New HydrogenBonding Stationary Phases for Liquid Chromatography Introduction

New Hydrogen-Bonding Stationary Phases for Liquid Chromatography

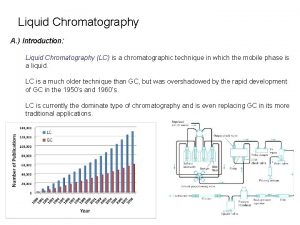
Introduction Traditional liquid chromatography techniques include reversed phase (RP), normal-phase (NP), and ion-exchange (IE) based mechanism of separation. Secondary interactions such as hydrogen bonding, dipole-dipole, Pi-Pi interactions often contribute to a small extent to each of those techniques. We recently developed a new approach and corresponding stationary phase which allows conducting separation entirely based on the hydrogen bonding (HB) properties of molecules. This technique is extending the separation scope to many different molecules and it is based on presence of polar groups in the analytes. Many classes of compounds can be analyzed and separated in a simple mobile phase composition in both gradient and isocratic modes with unique selectivity. Some traditionally difficult separation methods can be easily achieved using this new technique. Some examples and key points of method development using hydrogen-bonding mechanism will be discussed.
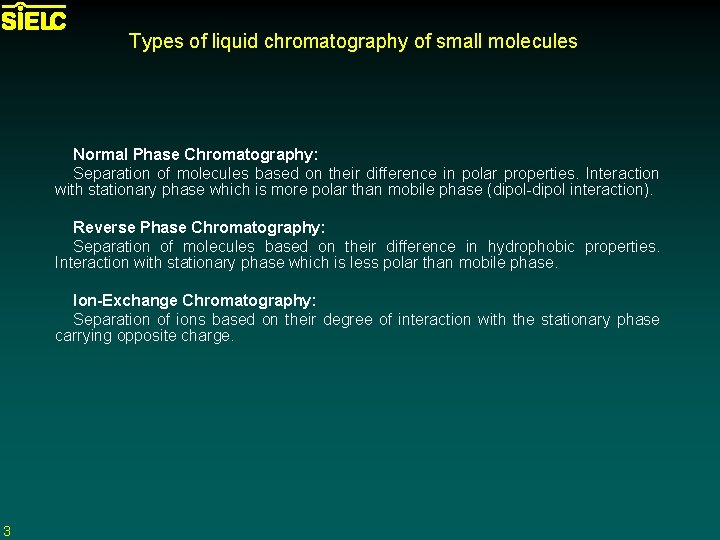
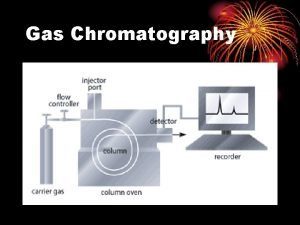
Types of liquid chromatography of small molecules Normal Phase Chromatography: Separation of molecules based on their difference in polar properties. Interaction with stationary phase which is more polar than mobile phase (dipol-dipol interaction). Reverse Phase Chromatography: Separation of molecules based on their difference in hydrophobic properties. Interaction with stationary phase which is less polar than mobile phase. Ion-Exchange Chromatography: Separation of ions based on their degree of interaction with the stationary phase carrying opposite charge. 3
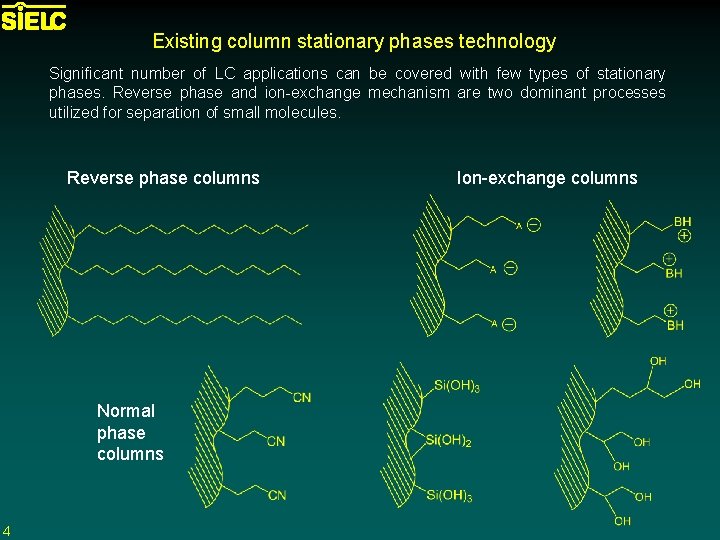
Existing column stationary phases technology Significant number of LC applications can be covered with few types of stationary phases. Reverse phase and ion-exchange mechanism are two dominant processes utilized for separation of small molecules. Reverse phase columns Normal phase columns 4 Ion-exchange columns
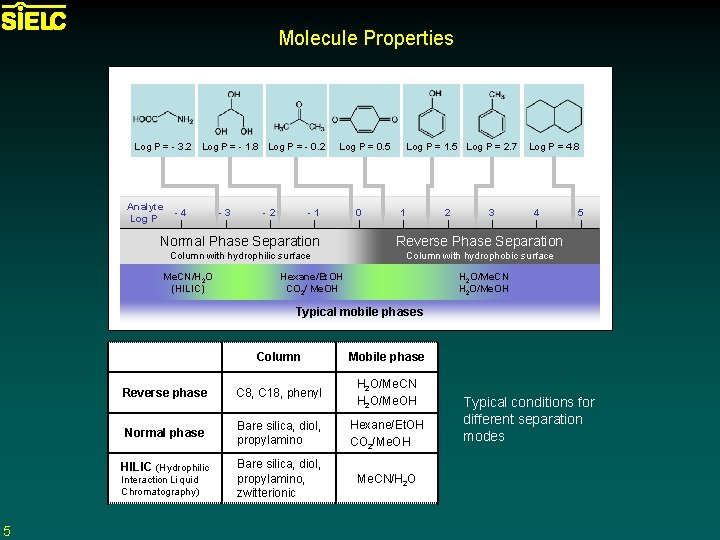
