UNIT 8 TEMPERATURE HEAT THERMODYNAMICS CONCEPTUAL WEDNESDAY 424









- Slides: 17

UNIT 8: TEMPERATURE, HEAT, & THERMODYNAMICS

CONCEPTUAL – WEDNESDAY 4/24 • Physics Fun Fact! • Warm Up Problem #1 & #2 • Discuss pre-lab topics • Penny Lab! • Friday 4/26: Finish Unit 8/Quiz

ACADEMIC – TUESDAY 4/23 • Happy Earth Day! – Video? • Begin Unit 8 – Heat/Temperature • Unit 8 Notes • Temperature Formative (In class) • Heat Practice Problems (In Notes) • Penny Lab Next Class!

UNIT 8 • 4/22 – 4/23: Notes/Practice • 4/24 – 4/25: Continue Notes & Practice/Penny Lab • 4/26 – 4/29: Conclude Unit 8/Quiz • Quiz will be the Final Unit 8 Summative since this is a short unit.

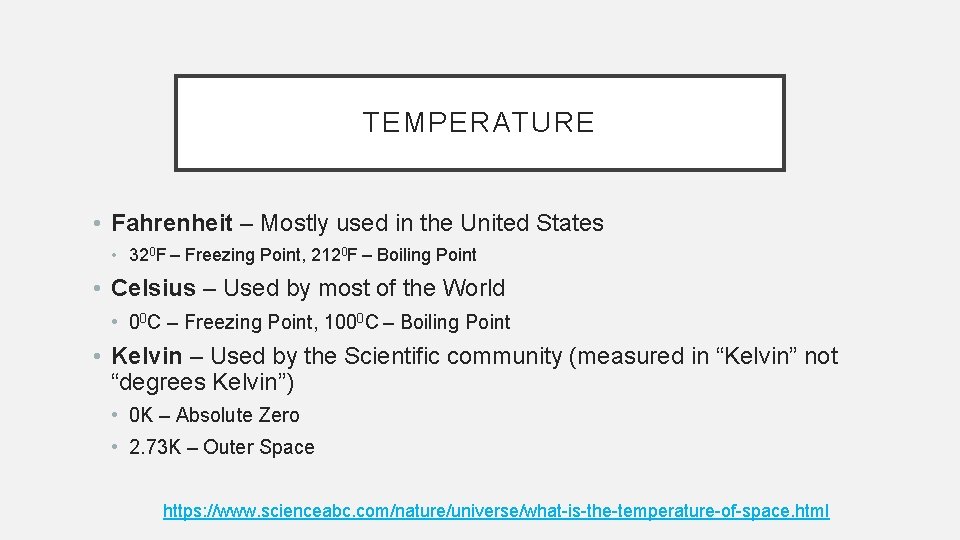
TEMPERATURE • Fahrenheit – Mostly used in the United States • 320 F – Freezing Point, 2120 F – Boiling Point • Celsius – Used by most of the World • 00 C – Freezing Point, 1000 C – Boiling Point • Kelvin – Used by the Scientific community (measured in “Kelvin” not “degrees Kelvin”) • 0 K – Absolute Zero • 2. 73 K – Outer Space https: //www. scienceabc. com/nature/universe/what-is-the-temperature-of-space. html

ABSOLUTE ZERO • The theoretical coldest temperature possible. • Absolute Zero has never been reached by humans • 1997 – Nobel Prize winners accomplished T = 0. 0001 K • 2003 – NASA accomplished one-half billionth of 1 K • 2013 – Scientists theorize “Negative Temperature” • 2017 – Scientists Accomplished 50 -trillionth of 1 K (0. 000005 K) http: //scienceline. ucsb. edu/getkey. php? key=225 (1997) https: //www. nasa. gov/vision/earth/technologies/biggest_chill. html (2003) https: //www. livescience. com/25959 -atoms-colder-than-absolute-zero. html (Negative Temp) https: //www. sciencealert. com/we-now-have-a-new-record-on-chilling-molecules-close-to-absolute-zero (2017

