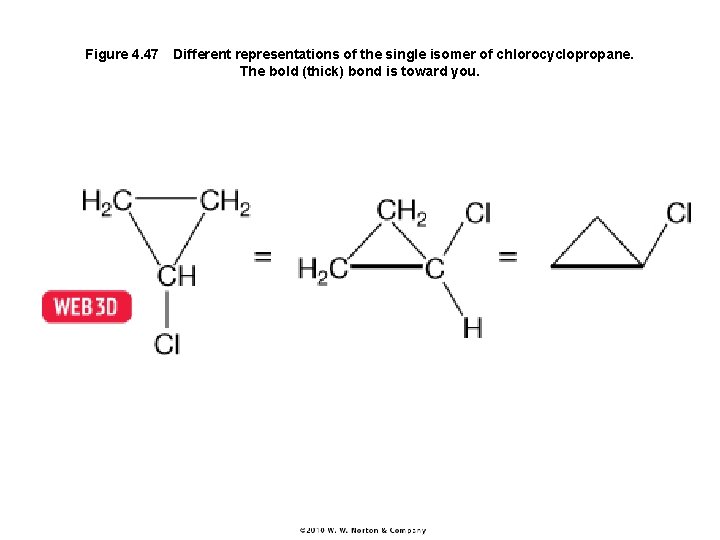
Figure 4. 47 Different representations of the single isomer of chlorocyclopropane. The bold (thick) bond is toward you.

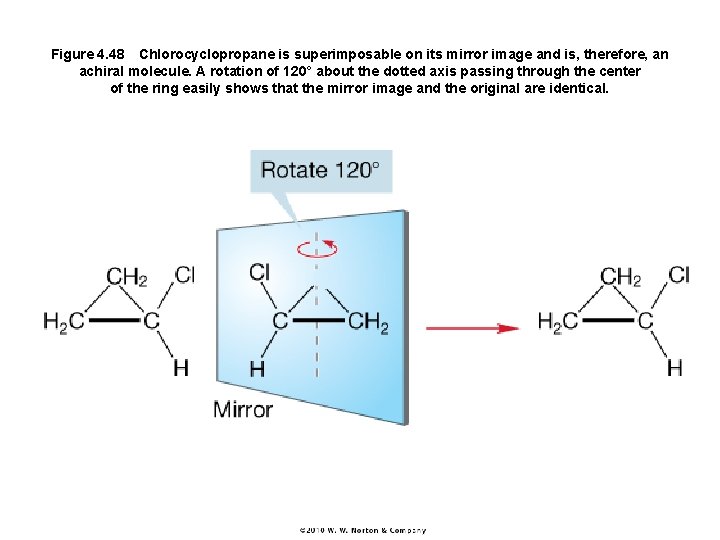
Figure 4. 48 Chlorocyclopropane is superimposable on its mirror image and is, therefore, an achiral molecule. A rotation of 120° about the dotted axis passing through the center of the ring easily shows that the mirror image and the original are identical.

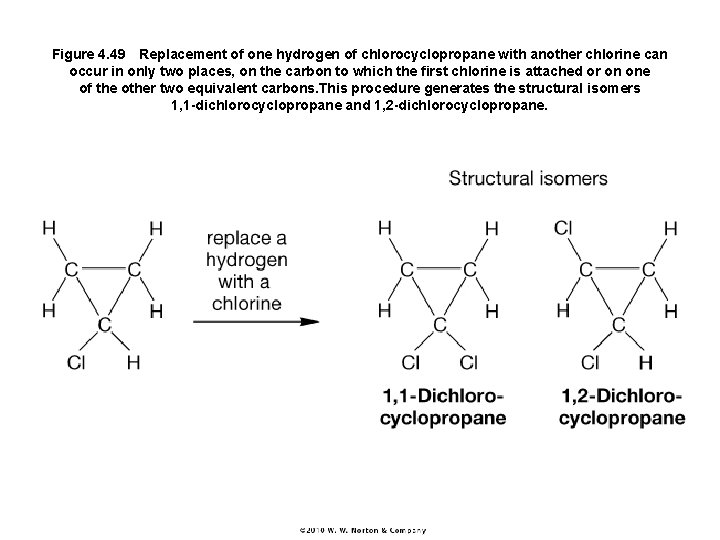
Figure 4. 49 Replacement of one hydrogen of chlorocyclopropane with another chlorine can occur in only two places, on the carbon to which the first chlorine is attached or on one of the other two equivalent carbons. This procedure generates the structural isomers 1, 1 -dichlorocyclopropane and 1, 2 -dichlorocyclopropane.

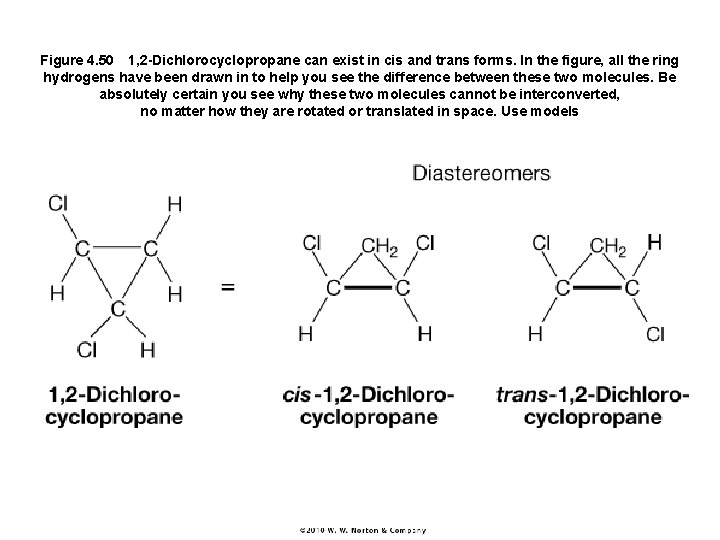
Figure 4. 50 1, 2 -Dichlorocyclopropane can exist in cis and trans forms. In the figure, all the ring hydrogens have been drawn in to help you see the difference between these two molecules. Be absolutely certain you see why these two molecules cannot be interconverted, no matter how they are rotated or translated in space. Use models

