Chapter 5 Temperature and Heat Temperature n Temperature




































- Slides: 86

Chapter 5 Temperature and Heat

Temperature n Temperature is loosely defined as a measure of the hotness or coldness of a substance. This is a very subjective definition. n We will provide a better definition, shortly. n n There are three common temperature scales: n Kelvin, n Celsius, and n Fahrenheit. 2

Temperature, cont’d n The temperature scales can be compared by examining the freezing and boiling points of water. These are determined by the atomic structure of water. n We must do the comparisons at the same pressure since theses phase transitions depend on pressure. n n Especially the boiling point. 3

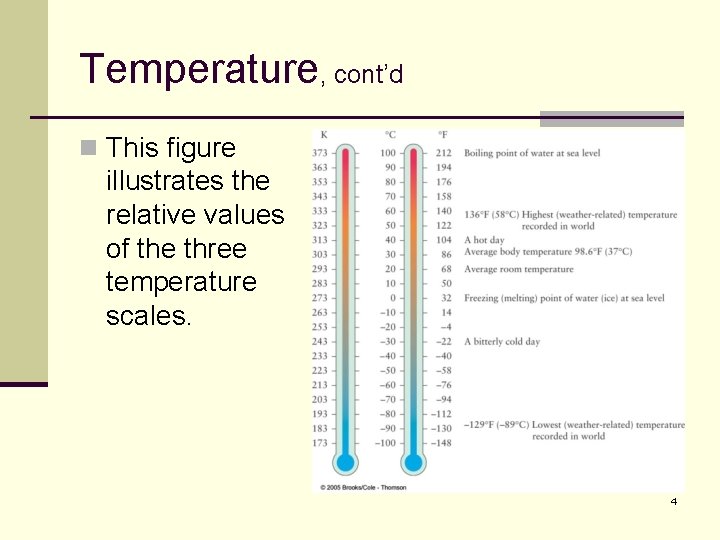
Temperature, cont’d n This figure illustrates the relative values of the three temperature scales. 4

Temperature, cont’d n We define absolute zero as the coldest temperature. n It is better defined as the temperature at which all the random motion of matter is halted. n The lowest value on the Kelvin scale is absolute zero. n So, 0 K corresponds to absolute zero. n This is -273. 15°C or -459. 67°K. 5

Temperature, cont’d n The freezing point of water is defined to be 0°C. n This corresponds to 32°F and 273 K. n The boiling point of water is defined to be 100°C. n This corresponds to 212°F and 373 K. n Note that the Kelvin scale is the only scale that is never negative. n There is an absolute English scale: Rankine. 6

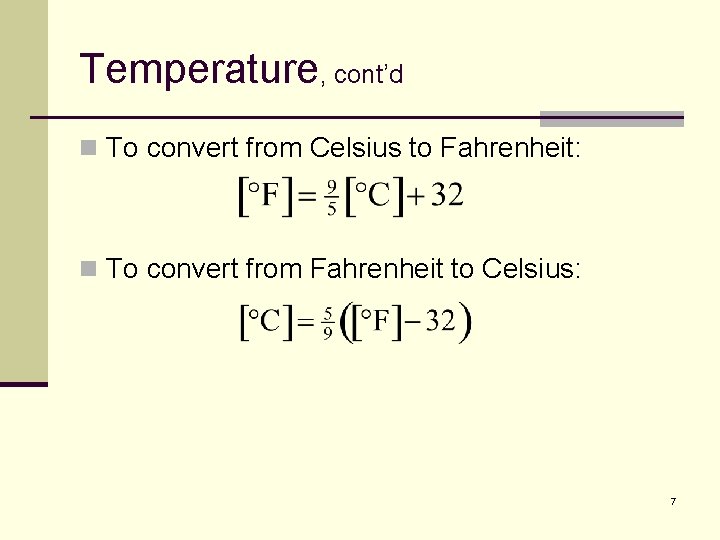
Temperature, cont’d n To convert from Celsius to Fahrenheit: n To convert from Fahrenheit to Celsius: 7

