Heat Review A When you touch ice heat






- Slides: 23

Heat Review A. When you touch ice, heat is transferred from 1) your hand to the ice 2) the ice to your hand B. When you drink a hot cup of coffee, heat is transferred from 1) your mouth to the coffee 2) the coffee to your mouth General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

Heat Review A. When you touch ice, heat is transferred from 1) your hand to the ice B. When you drink a hot cup of coffee, heat is transferred from 2) the coffee to your mouth General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2

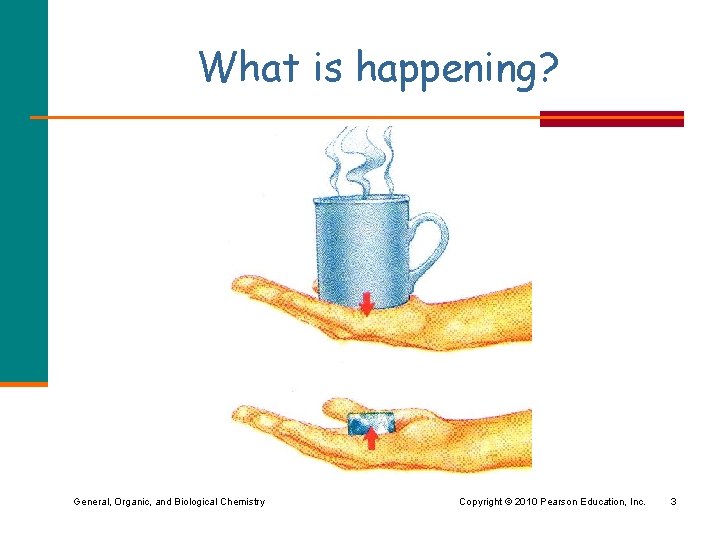
What is happening? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

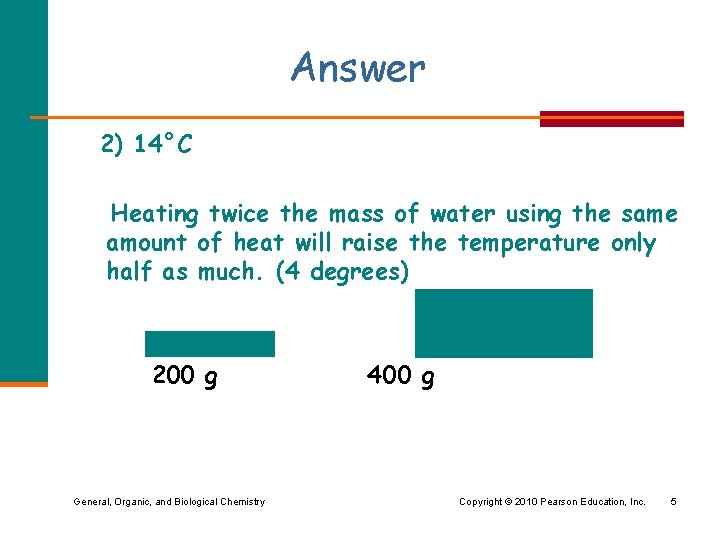
What do you think? When you heat 200 g of water for 1 minute, the water temperature rises from 10°C to 18°C. 200 g 400 g If you heat 400 g of water at 10°C in the same pan with the same amount of heat for 1 minute, what would you expect the final temperature to be? 1) 10 °C 2) 14°C 3) 18°C General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

Answer 2) 14°C Heating twice the mass of water using the same amount of heat will raise the temperature only half as much. (4 degrees) 200 g 400 g General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5

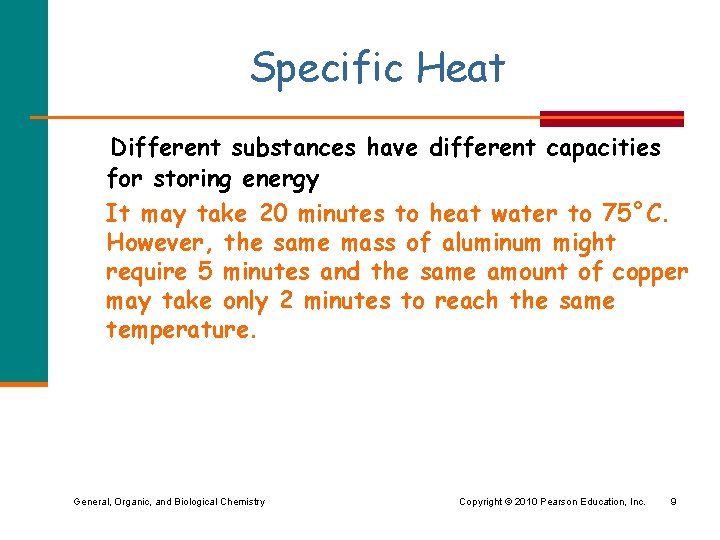
Specific Heat l Why do some foods stay hot longer than others? l Why is the beach sand hot, but the water is cool on the same hot day? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6