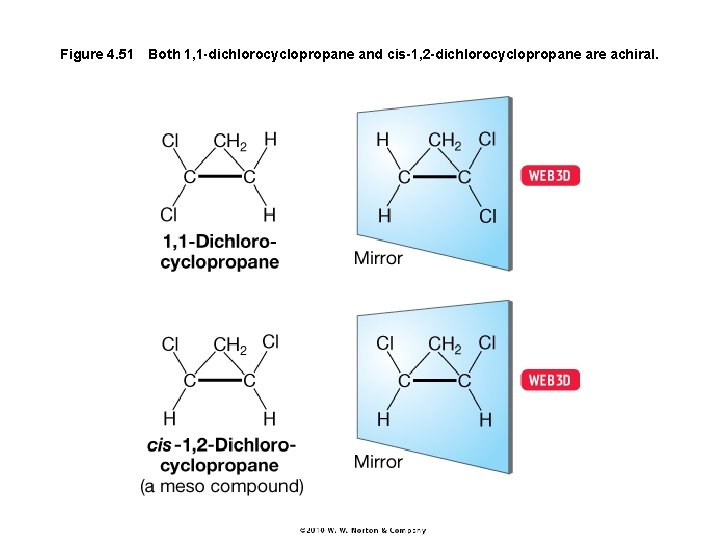
Figure 4. 51 Both 1, 1 -dichlorocyclopropane and cis-1, 2 -dichlorocyclopropane are achiral.

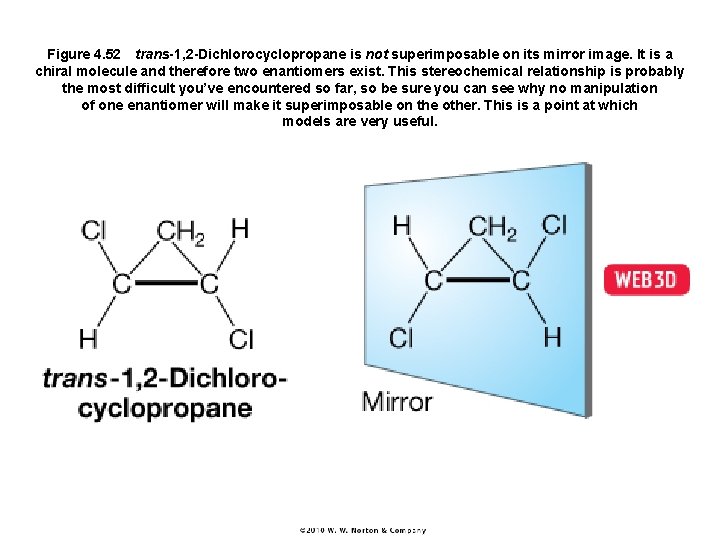
Figure 4. 52 trans-1, 2 -Dichlorocyclopropane is not superimposable on its mirror image. It is a chiral molecule and therefore two enantiomers exist. This stereochemical relationship is probably the most difficult you’ve encountered so far, so be sure you can see why no manipulation of one enantiomer will make it superimposable on the other. This is a point at which models are very useful.

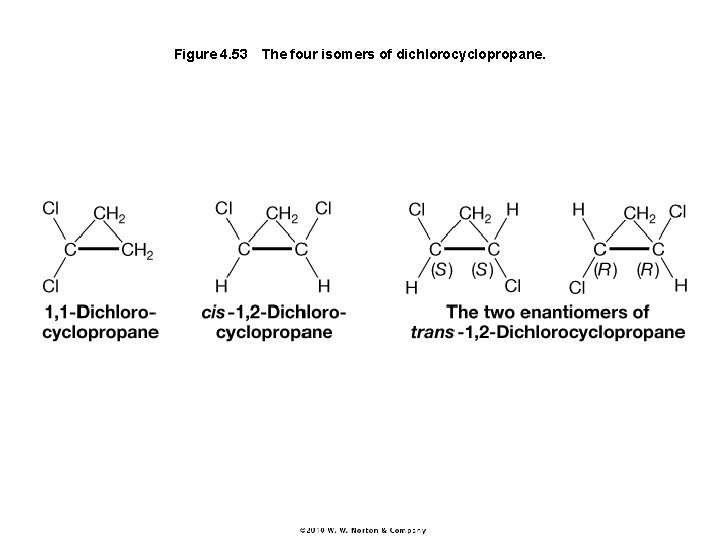
Figure 4. 53 The four isomers of dichlorocyclopropane.

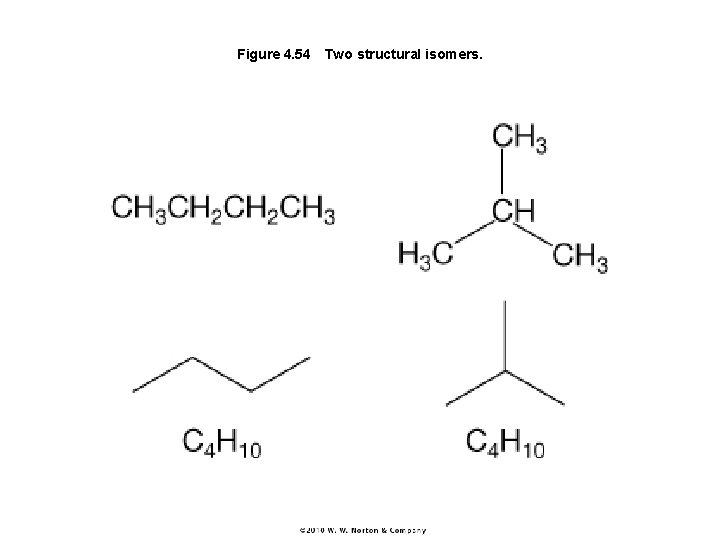
Figure 4. 54 Two structural isomers.