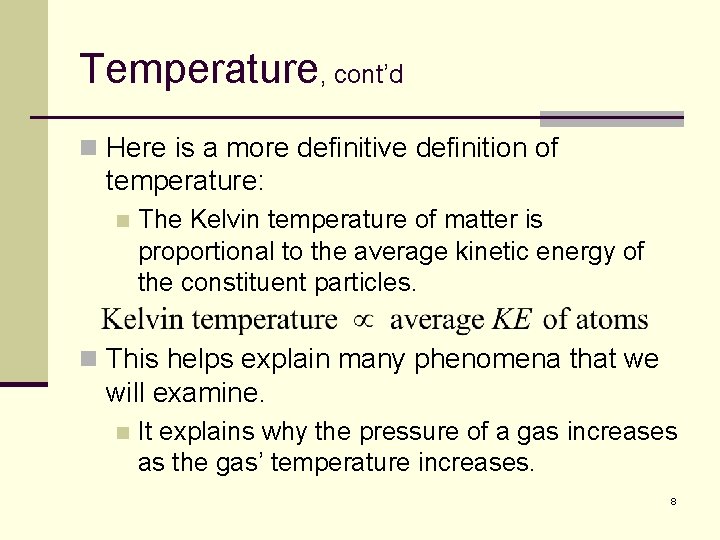
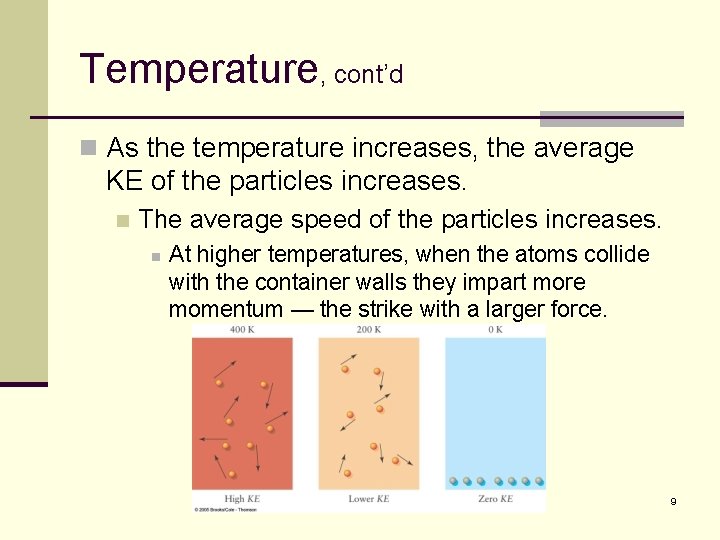
Temperature, cont’d n Here is a more definitive definition of temperature: n The Kelvin temperature of matter is proportional to the average kinetic energy of the constituent particles. n This helps explain many phenomena that we will examine. n It explains why the pressure of a gas increases as the gas’ temperature increases. 8

Temperature, cont’d n As the temperature increases, the average KE of the particles increases. n The average speed of the particles increases. n At higher temperatures, when the atoms collide with the container walls they impart more momentum — the strike with a larger force. 9

Thermal expansion n We know that the average KE of atoms increases with high temperature. n We saw what this means for gases. n The pressure increases as the temperature rises. n What about solids? n The solid’s atoms are not free to move like in a gas. n But they can vibrate. 10

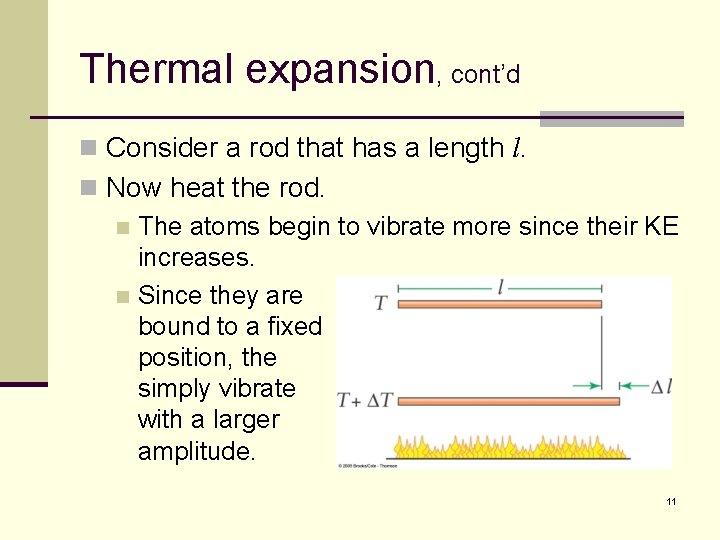
Thermal expansion, cont’d n Consider a rod that has a length l. n Now heat the rod. n The atoms begin to vibrate more since their KE increases. n Since they are bound to a fixed position, the simply vibrate with a larger amplitude. 11