CONVERTING TEMPERATURE Kelvin When converting to Kelvin, first find temperature in degrees Celsius. K = o. C + 273 Complete the opposite to convert temperature from Kelvin o. C = K - 273

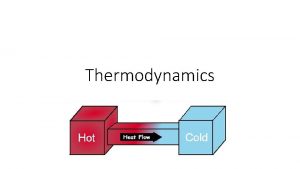
HEAT • A Form of energy that can be transferred between objects or created at the loss of another form of energy. Example: Stored Chemical Energy allows for Heat to be created when someone rubs their hands together. Question: In order to heat up an object, what factors contribute to how much Energy will be needed?

HEAT CONT. Answer: • How much mass does the object have? (greater mass requires more energy) • How much do we need to heat it? (∆T – Change in Temperature) • How easy is it to heat? (Object like metal heat more easily than water) ^^ Something we call Specific Heat Capacity Heat is represented by Variable: Q SI Unit: [ J ] – Joule Unit of Calories can also be used. This related to food Calories. https: //www. physicsclassroom. com/class/thermal. P/Lesson-1/What-is-Heat http: //misterguch. brinkster. net/chapter 16. pdf

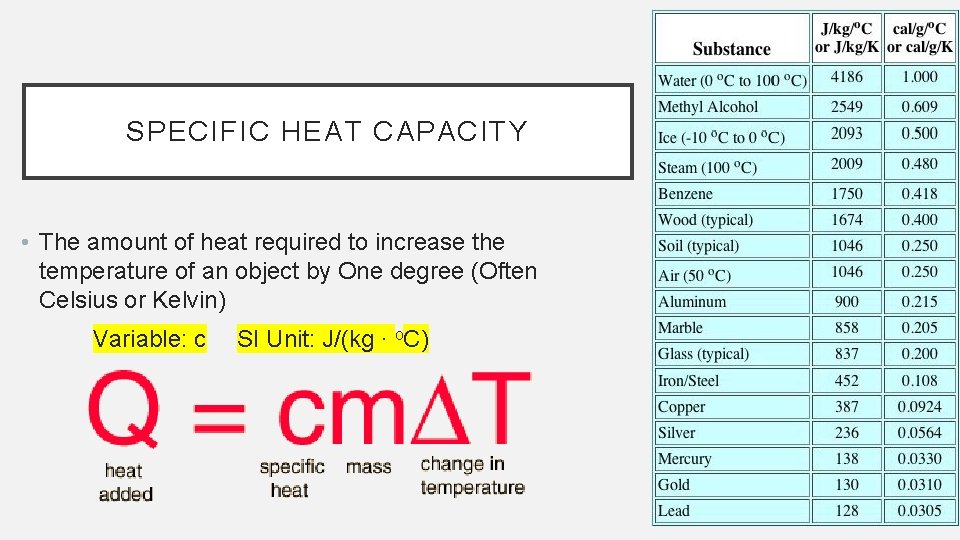
SPECIFIC HEAT CAPACITY • The amount of heat required to increase the temperature of an object by One degree (Often Celsius or Kelvin) Variable: c SI Unit: J/(kg ∙ o. C)

CALORIES AS ENERGY • In Physics, One calorie (cal) = 4. 186 Joules • In Nutrition, One Calorie (Cal or kcal) = 4, 186 Joules 1 Cal = 1, 000 cal • A kilocalorie (kcal) is the amount of heat required to raise the temperature of 1 kilogram of water one degree Celsius. • To run a marathon, a 55 kg runner needs 8, 740 k. J of energy to make it to the finish line. If a Cliff Bar has 260 Calories, exactly how many will the runner need to eat to complete the marathon?

https: //www. youtube. com/watch? v=REh. Iq. Ra 3 z. O 4 THERMAL EXPANSION • The tendency for an object to change is area, volume, or shape as its temperature changes. https: //www. popularmechanics. com/technology/infrastruct ure/a 26090529/rail-company-tracks-fire-chicago/ https: //www. dnainfo. com/chicago/20170109/downtown/thi s-is-why-metra-sets-its-tracks-on-fire-really-cold-weather

