Why do penguins huddle Specific Heat Capacity Task








- Slides: 12

Why do penguins huddle?

Specific Heat Capacity

Task Unjumble the key words for today's lesson below, and then discuss with a partner what you think today's lesson objectives will be rmomtheteer athe rneyge mpreeteatur torconduc inlatorsu

Keep your cool! • Learn to distinguish between heat (as energy) and temperature • Recognise the need for a temperature scale • Be able to name conductors and insulators and know what they do. • Know what specific heat capacity is and how we use it.

What is heat? Heat is a type of energy. Heat is the name for the type of kinetic energy possessed by particles. Heat energy is measured in joules (J). How many joules are there in a kilojoule (k. J)? If something gains a lot of heat energy, it becomes hot… …so what is temperature?

What is temperature? Temperature is a measure of how hot or cold an object is. It is not the total amount of energy contained in the object. ) Temperature is measured in degrees Celsius (°C). The freezing point of water is defined as 0°C (at 1 atm. ). The boiling point of water is defined as 100°C (at 1 atm. ). Temperature can be measured by a variety of different thermometers. These include liquid in glass, digital, thermocouple and bimetal strip thermometers.

Task Using you traffic light cards display whether you are happy with the meanings of the below key words • Temperature • Celsius • Joule Now show your traffic light cards if you are confident using a thermometer.

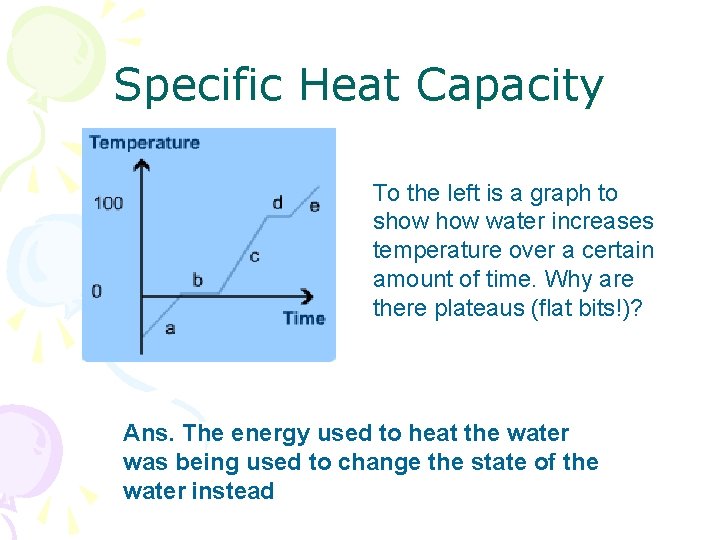
Specific Heat Capacity To the left is a graph to show water increases temperature over a certain amount of time. Why are there plateaus (flat bits!)? Ans. The energy used to heat the water was being used to change the state of the water instead

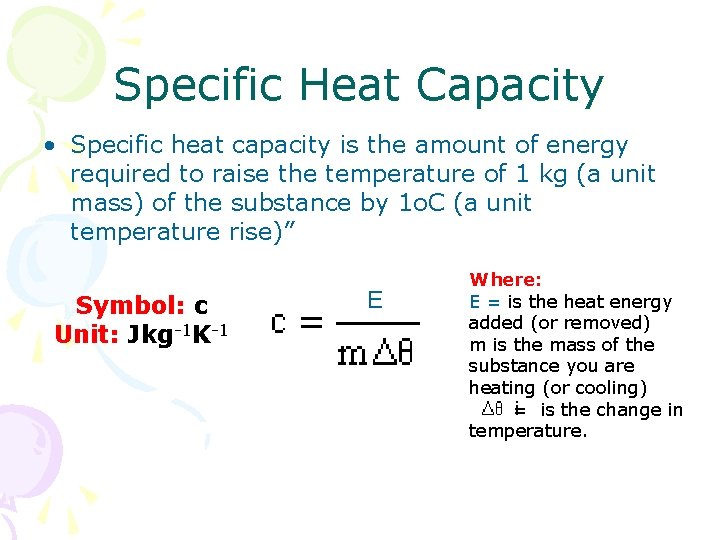
Specific Heat Capacity • Specific heat capacity is the amount of energy required to raise the temperature of 1 kg (a unit mass) of the substance by 1 o. C (a unit temperature rise)” Symbol: c Unit: Jkg-1 K-1 E Where: E = is the heat energy added (or removed) m is the mass of the substance you are heating (or cooling) = is the change in temperature.

Construct Your Knowledge - Specific Heat Capacity Practical • Using the task sheet provided you are going to find the specific heat capacity of a metal block.

Apply Your Knowledge 1) E = 500 J M = 1 kg = 50 C c = ? Answer - 10 J/Kg c 2) E = ? M = 10 kg =20 C c = 900 Answer - 180000 J or (180 KJ)

Review Complete your learning logs describing what you have learned this lesson.