Molecule Properties Log P = - 3. 2 Log P = - 1. 8 Log P = - 0. 2 Analyte Log P -4 -3 -2 Log P = 1. 5 Log P = 2. 7 Log P = 0. 5 -1 0 1 2 3 Log P = 4. 8 4 Normal Phase Separation Reverse Phase Separation Column with hydrophilic surface Column with hydrophobic surface Me. CN/H 2 O (HILIC) Hexane/Et. OH CO 2/ Me. OH 5 H 2 O/Me. CN H 2 O/Me. OH Typical mobile phases Column Mobile phase Reverse phase C 8, C 18, phenyl H 2 O/Me. CN H 2 O/Me. OH Normal phase Bare silica, diol, propylamino Hexane/Et. OH CO 2/Me. OH Bare silica, diol, propylamino, zwitterionic Me. CN/H 2 O HILIC (Hydrophilic Interaction Liquid Chromatography) 5 Typical conditions for different separation modes
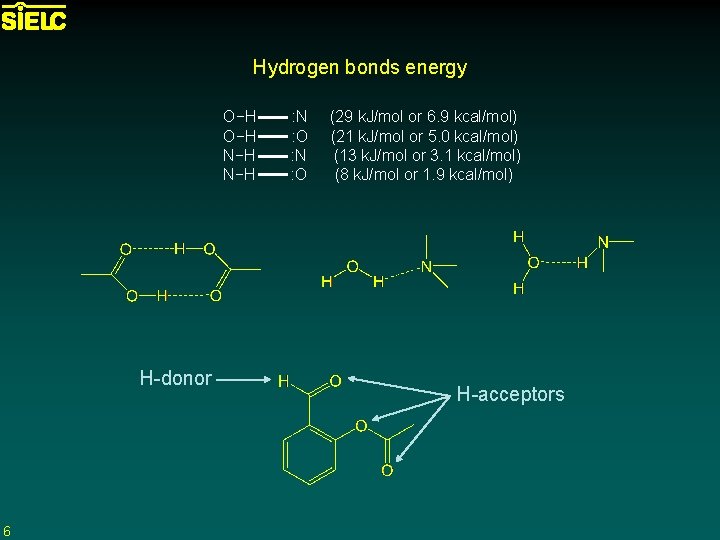
Hydrogen bonds energy O−H : N (29 k. J/mol or 6. 9 kcal/mol) O−H : O (21 k. J/mol or 5. 0 kcal/mol) N−H : N (13 k. J/mol or 3. 1 kcal/mol) N−H : O (8 k. J/mol or 1. 9 kcal/mol) H-donor 6 H-acceptors
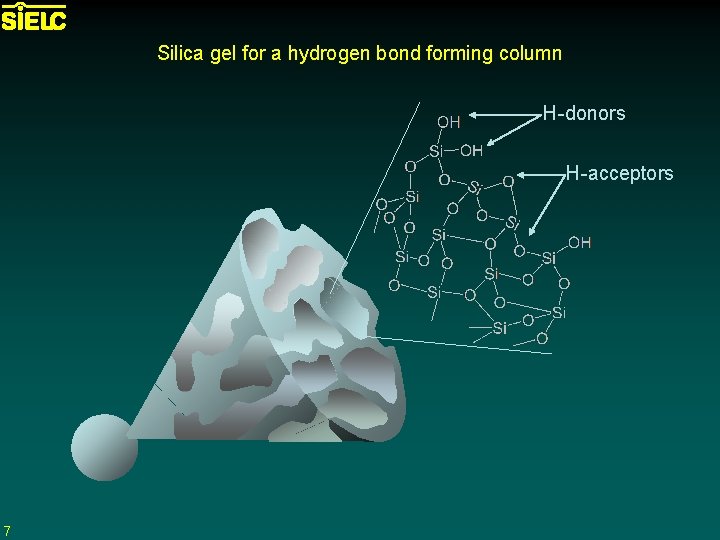
Silica gel for a hydrogen bond forming column H-donors H-acceptors 7

Silica gel for a hydrogen bond forming column Advantages • • Available material Many versions available Stable at elevated temperature Chemically stable under acidic conditions Disadvantages • Soluble in Me. OH • Has acidic properties • Adsorbs water which affects silica gel properties • Chemically unstable under basic conditions • There are several different functional groups on silica surface. Their distribution is not consistent, their strength of formation of hydrogen bonding is different. 8

Functional group candidates for SHARC separation 9
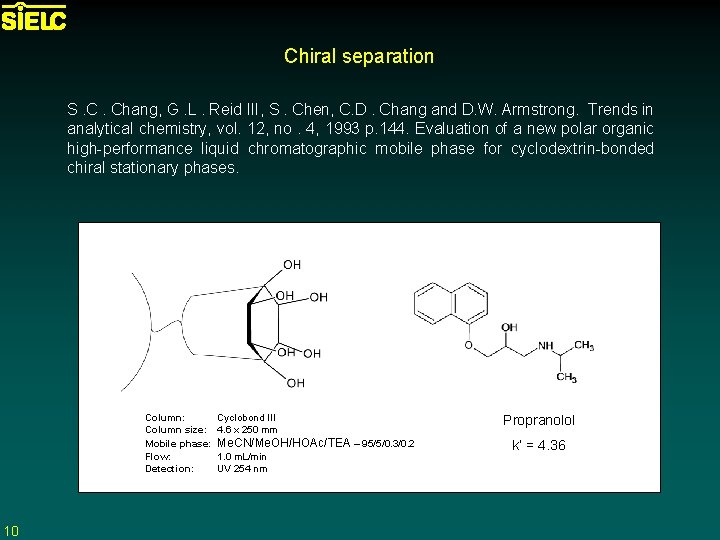
Chiral separation S. C. Chang, G. L. Reid III, S. Chen, C. D. Chang and D. W. Armstrong. Trends in analytical chemistry, vol. 12, no. 4, 1993 p. 144. Evaluation of a new polar organic high-performance liquid chromatographic mobile phase for cyclodextrin-bonded chiral stationary phases. Column: Cyclobond III Column size: 4. 6 x 250 mm Mobile phase: Me. CN/Me. OH/HOAc/TEA – 95/5/0. 3/0. 2 Flow: 1. 0 m. L/min Detection: UV 254 nm 10 Propranolol k’ = 4. 36
Hydrogen Bonding Stationary Phase A stationary phase that provides strong hydrogen bonding (HB) donor type, acceptor type, or both types with molecules possessing a polar functional group(s). Hydrogen Bonding LC Separation A separation technique based on the degree of hydrogen bond formation of the molecules with the polar stationary phase. It is possible in solvents such as Me. CN/Hexane/CO 2 with the addition of a strong polar solvent capable of competing for HB formation.