Specific Heat Some things heat up or cool down faster than others. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7

Specific Heat This is why land heats up quickly during the day and cools quickly at night and why water takes longer. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

Specific Heat Different substances have different capacities for storing energy It may take 20 minutes to heat water to 75°C. However, the same mass of aluminum might require 5 minutes and the same amount of copper may take only 2 minutes to reach the same temperature. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9

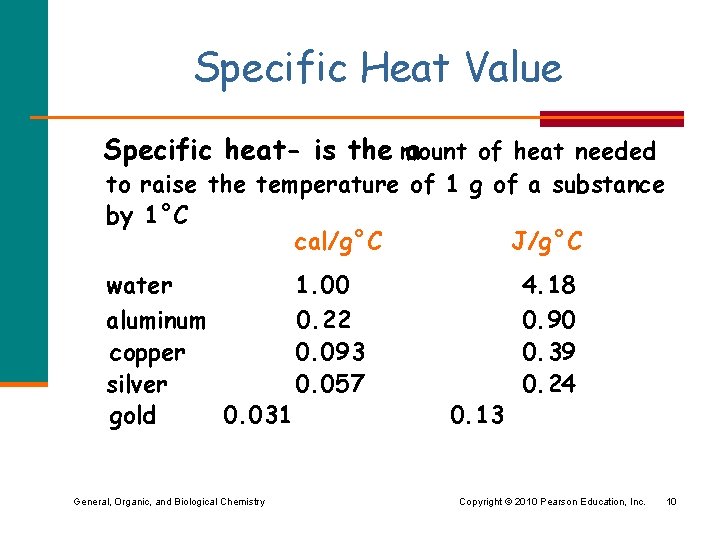
Specific Heat Value Specific heat- is the mount a of heat needed to raise the temperature of 1 g of a substance by 1°C cal/g°C J/g°C water 1. 00 aluminum 0. 22 copper 0. 093 silver 0. 057 gold 0. 031 General, Organic, and Biological Chemistry 0. 13 4. 18 0. 90 0. 39 0. 24 Copyright © 2010 Pearson Education, Inc. 10

Review A. A substance with a large specific heat 1) heats up quickly 2) heats up slowly B. When ocean water cools, the surrounding air 1) cools 2) warms 3) stays the same C. Sand in the desert is hot in the day, and cool at night. Sand must have a 1) high specific heat 2) low specific heat General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11

Answers A. A substance with alarge specific heat 2) heats up slowly B. When ocean water cools, the surrounding air 2) warms C. Sand in the desert is hot in the day, and cool at night. Sand must have a 2) low specific heat General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12

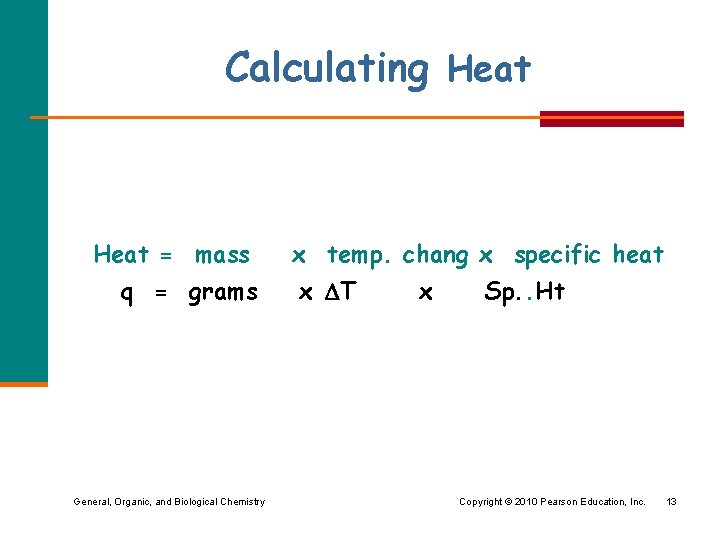
Calculating Heat = mass q = grams General, Organic, and Biological Chemistry x temp. chang x specific heat x T x Sp. . Ht Copyright © 2010 Pearson Education, Inc. 13