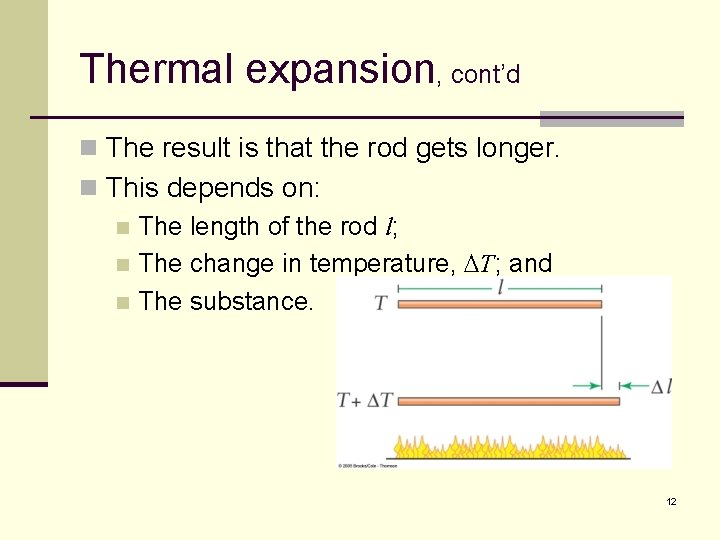
Thermal expansion, cont’d n The result is that the rod gets longer. n This depends on: n The length of the rod l; n The change in temperature, DT; and n The substance. 12

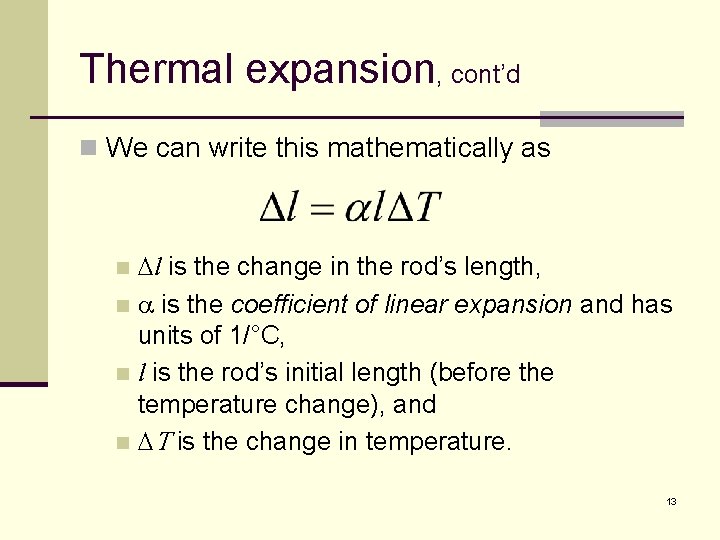
Thermal expansion, cont’d n We can write this mathematically as Dl is the change in the rod’s length, n a is the coefficient of linear expansion and has units of 1/°C, n l is the rod’s initial length (before the temperature change), and n DT is the change in temperature. n 13

Example 5. 1 The center span of a steel bridge is 1, 200 meters long on a winter day when the temperature if -5°C. How much longer is the span on a summer day when the temperature is 35°C? 14

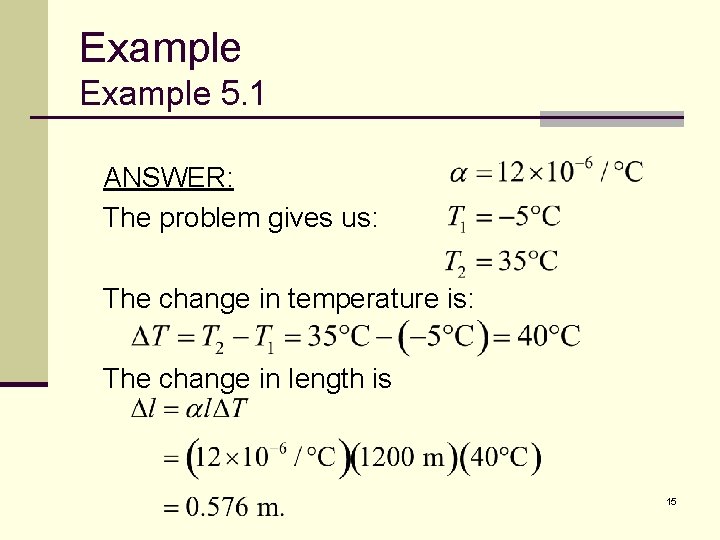
Example 5. 1 ANSWER: The problem gives us: The change in temperature is: The change in length is 15