SHARC stands for: Specific Hydrogen-bond Adsorption Resolution Chromatography 12
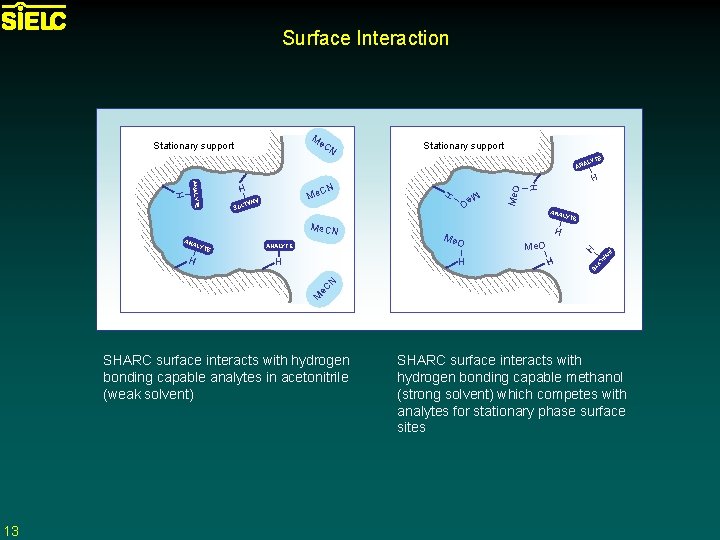
Surface Interaction Me CN Stationary support E LYT H O Me H ANA LYT E E H AL YT H H Me. O M e. C N E H Me O ANALYTE AN H YTE H LYT H ANA L Me. CN ANA H ANALYTE H CN Me Me. O ANA SHARC surface interacts with hydrogen bonding capable analytes in acetonitrile (weak solvent) 13 SHARC surface interacts with hydrogen bonding capable methanol (strong solvent) which competes with analytes for stationary phase surface sites
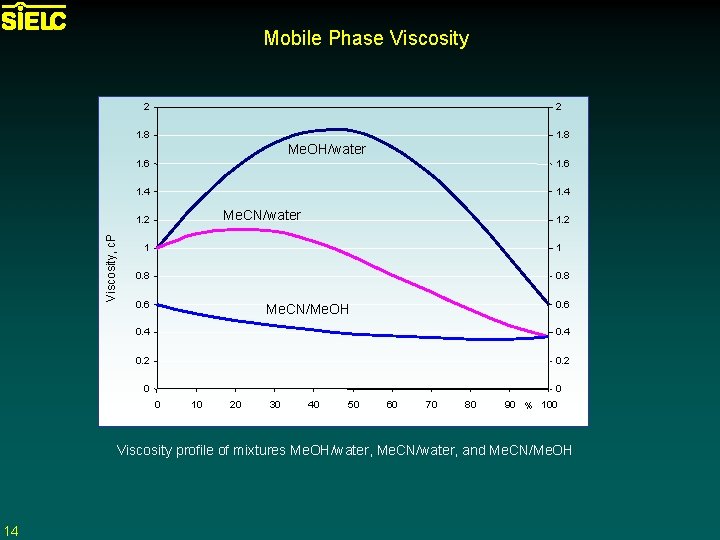
Mobile Phase Viscosity 2 2 1. 8 Me. OH/water 1. 6 1. 4 Me. CN/water Viscosity, c. P 1. 2 1 1 0. 8 0. 6 Me. CN/Me. OH 0. 4 0. 2 0 0 0 10 20 30 40 50 60 70 80 90 % 100 Viscosity profile of mixtures Me. OH/water, Me. CN/water, and Me. CN/Me. OH 14
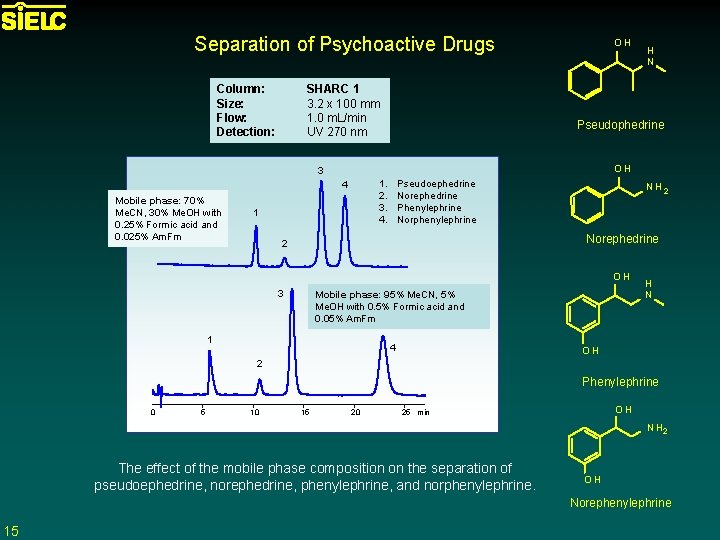
Separation of Psychoactive Drugs Column: Size: Flow: Detection: SHARC 1 3. 2 x 100 mm 1. 0 m. L/min UV 270 nm OH Pseudophedrine OH 3 1. 2. 3. 4. 4 Mobile phase: 70% Me. CN, 30% Me. OH with 0. 25% Formic acid and 0. 025% Am. Fm H N 1 Pseudoephedrine Norephedrine Phenylephrine Norphenylephrine NH 2 Norephedrine 2 OH 3 Mobile phase: 95% Me. CN, 5% Me. OH with 0. 5% Formic acid and 0. 05% Am. Fm 1 4 H N OH 2 Phenylephrine 0 5 10 15 20 OH 25 min N H 2 The effect of the mobile phase composition on the separation of pseudoephedrine, norephedrine, phenylephrine, and norphenylephrine. OH Norephenylephrine 15
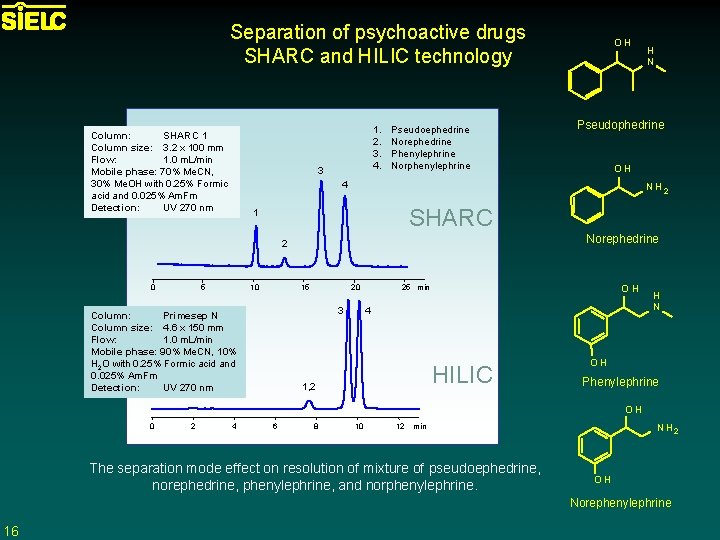