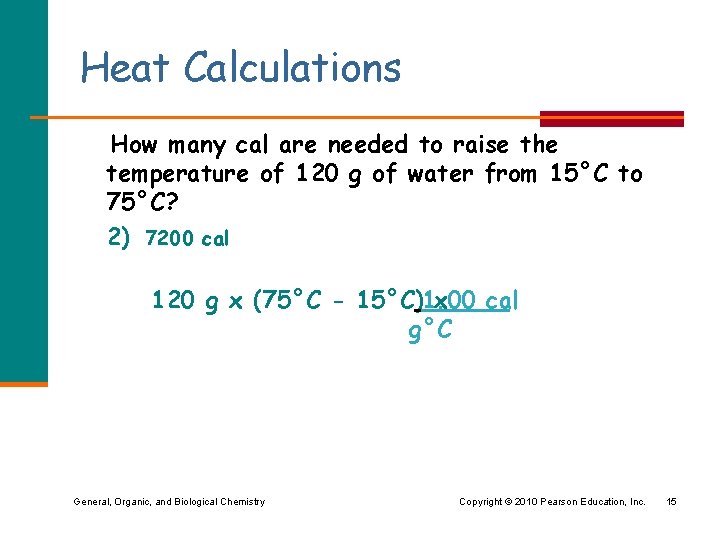
Heat Calculations How many cal are needed to raise the temperature of 120 g of water from 15°C to 75°C? 1) 1800 cal 2) 7200 cal 3) 9000 cal General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 14

Heat Calculations How many cal are needed to raise the temperature of 120 g of water from 15°C to 75°C? 2) 7200 cal 120 g x (75°C - 15°C)1. 00 x cal g°C General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 15

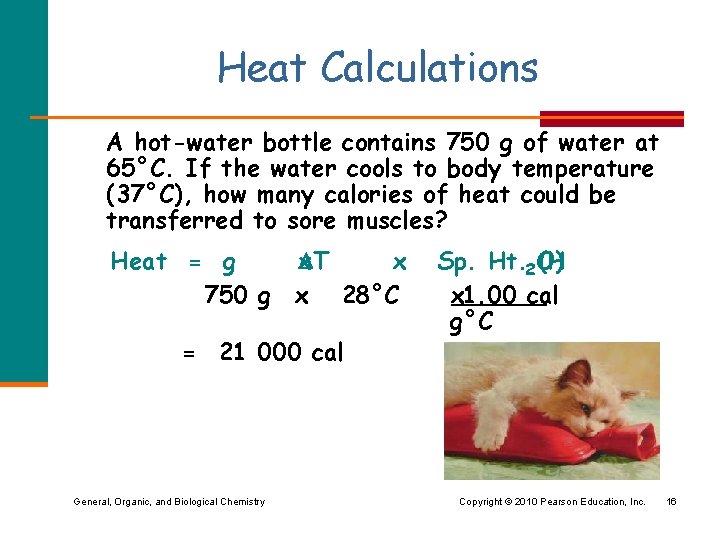
Heat Calculations A hot-water bottle contains 750 g of water at 65°C. If the water cools to body temperature (37°C), how many calories of heat could be transferred to sore muscles? Heat = g 750 g x T x x 28°C = 21 000 cal General, Organic, and Biological Chemistry Sp. Ht. 2 O) (H x 1. 00 cal g°C Copyright © 2010 Pearson Education, Inc. 16

The variation in temperature between night and day on Mars is 150 degrees Fahrenheit, but on Earth it isn’t……. Why? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 17