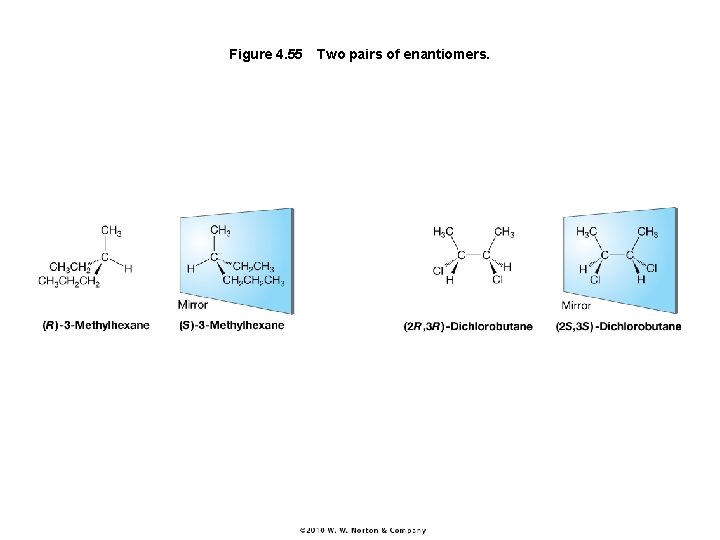
Figure 4. 55 Two pairs of enantiomers.

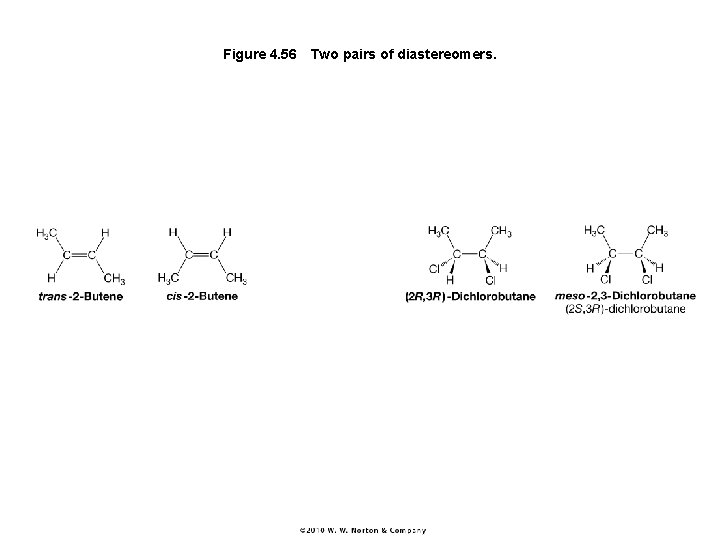
Figure 4. 56 Two pairs of diastereomers.

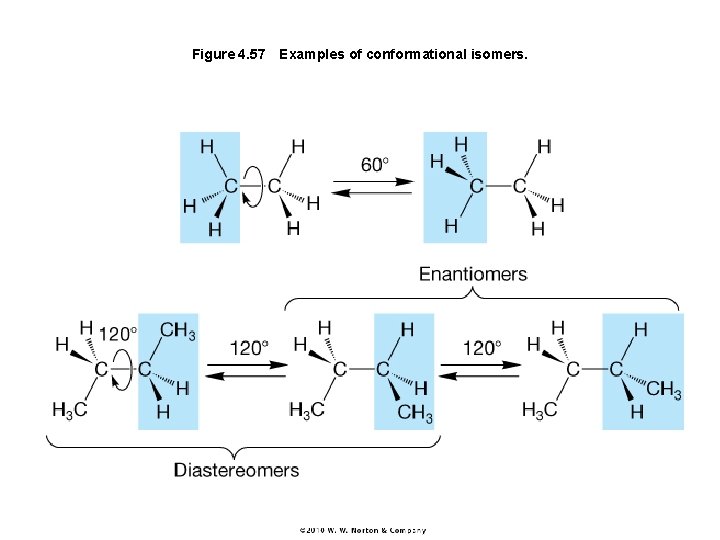
Figure 4. 57 Examples of conformational isomers.

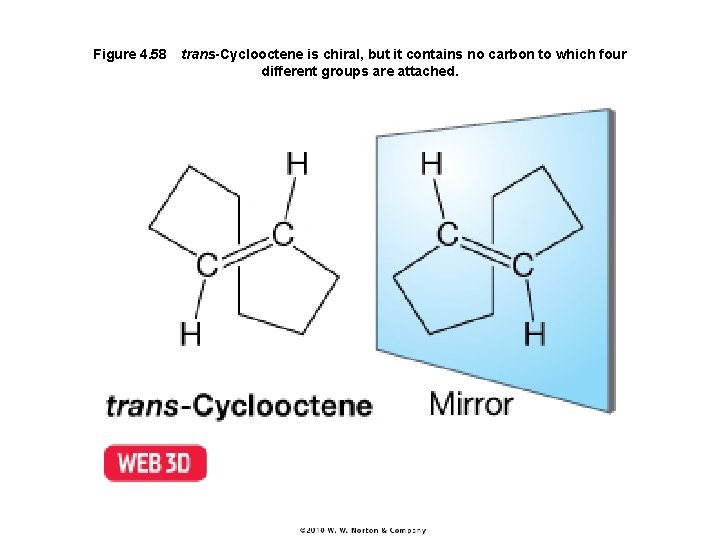
Figure 4. 58 trans-Cyclooctene is chiral, but it contains no carbon to which four different groups are attached.