Separation of psychoactive drugs SHARC and HILIC technology Column: SHARC 1 Column size: 3. 2 x 100 mm Flow: 1. 0 m. L/min Mobile phase: 70% Me. CN, 30% Me. OH with 0. 25% Formic acid and 0. 025% Am. Fm Detection: UV 270 nm 1. 2. 3. 4. 3 Pseudoephedrine Norephedrine Phenylephrine Norphenylephrine OH NH 2 SHARC 1 5 H N Pseudophedrine 4 Norephedrine 2 0 OH 10 15 20 3 Column: Primesep N Column size: 4. 6 x 150 mm Flow: 1. 0 m. L/min Mobile phase: 90% Me. CN, 10% H 2 O with 0. 25% Formic acid and 0. 025% Am. Fm Detection: UV 270 nm 25 min OH 4 HILIC 1, 2 H N OH Phenylephrine OH 0 2 4 6 8 10 12 min The separation mode effect on resolution of mixture of pseudoephedrine, norephedrine, phenylephrine, and norphenylephrine. N H 2 OH Norephenylephrine 16
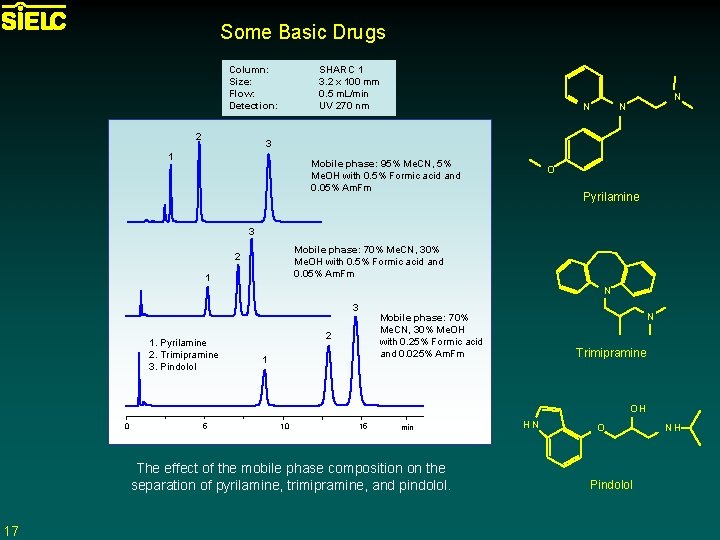
Some Basic Drugs Column: Size: Flow: Detection: 2 SHARC 1 3. 2 x 100 mm 0. 5 m. L/min UV 270 nm N N N 3 1 Mobile phase: 95% Me. CN, 5% Me. OH with 0. 5% Formic acid and 0. 05% Am. Fm O Pyrilamine 3 Mobile phase: 70% Me. CN, 30% Me. OH with 0. 5% Formic acid and 0. 05% Am. Fm 2 1 N 3 1. Pyrilamine 2. Trimipramine 3. Pindolol N Mobile phase: 70% Me. CN, 30% Me. OH with 0. 25% Formic acid and 0. 025% Am. Fm 2 1 Trimipramine OH 0 5 10 15 min The effect of the mobile phase composition on the separation of pyrilamine, trimipramine, and pindolol. 17 HN O Pindolol NH
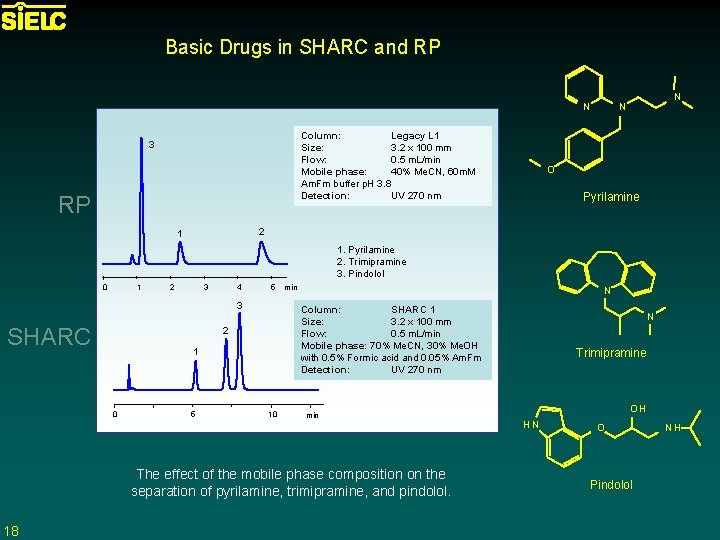
Basic Drugs in SHARC and RP N Column: Legacy L 1 Size: 3. 2 x 100 mm Flow: 0. 5 m. L/min Mobile phase: 40% Me. CN, 60 m. M Am. Fm buffer p. H 3. 8 Detection: UV 270 nm 3 RP N N O Pyrilamine 2 1 1. Pyrilamine 2. Trimipramine 3. Pindolol 1 0 2 3 4 5 3 SHARC 1 5 N Column: SHARC 1 Size: 3. 2 x 100 mm Flow: 0. 5 m. L/min Mobile phase: 70% Me. CN, 30% Me. OH with 0. 5% Formic acid and 0. 05% Am. Fm Detection: UV 270 nm 2 0 min 10 min The effect of the mobile phase composition on the separation of pyrilamine, trimipramine, and pindolol. 18 N Trimipramine OH HN O Pindolol NH