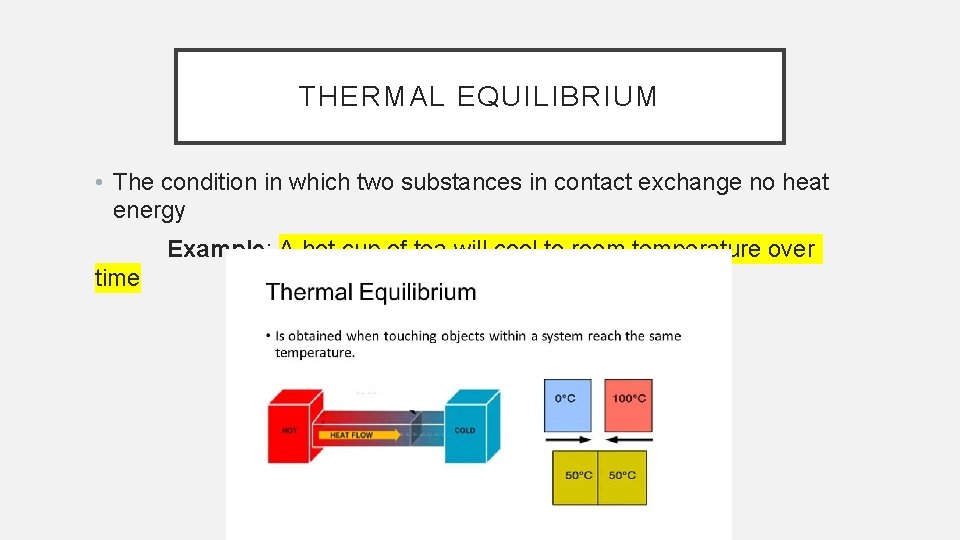
THERMAL EQUILIBRIUM • The condition in which two substances in contact exchange no heat energy Example: A hot cup of tea will cool to room temperature over time

HEAT QUESTION #1 • In a half hour, a 65 kg jogger can generate 8. 0 x 105 J of heat. This heat is naturally removed from the human body as it regulates temperature. If the heat were not removed, how much would the jogger’s body Temperature increase? Specific Heat Capacity of the human body = 3, 500 J/(kg ∙ o. C) Convert your answer to degrees Fahrenheit and formulate the impact this would have on the human body.

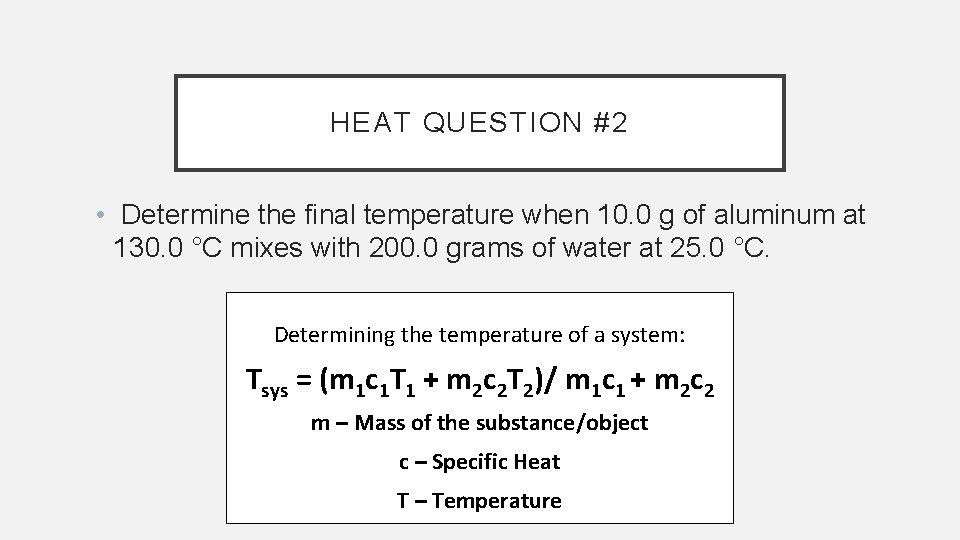
HEAT QUESTION #2 • Determine the final temperature when 10. 0 g of aluminum at 130. 0 °C mixes with 200. 0 grams of water at 25. 0 °C. Determining the temperature of a system: Tsys = (m 1 c 1 T 1 + m 2 c 2 T 2)/ m 1 c 1 + m 2 c 2 m – Mass of the substance/object c – Specific Heat T – Temperature