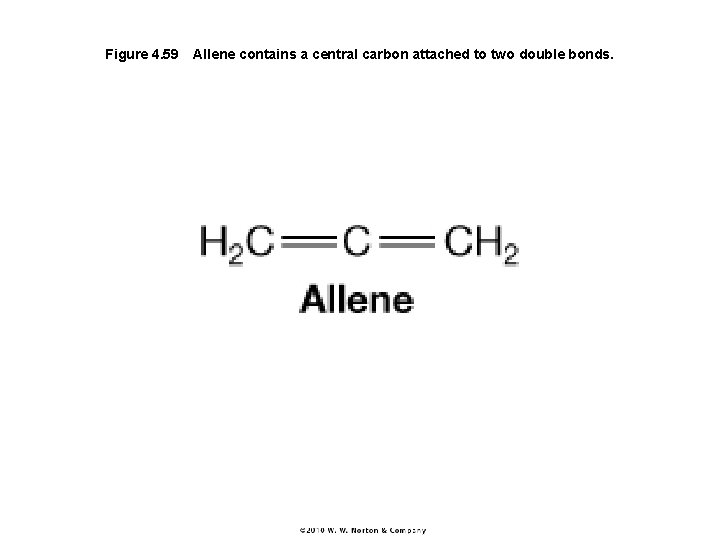
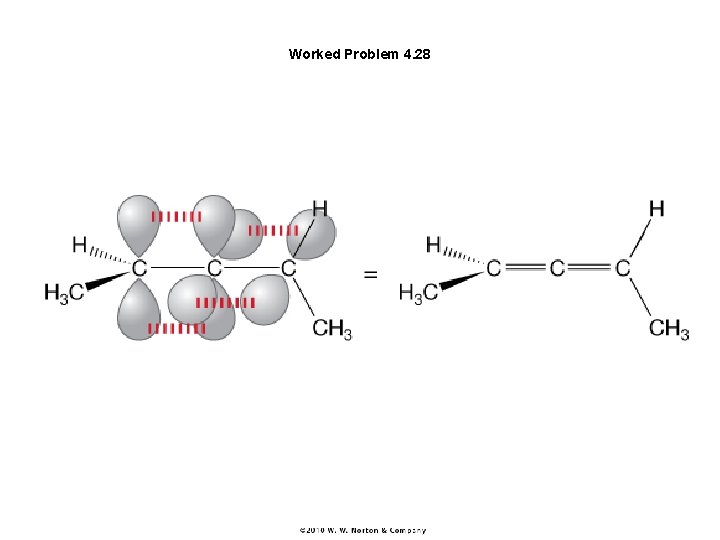
Figure 4. 59 Allene contains a central carbon attached to two double bonds.

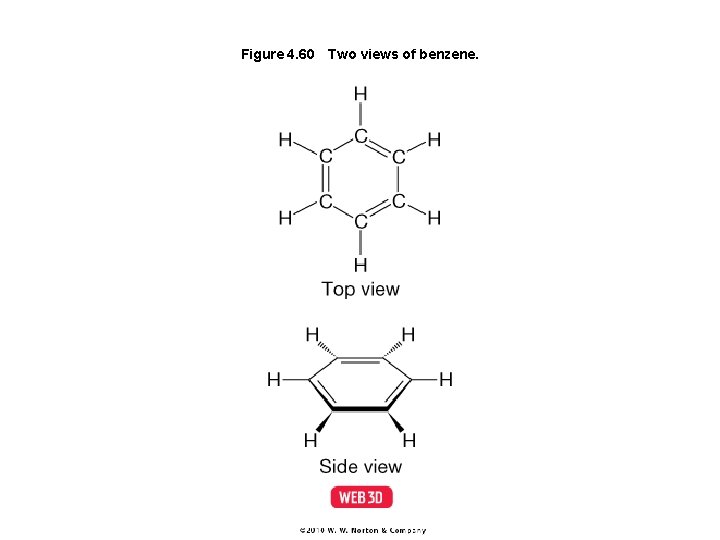
Figure 4. 60 Two views of benzene.

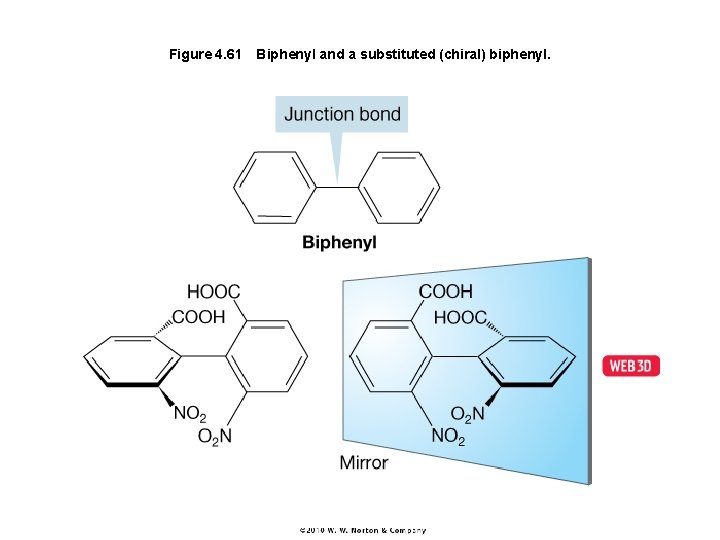
Figure 4. 61 Biphenyl and a substituted (chiral) biphenyl.