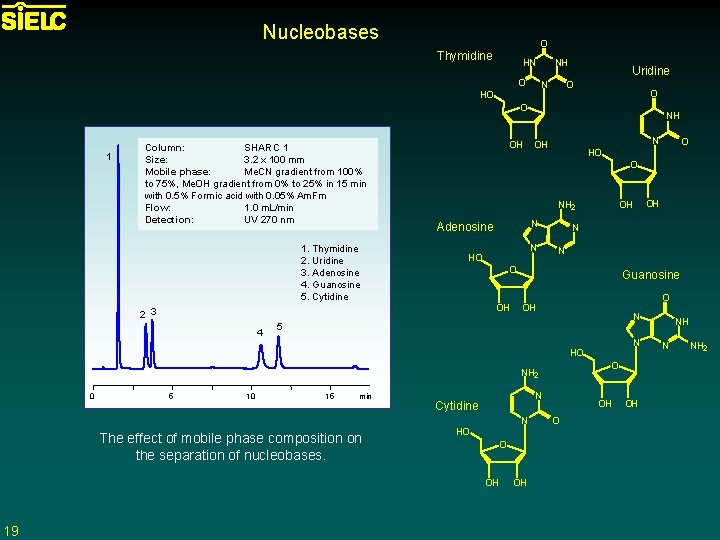
Nucleobases O Thymidine HN O NH N HO Uridine O O O 1 Column: SHARC 1 Size: 3. 2 x 100 mm Mobile phase: Me. CN gradient from 100% to 75%, Me. OH gradient from 0% to 25% in 15 min with 0. 5% Formic acid with 0. 05% Am. Fm Flow: 1. 0 m. L/min Detection: UV 270 nm 1. Thymidine 2. Uridine 3. Adenosine 4. Guanosine 5. Cytidine OH NH 2 N Adenosine N HO N N O OH Guanosine O OH N 5 N O NH 2 5 10 15 min N Cytidine N The effect of mobile phase composition on the separation of nucleobases. HO O OH 19 OH OH HO 0 O HO O 2 3 4 NH OH OH O OH NH N NH 2

Nucleobases: Reverse Mode and SHARC mode O Thymidine HN O 3 2 4 1 N HO Column: Legacy L 1 Size: 3. 2 x 100 mm Mobile phase: Me. CN/H 2 O 5/95 with 0. 5% Formic acid with 0. 05% Am. Fm Flow: 1. 0 m. L/min Detection: UV 270 nm NH Uridine O O O 1. Thymidine 2. Uridine 3. Adenosine 4. Guanosine 5. Cytidine OH NH N OH O HO O 5 1 RP Column: SHARC 1 Size: 3. 2 x 100 mm Mobile phase: Me. CN gradient from 100% to 75%, Me. OH gradient from 0% to 25% in 15 min with 0. 5% Formic acid with 0. 05% Am. Fm Flow: 1. 0 m. L/min Detection: UV 270 nm 2 3 4 NH 2 N Adenosine N HO N N O 5 OH Guanosine O OH N SHARC 0 5 10 N N HO O OH 20 O NH 2 Cytidine The effect of mobile phase composition on the separation of nucleobases. N HO min OH OH O OH NH N NH 2
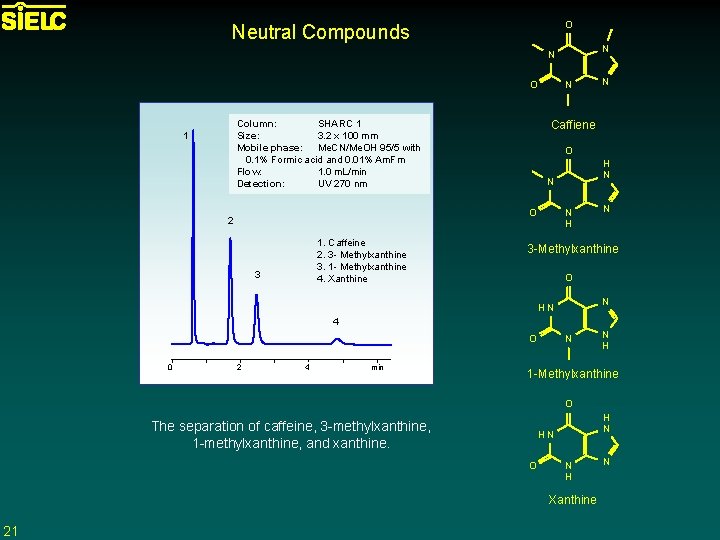
O Neutral Compounds N N O O 1. Caffeine 2. 3 - Methylxanthine 3. 1 - Methylxanthine 4. Xanthine 3 H N N O 2 N Caffiene Column: SHARC 1 Size: 3. 2 x 100 mm Mobile phase: Me. CN/Me. OH 95/5 with 0. 1% Formic acid and 0. 01% Am. Fm Flow: 1. 0 m. L/min Detection: UV 270 nm 1 N N H N 3 -Methylxanthine O N HN 4 O 0 2 4 min N N H 1 -Methylxanthine O The separation of caffeine, 3 -methylxanthine, 1 -methylxanthine, and xanthine. H N HN O N H Xanthine 21 N