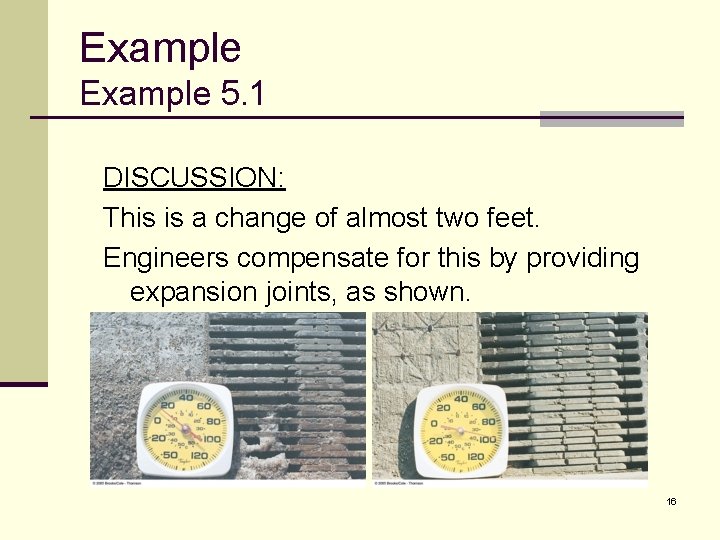
Example 5. 1 DISCUSSION: This is a change of almost two feet. Engineers compensate for this by providing expansion joints, as shown. 16

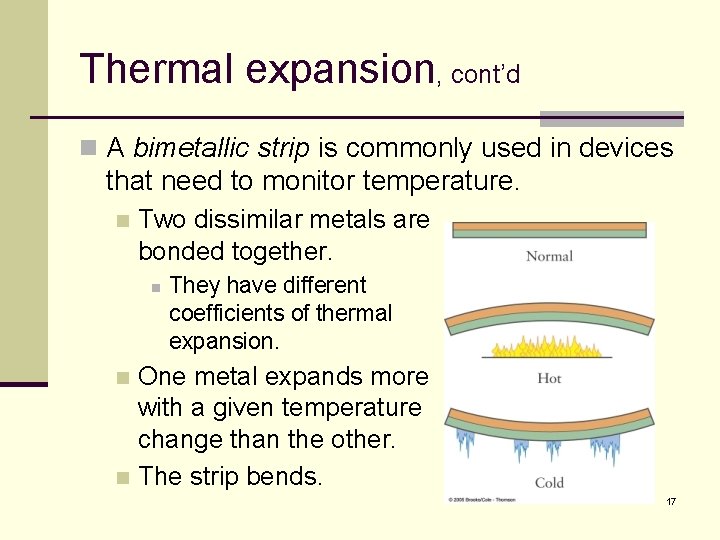
Thermal expansion, cont’d n A bimetallic strip is commonly used in devices that need to monitor temperature. n Two dissimilar metals are bonded together. n They have different coefficients of thermal expansion. One metal expands more with a given temperature change than the other. n The strip bends. n 17

Thermal expansion, cont’d n An analog thermostat is a common example of a bimetallic strip. 18

Thermal expansion, cont’d n Liquids also undergo thermal expansion with a temperature increase. n We typically deal with the volume expansion. n Consider water as a “special” example. n A volume of water increases with an increase in temperature above 4°C. n Between 0° and 4°C, water contracts with an increase in temperature. n Water is most dense at 4°C. 19

Thermal expansion, cont’d n Gases also expand with an increase in temperature. n For a given pressure, the volume of a gas is proportional to its temperature: n This means that if you heat a balloon, the volume will increase. n It is (almost) constant pressure since the balloon is capable of expanding. 20

Thermal expansion, cont’d n Instead of a balloon, consider a gas in a metal container of fixed volume, e. g. , a can of beans. n The pressure, volume and temperature are related through: n Heating the can increases the pressure. n Get the can hot enough, it will explode. 21