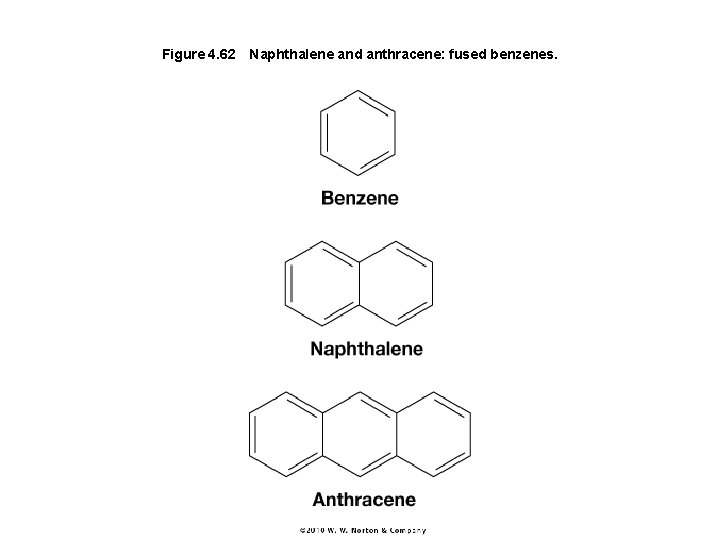
Figure 4. 62 Naphthalene and anthracene: fused benzenes.

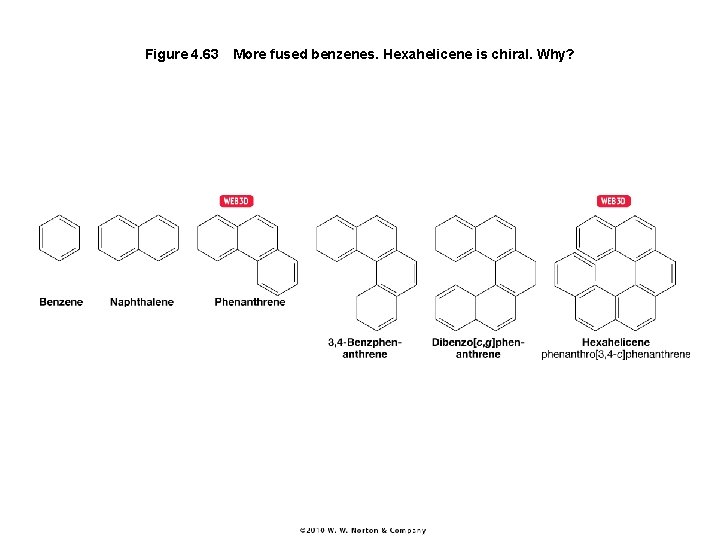
Figure 4. 63 More fused benzenes. Hexahelicene is chiral. Why?

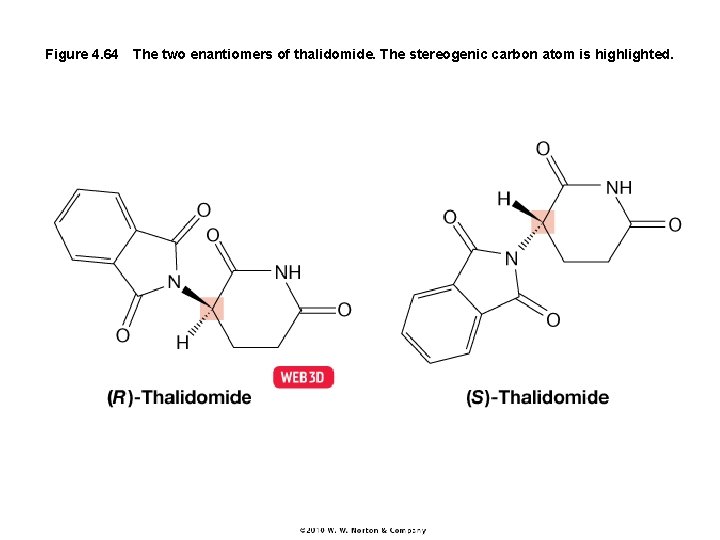
Figure 4. 64 The two enantiomers of thalidomide. The stereogenic carbon atom is highlighted.