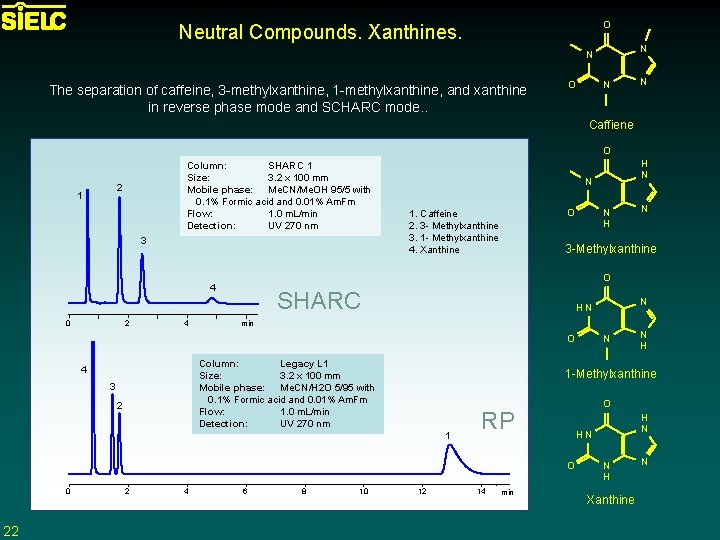
O Neutral Compounds. Xanthines. N N The separation of caffeine, 3 -methylxanthine, 1 -methylxanthine, and xanthine in reverse phase mode and SCHARC mode. . O N N Caffiene O Column: SHARC 1 Size: 3. 2 x 100 mm Mobile phase: Me. CN/Me. OH 95/5 with 0. 1% Formic acid and 0. 01% Am. Fm Flow: 1. 0 m. L/min Detection: UV 270 nm 2 1 3 2 4 N O 1. Caffeine 2. 3 - Methylxanthine 3. 1 - Methylxanthine 4. Xanthine N H 3 -Methylxanthine SHARC min Column: Legacy L 1 Size: 3. 2 x 100 mm Mobile phase: Me. CN/H 2 O 5/95 with 0. 1% Formic acid and 0. 01% Am. Fm Flow: 1. 0 m. L/min Detection: UV 270 nm 3 2 22 4 6 8 10 N H O RP 12 14 min H N HN O 2 N 1 -Methylxanthine 1 0 N HN O 4 0 H N N H Xanthine N
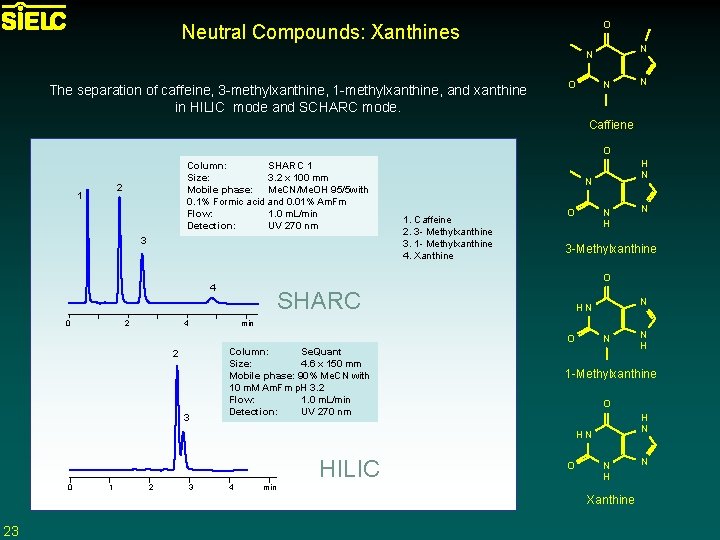
O Neutral Compounds: Xanthines N N The separation of caffeine, 3 -methylxanthine, 1 -methylxanthine, and xanthine in HILIC mode and SCHARC mode. O N N Caffiene O Column: SHARC 1 Size: 3. 2 x 100 mm Mobile phase: Me. CN/Me. OH 95/5 with 0. 1% Formic acid and 0. 01% Am. Fm Flow: 1. 0 m. L/min Detection: UV 270 nm 2 1 3 2 N 1. Caffeine 2. 3 - Methylxanthine 3. 1 - Methylxanthine 4. Xanthine O N H 3 -Methylxanthine SHARC 4 N HN min O 2 3 Column: Se. Quant Size: 4. 6 x 150 mm Mobile phase: 90% Me. CN with 10 m. M Am. Fm p. H 3. 2 Flow: 1. 0 m. L/min Detection: UV 270 nm N HILIC 1 2 3 4 O H N O N H min Xanthine 23 N H 1 -Methylxanthine HN 0 N O 4 0 H N N
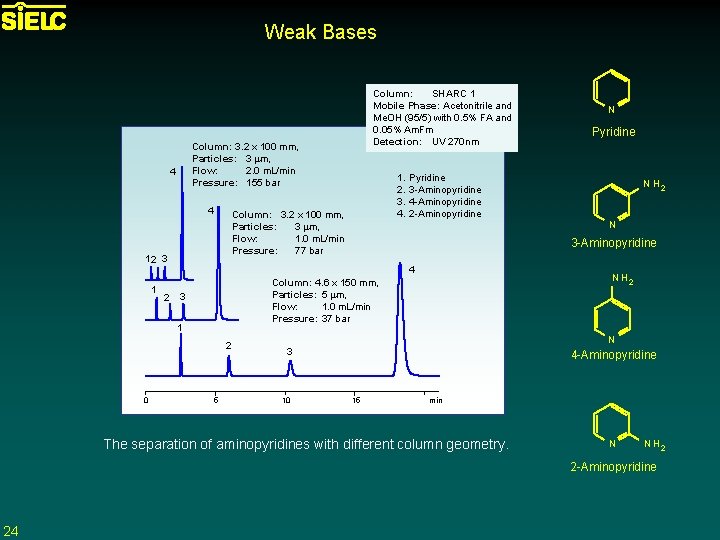