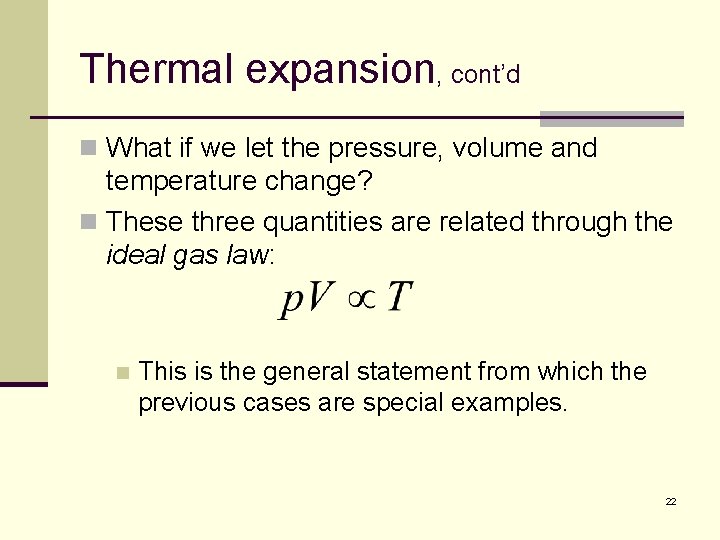
Thermal expansion, cont’d n What if we let the pressure, volume and temperature change? n These three quantities are related through the ideal gas law: n This is the general statement from which the previous cases are special examples. 22

First law of thermodynamics n So far, we have discussed only one way to increase an objects temperature: n expose it to something that has a higher temperature. n There is another possibility: n Do work on it. 23

First law of thermodynamics, cont’d n Consider piston pushing on a gas in a cylinder. n Forcing the piston down requires a force. n Applying this force through a distance means you are doing work. n The work is done against the gas. n n You compress the gas. The gas gets hot. 24

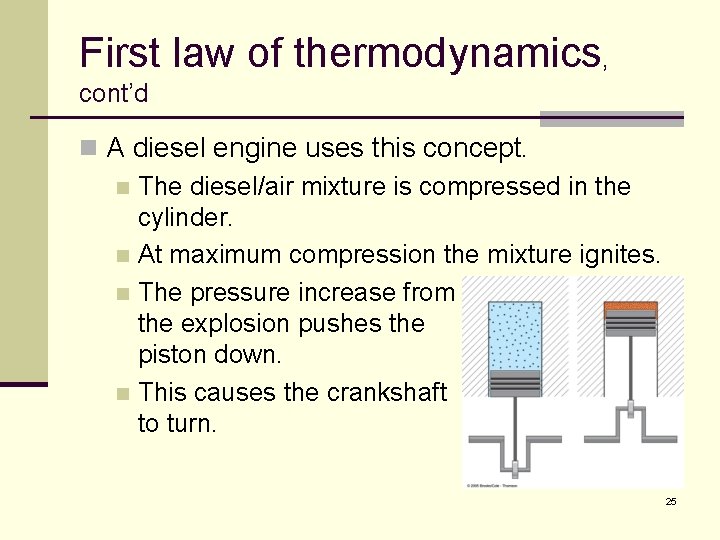
First law of thermodynamics, cont’d n A diesel engine uses this concept. n The diesel/air mixture is compressed in the cylinder. n At maximum compression the mixture ignites. n The pressure increase from the explosion pushes the piston down. n This causes the crankshaft to turn. 25

First law of thermodynamics, cont’d n Compressing a gas increases its temperature. n We noted last chapter that temperature is related to the average kinetic energy of the gas atoms/molecules. n So compressing the gas increases its internal energy. 26

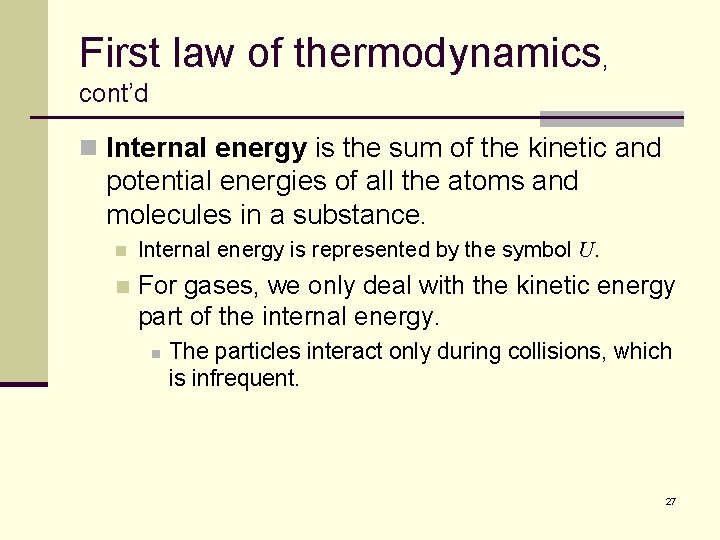
First law of thermodynamics, cont’d n Internal energy is the sum of the kinetic and potential energies of all the atoms and molecules in a substance. n Internal energy is represented by the symbol U. n For gases, we only deal with the kinetic energy part of the internal energy. n The particles interact only during collisions, which is infrequent. 27