PENNY LAB Objective: Observe Thermodynamic changes in a controlled system and verify your findings using equations learned previously. Pre-Lab: • Process and Materials • Lab Safety • Discuss potential error during experiment

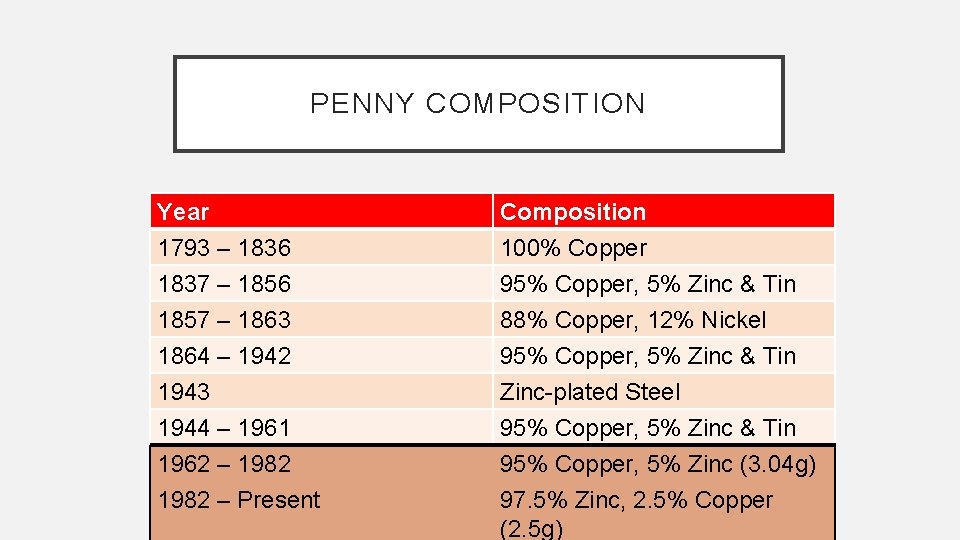
PENNY COMPOSITION Year 1793 – 1836 1837 – 1856 1857 – 1863 Composition 100% Copper 95% Copper, 5% Zinc & Tin 88% Copper, 12% Nickel 1864 – 1942 1943 1944 – 1961 1962 – 1982 – Present 95% Copper, 5% Zinc & Tin Zinc-plated Steel 95% Copper, 5% Zinc & Tin 95% Copper, 5% Zinc (3. 04 g) 97. 5% Zinc, 2. 5% Copper (2. 5 g)
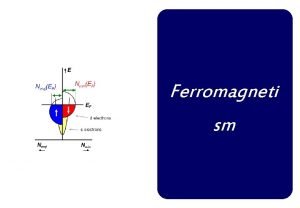
 Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Ferrimagnetism
Ferrimagnetism Endothermic equation
Endothermic equation Lesson 10: thermodynamics unit review
Lesson 10: thermodynamics unit review Pi 424 atualizada
Pi 424 atualizada Mathew v eldridge
Mathew v eldridge Ece 424
Ece 424 Arinc 424 format
Arinc 424 format Econ 424
Econ 424 Ece 424
Ece 424 Ece 424
Ece 424 Ece 424
Ece 424 Arinc 424 waypoint format
Arinc 424 waypoint format Qg 424
Qg 424 Rn424
Rn424 Ece 424
Ece 424 Ece 424
Ece 424