Weak Bases Column: 3. 2 x 100 mm, Particles: 3 mm, Flow: 2. 0 m. L/min Pressure: 155 bar 4 4 Pyridine 3 -Aminopyridine 4 -Aminopyridine 2 -Aminopyridine 4 2 3 2 5 N Pyridine N H 2 N 3 -Aminopyridine N H 2 Column: 4. 6 x 150 mm, Particles: 5 mm, Flow: 1. 0 m. L/min Pressure: 37 bar 1 0 1. 2. 3. 4. Column: 3. 2 x 100 mm, Particles: 3 mm, Flow: 1. 0 m. L/min Pressure: 77 bar 12 3 1 Column: SHARC 1 Mobile Phase: Acetonitrile and Me. OH (95/5) with 0. 5% FA and 0. 05% Am. Fm Detection: UV 270 nm N 3 10 4 -Aminopyridine 15 min The separation of aminopyridines with different column geometry. N N H 2 2 -Aminopyridine 24
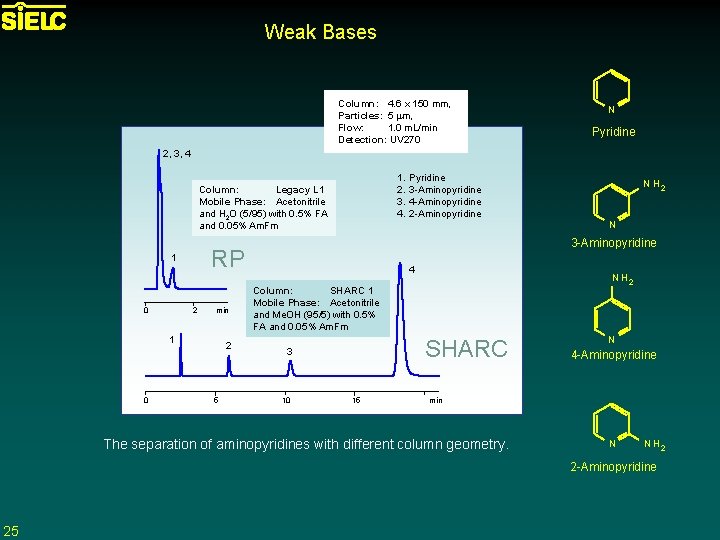
Weak Bases Column: 4. 6 x 150 mm, Particles: 5 mm, Flow: 1. 0 m. L/min Detection: UV 270 N Pyridine 2, 3, 4 1. 2. 3. 4. Column: Legacy L 1 Mobile Phase: Acetonitrile and H 2 O (5/95) with 0. 5% FA and 0. 05% Am. Fm 0 2 min 1 0 2 5 N H 2 N 3 -Aminopyridine RP 1 Pyridine 3 -Aminopyridine 4 -Aminopyridine 2 -Aminopyridine 4 N H 2 Column: SHARC 1 Mobile Phase: Acetonitrile and Me. OH (95/5) with 0. 5% FA and 0. 05% Am. Fm SHARC 3 10 15 N 4 -Aminopyridine min The separation of aminopyridines with different column geometry. N N H 2 2 -Aminopyridine 25
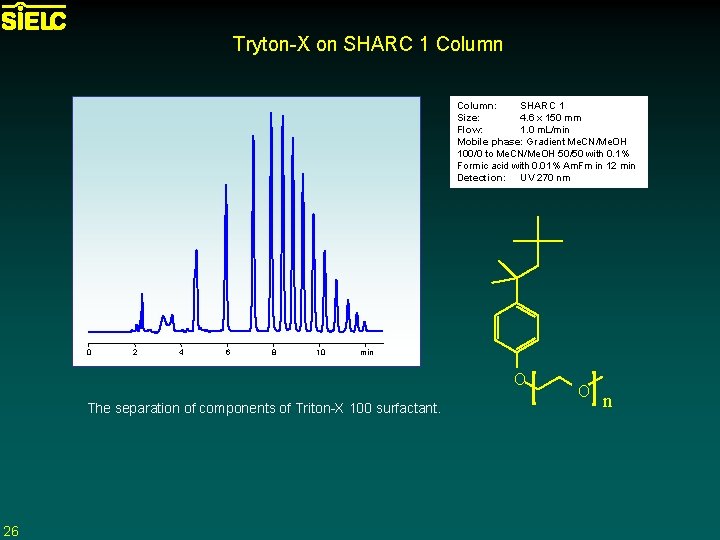
Tryton-X on SHARC 1 Column: SHARC 1 Size: 4. 6 x 150 mm Flow: 1. 0 m. L/min Mobile phase: Gradient Me. CN/Me. OH 100/0 to Me. CN/Me. OH 50/50 with 0. 1% Formic acid with 0. 01% Am. Fm in 12 min Detection: UV 270 nm 0 2 4 6 8 10 min O The separation of components of Triton-X 100 surfactant. 26 O n
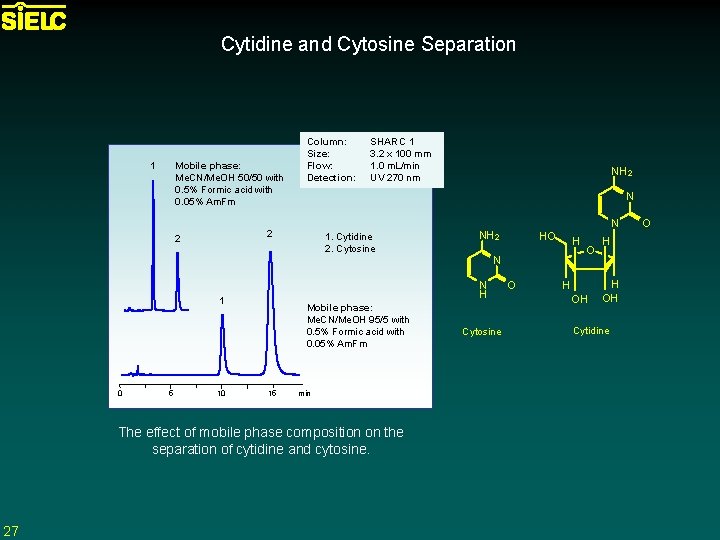
Cytidine and Cytosine Separation 1 Mobile phase: Me. CN/Me. OH 50/50 with 0. 5% Formic acid with 0. 05% Am. Fm 10 1. Cytidine 2. Cytosine Mobile phase: Me. CN/Me. OH 95/5 with 0. 5% Formic acid with 0. 05% Am. Fm 15 min The effect of mobile phase composition on the separation of cytidine and cytosine. 27 NH 2 N NH 2 HO H N N H 1 5 SHARC 1 3. 2 x 100 mm 1. 0 m. L/min UV 270 nm N 2 2 0 Column: Size: Flow: Detection: Cytosine O O H OH H H OH Cytidine O