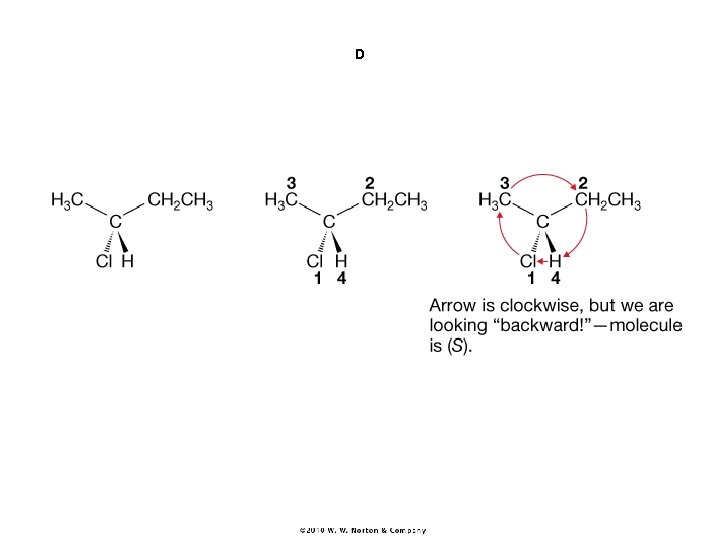
B

C

D

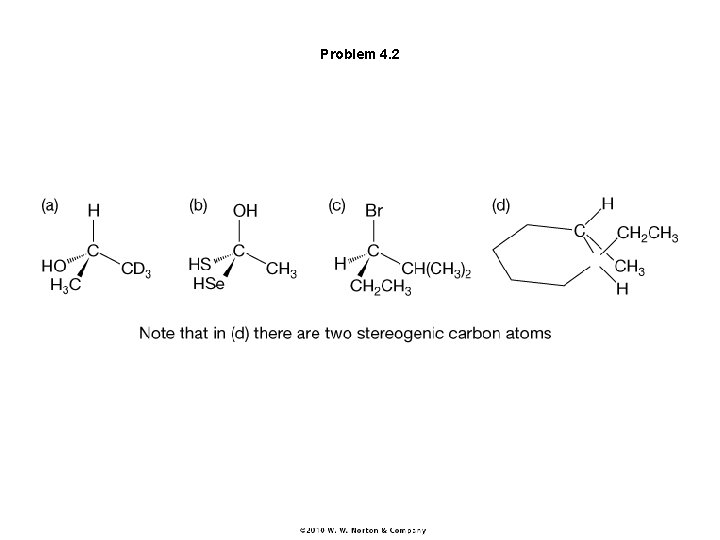
Problem 4. 2

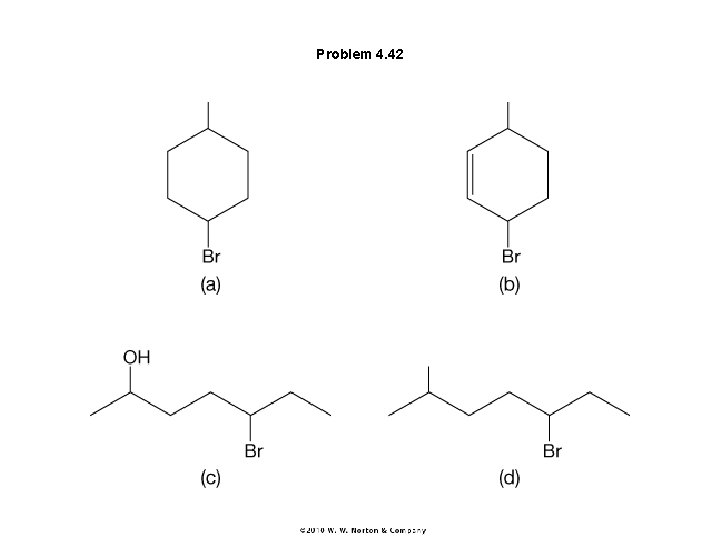
Problem 4. 42

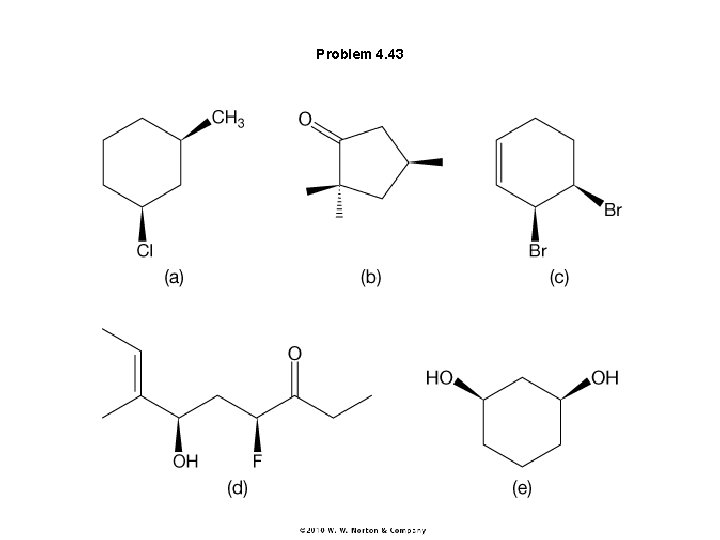
Problem 4. 43

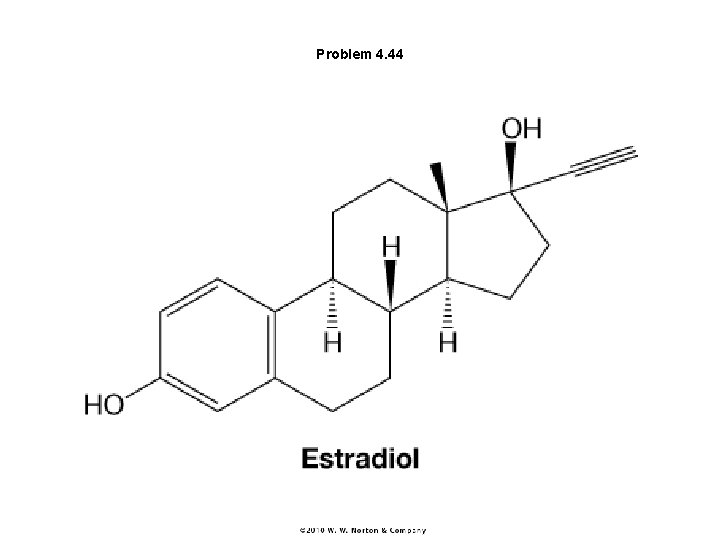
Problem 4. 44