First law of thermodynamics, cont’d n Heat is a form of energy that is transferred between two substances because they have different temperatures. An object does not have heat. n An object transfers heat when its temperature is raised by contact with a hotter object or lowered by contact with a cooler object. n Heat is represented by the symbol Q. n 28

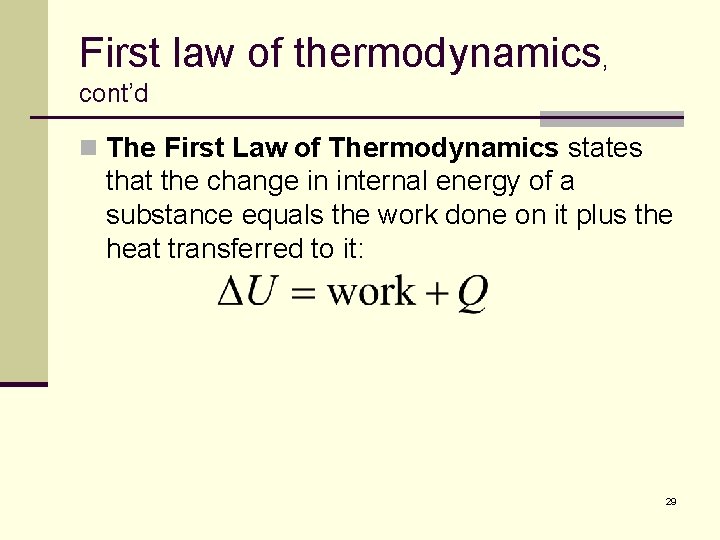
First law of thermodynamics, cont’d n The First Law of Thermodynamics states that the change in internal energy of a substance equals the work done on it plus the heat transferred to it: 29

First law of thermodynamics, cont’d n Recall that work can be positive or negative. n When a gas is compressed, positive work is done on the gas. n n The change in internal energy is positive — the gas’ internal energy increases. When a gas expands, negative work is done on the gas. n The change in internal energy is negative — the gas’ internal energy decreases. 30

First law of thermodynamics, cont’d n Internal energy is important during phase transitions. When you boil water, you increase the water’s temperature and its internal energy. n As the water undergoes the phase change to water vapor, its internal energy increases but its temperature remains at 100°C. n n The energy added to the water to evaporate is applied to break the bonds holding the molecules together — not to the molecular kinetic energy. 31

Explorations in Physics Lecture 4 — Nov. 7, 2005 Chapters 5. 4 – 6. 6

Heat transfer n There are three types of heat transfer: n Conduction: the transfer of heat between atoms and molecules in direct contact. n Convection: the transfer of heat by buoyant mixing in a fluid. n Radiation: the transfer of heat by way of electromagnetic waves. 33

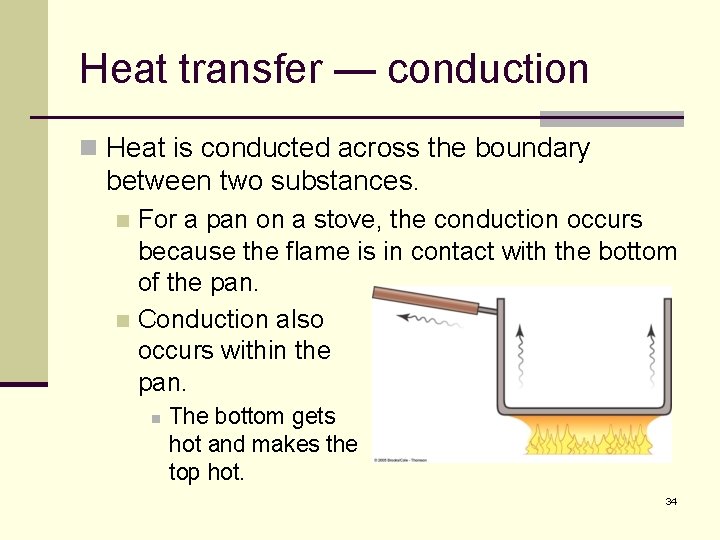
Heat transfer — conduction n Heat is conducted across the boundary between two substances. For a pan on a stove, the conduction occurs because the flame is in contact with the bottom of the pan. n Conduction also occurs within the pan. n n The bottom gets hot and makes the top hot. 34