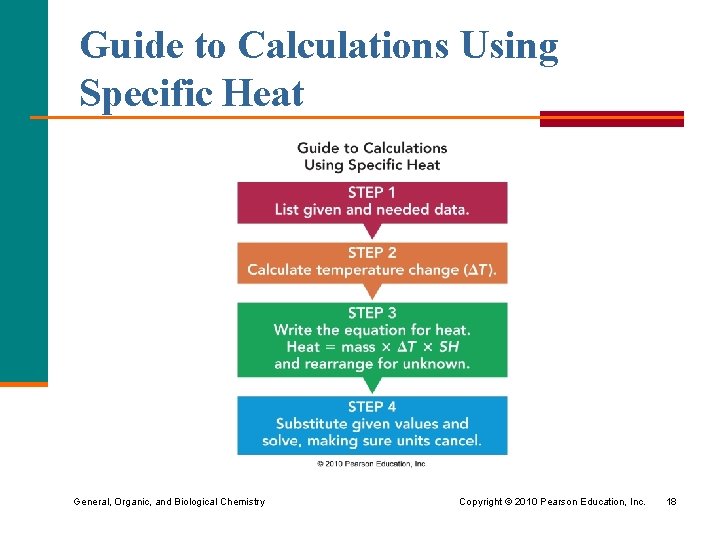
Guide to Calculations Using Specific Heat General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 18

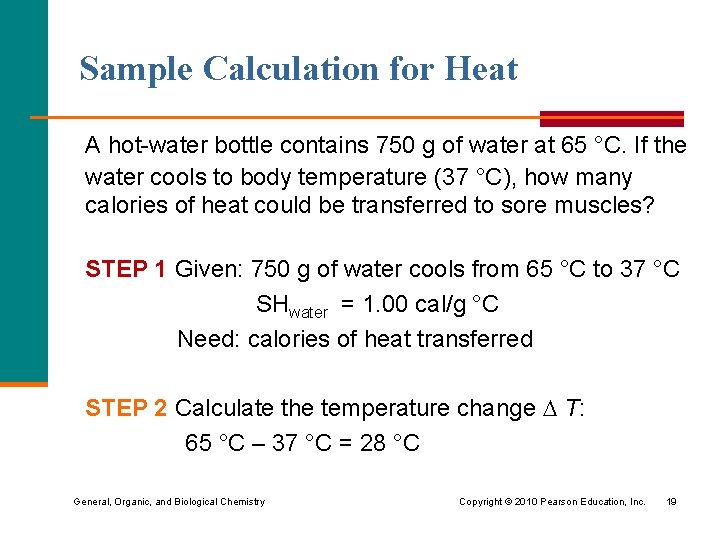
Sample Calculation for Heat A hot-water bottle contains 750 g of water at 65 °C. If the water cools to body temperature (37 °C), how many calories of heat could be transferred to sore muscles? STEP 1 Given: 750 g of water cools from 65 °C to 37 °C SHwater = 1. 00 cal/g °C Need: calories of heat transferred STEP 2 Calculate the temperature change T: 65 °C – 37 °C = 28 °C General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 19

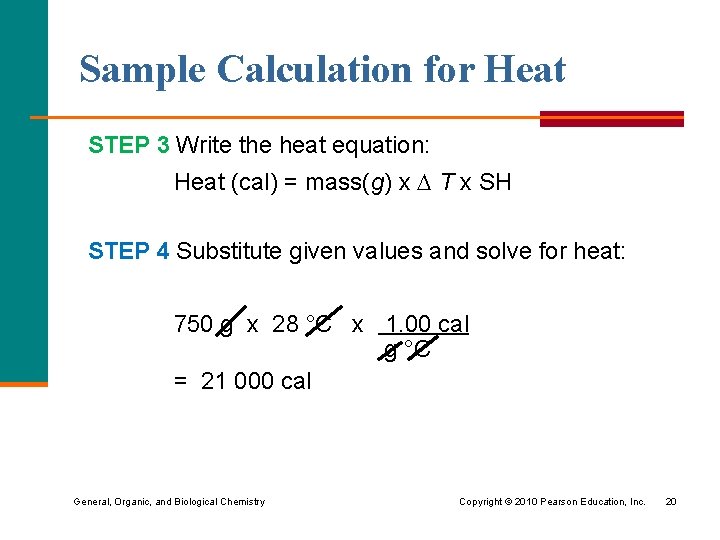
Sample Calculation for Heat STEP 3 Write the heat equation: Heat (cal) = mass(g) x T x SH STEP 4 Substitute given values and solve for heat: 750 g x 28 °C x 1. 00 cal g °C = 21 000 cal General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 20