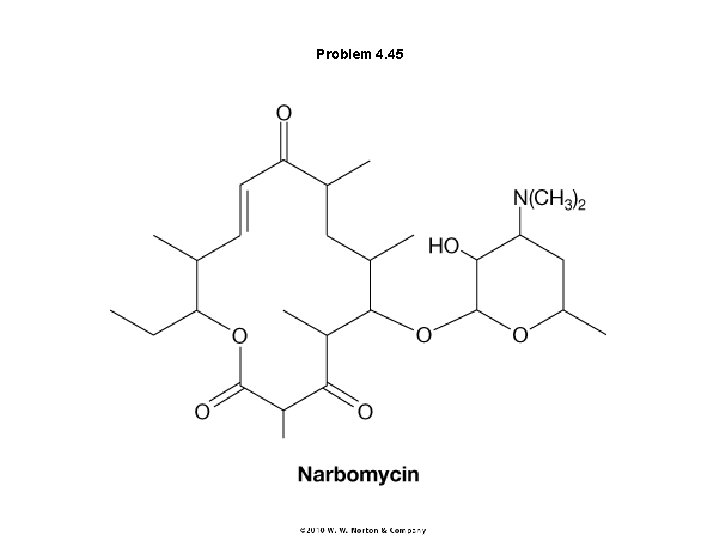
Problem 4. 45

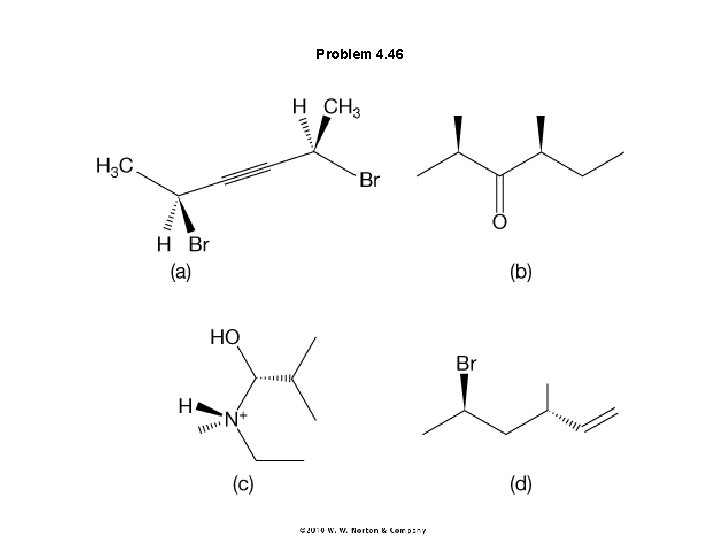
Problem 4. 46

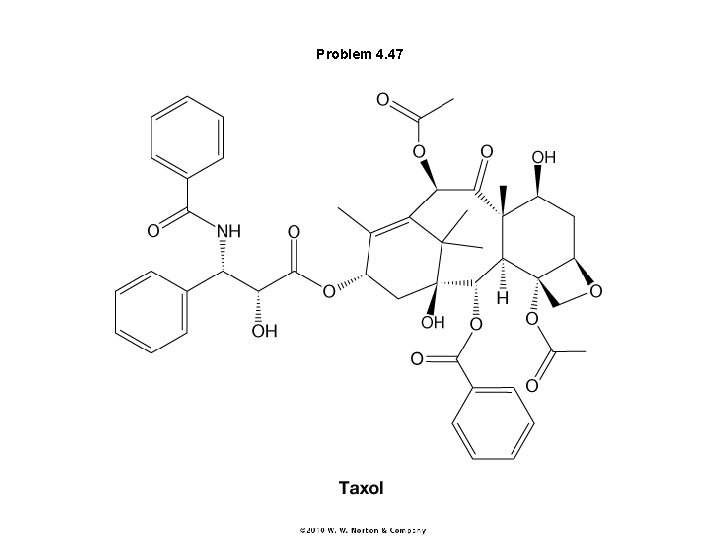
Problem 4. 47

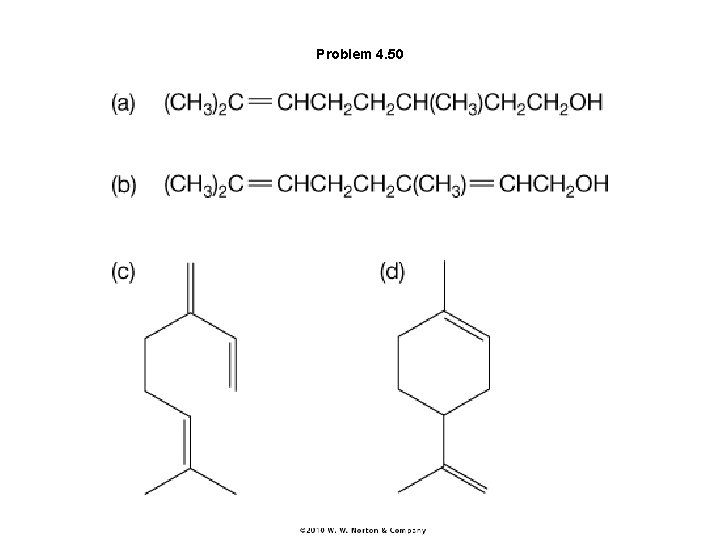
Problem 4. 50

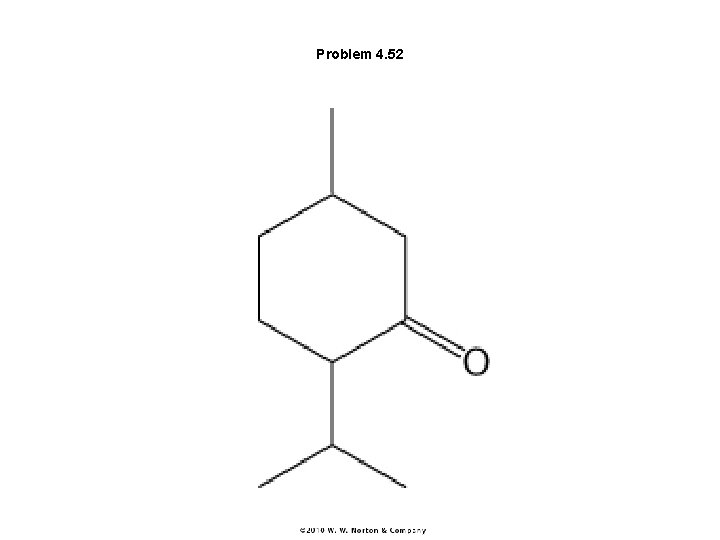
Problem 4. 52