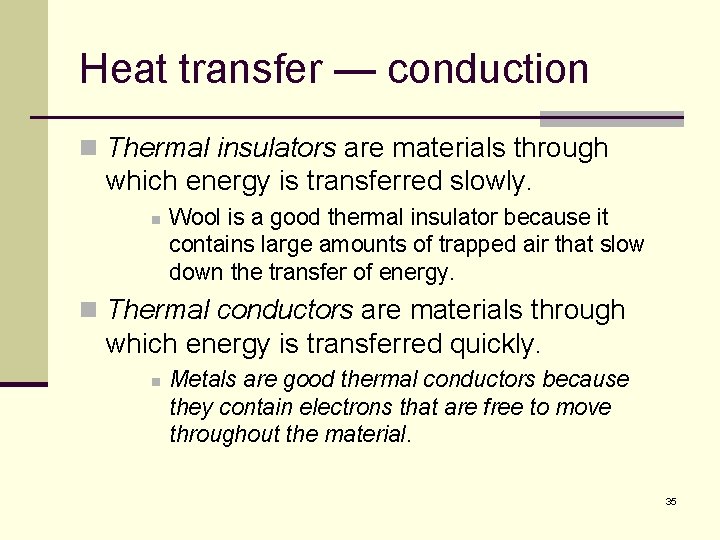
Heat transfer — conduction n Thermal insulators are materials through which energy is transferred slowly. n Wool is a good thermal insulator because it contains large amounts of trapped air that slow down the transfer of energy. n Thermal conductors are materials through which energy is transferred quickly. n Metals are good thermal conductors because they contain electrons that are free to move throughout the material. 35

Heat transfer — conduction n A hard-wood floor feels colder than a carpeted floor because the wood conducts heat more quickly from your foot than the carpet. n You can judge a good conductor if it feels colder than another substance at the same temperature. 36

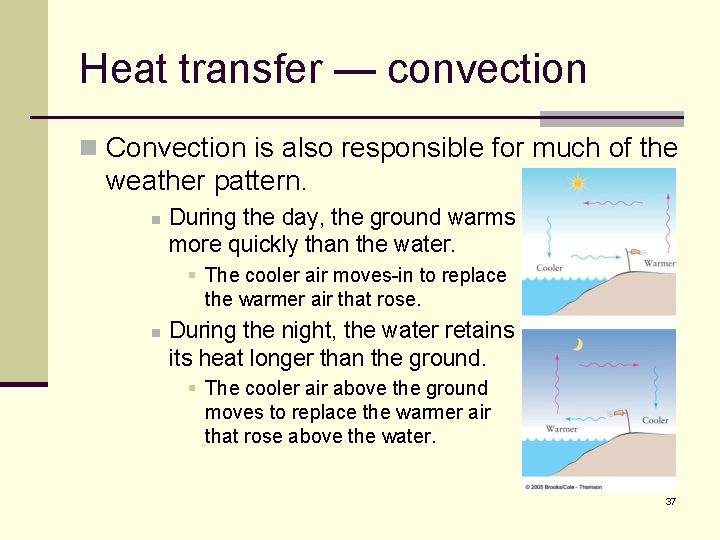
Heat transfer — convection n Convection is also responsible for much of the weather pattern. n During the day, the ground warms more quickly than the water. § The cooler air moves-in to replace the warmer air that rose. n During the night, the water retains its heat longer than the ground. § The cooler air above the ground moves to replace the warmer air that rose above the water. 37