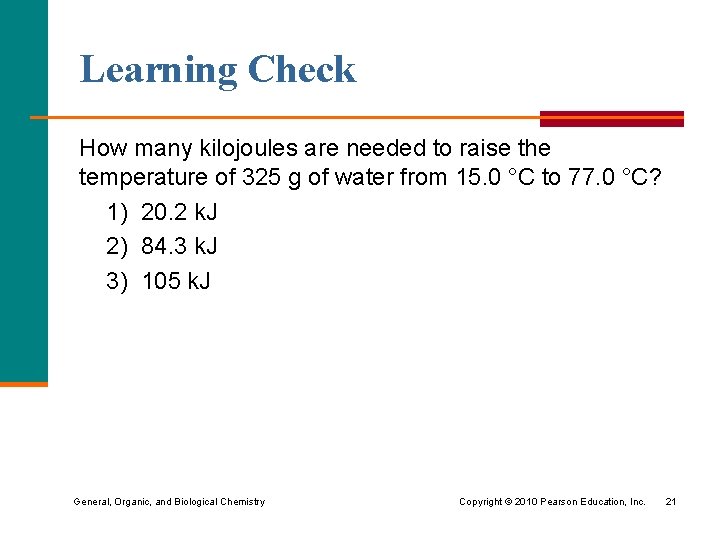
Learning Check How many kilojoules are needed to raise the temperature of 325 g of water from 15. 0 °C to 77. 0 °C? 1) 20. 2 k. J 2) 84. 3 k. J 3) 105 k. J General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 21

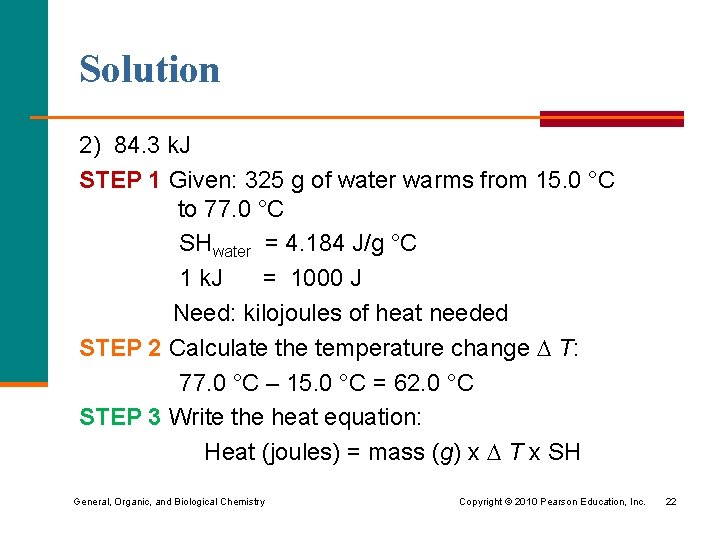
Solution 2) 84. 3 k. J STEP 1 Given: 325 g of water warms from 15. 0 °C to 77. 0 °C SHwater = 4. 184 J/g °C 1 k. J = 1000 J Need: kilojoules of heat needed STEP 2 Calculate the temperature change T: 77. 0 °C – 15. 0 °C = 62. 0 °C STEP 3 Write the heat equation: Heat (joules) = mass (g) x T x SH General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 22

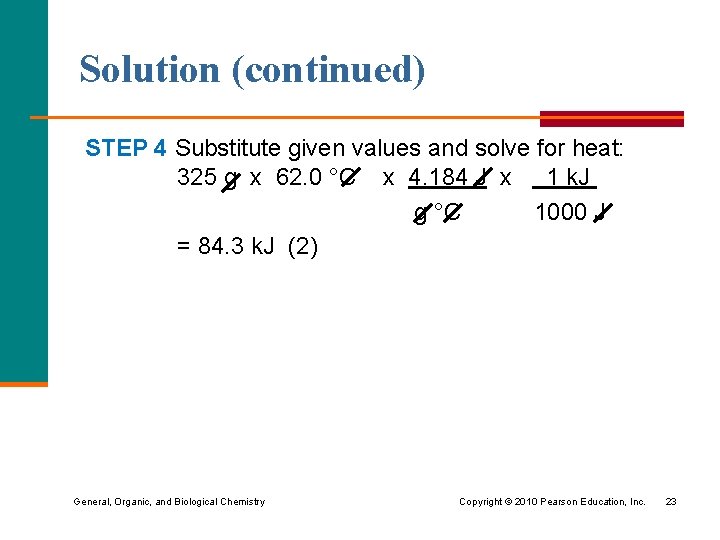
Solution (continued) STEP 4 Substitute given values and solve for heat: 325 g x 62. 0 °C x 4. 184 J x 1 k. J g °C 1000 J = 84. 3 k. J (2) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 23