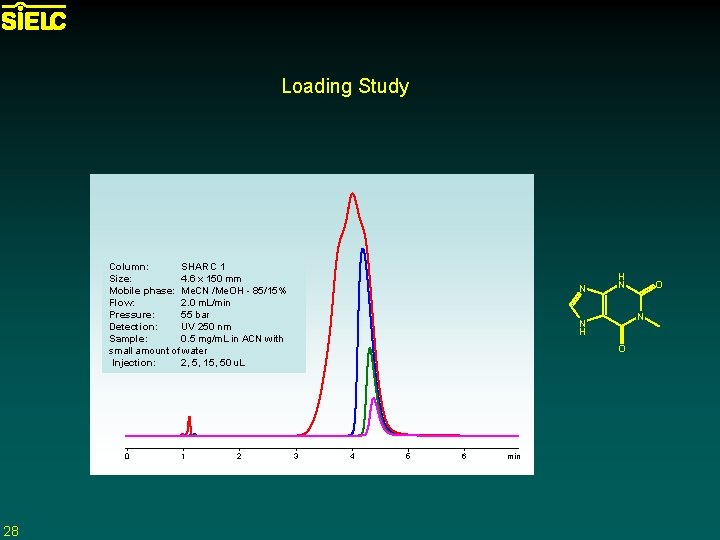
Loading Study Column: SHARC 1 Size: 4. 6 x 150 mm Mobile phase: Me. CN /Me. OH - 85/15% Flow: 2. 0 m. L/min Pressure: 55 bar Detection: UV 250 nm Sample: 0. 5 mg/m. L in ACN with small amount of water Injection: 2, 5, 15, 50 u. L 0 28 1 2 N H N N N H O 3 4 5 6 min O
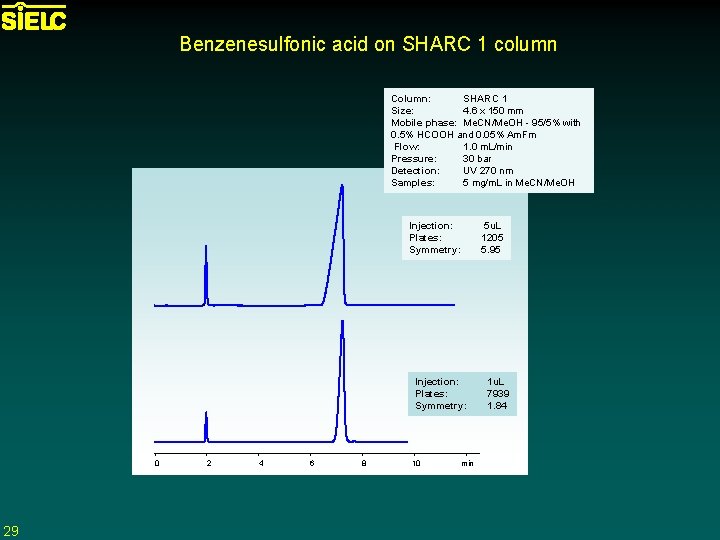
Benzenesulfonic acid on SHARC 1 column Column: SHARC 1 Size: 4. 6 x 150 mm Mobile phase: Me. CN/Me. OH - 95/5% with 0. 5% HCOOH and 0. 05% Am. Fm Flow: 1. 0 m. L/min Pressure: 30 bar Detection: UV 270 nm Samples: 5 mg/m. L in Me. CN/Me. OH Injection: Plates: Symmetry: 0 29 2 4 6 8 10 min 5 u. L 1205 5. 95 1 u. L 7939 1. 84

Conclusions 1. 2. 3. 4. 5. 6. 7. 8. Hydrogen bond strength based separation can be a powerful LC technique Different classes of compounds can be retained and resolved with a simple mixture of Me. OH/Me. CN/buffer as a MP Low viscosity of MP allows the use of smaller particles and increased flow rate using conventional HPLC equipment Unique selectivity can be obtained High selectivity toward structural isomers LC-MS friendly mobile phase and total organic provides high sensitivity Preparative separation friendly MP allows simple solvent removal. Variety of stationary phases to obtain optimum chromatography results

Sources www. sielc. com www. primesep. com www. hplcmethoddevelopment. com www. columnkit. com SIELC Technologies, Inc. 804 Seton Court Wheeling, IL 60090 847 229 -2629 (Phone) 847 655 -6079 (Fax)

Chicago New Year 2004 -2005
- Slides: 32