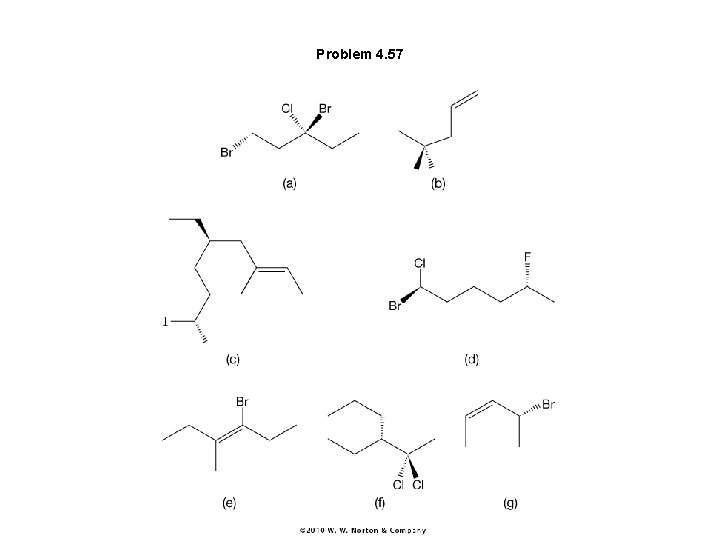
Problem 4. 57

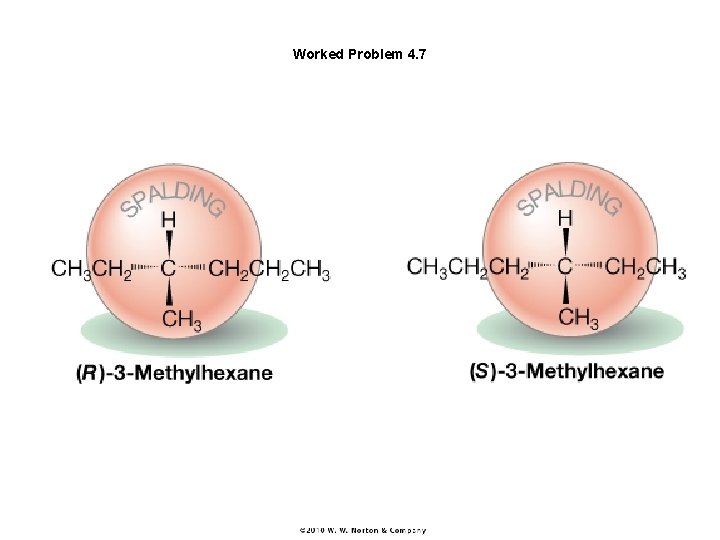
Worked Problem 4. 7

Worked Problem 4. 10

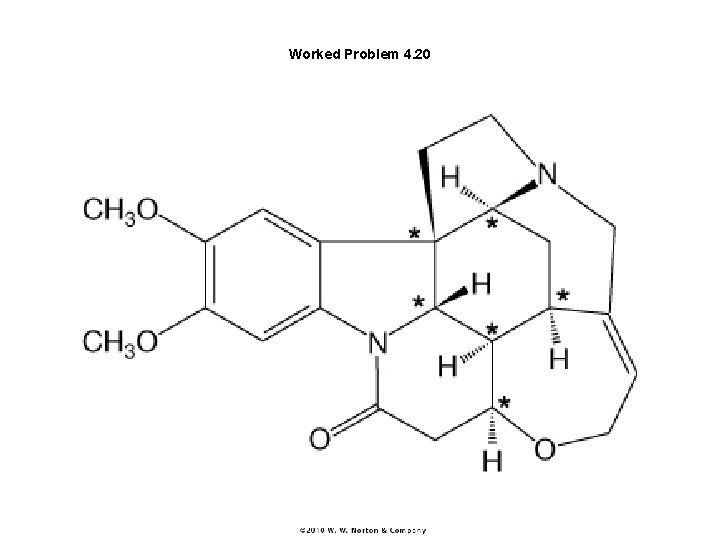
Worked Problem 4. 20

Worked Problem 4. 28

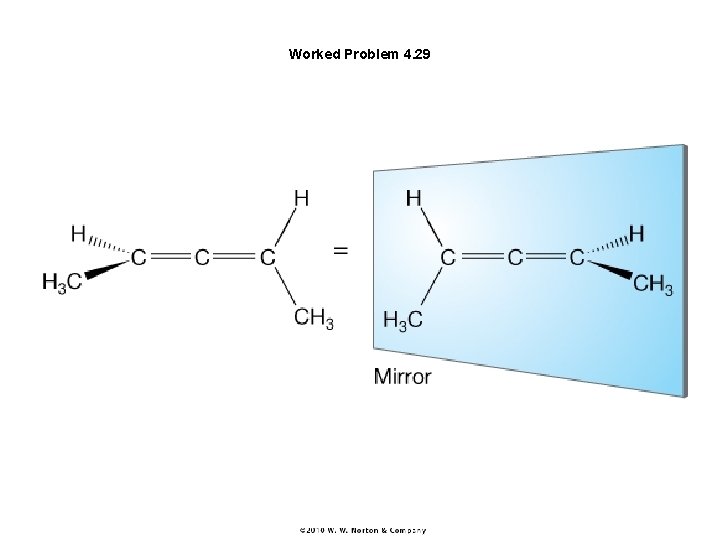
Worked Problem 4. 29