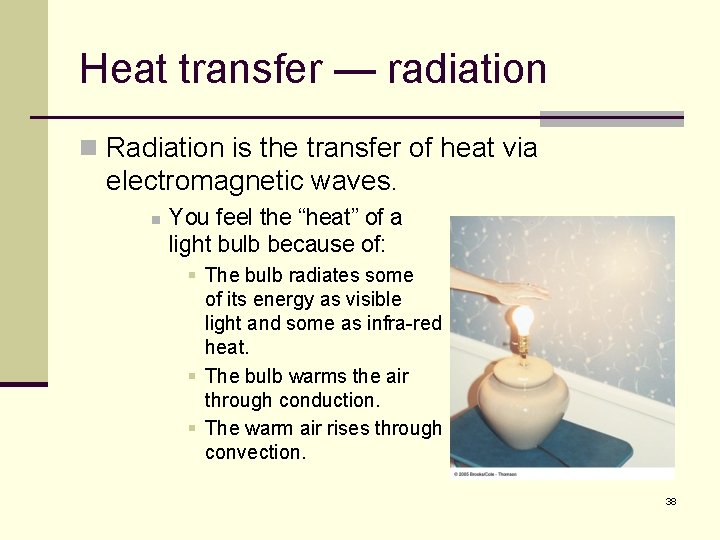
Heat transfer — radiation n Radiation is the transfer of heat via electromagnetic waves. n You feel the “heat” of a light bulb because of: § The bulb radiates some of its energy as visible light and some as infra-red heat. § The bulb warms the air through conduction. § The warm air rises through convection. 38

Specific heat capacity n Transferring heat to/from a substance changes its internal energy. n The substance’s change in temperature for a given change in internal energy depends on the type of substance. n It requires much more energy to raise the temperature of water than of air. 39

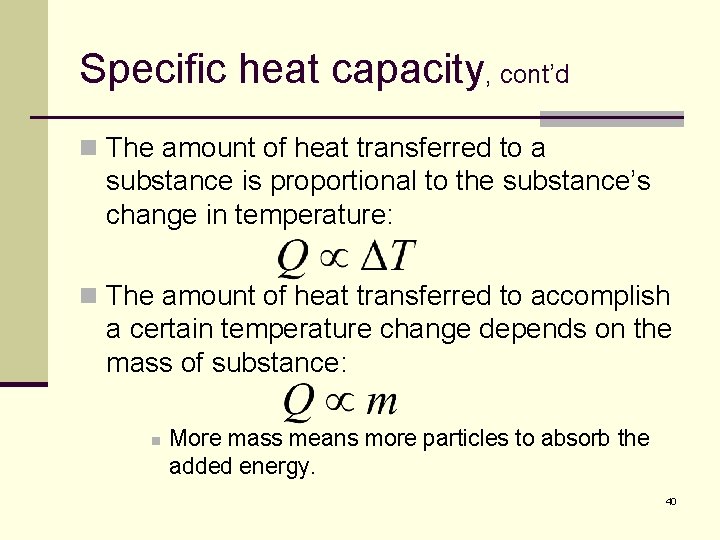
Specific heat capacity, cont’d n The amount of heat transferred to a substance is proportional to the substance’s change in temperature: n The amount of heat transferred to accomplish a certain temperature change depends on the mass of substance: n More mass means more particles to absorb the added energy. 40

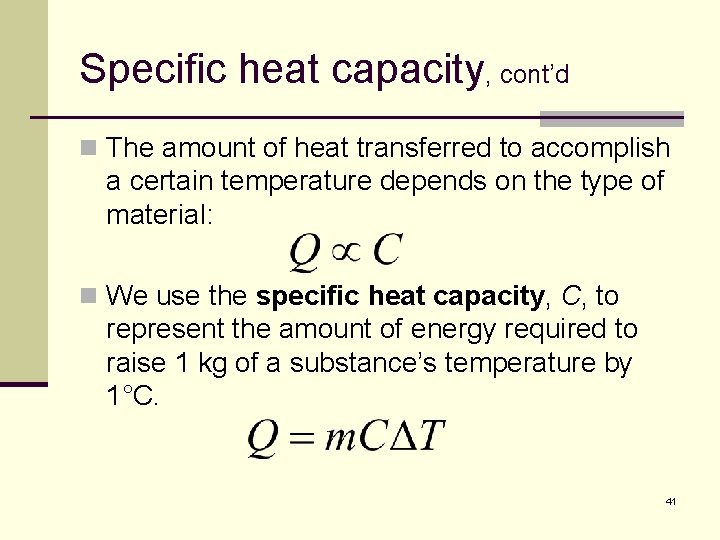
Specific heat capacity, cont’d n The amount of heat transferred to accomplish a certain temperature depends on the type of material: n We use the specific heat capacity, C, to represent the amount of energy required to raise 1 kg of a substance’s temperature by 1°C. 41

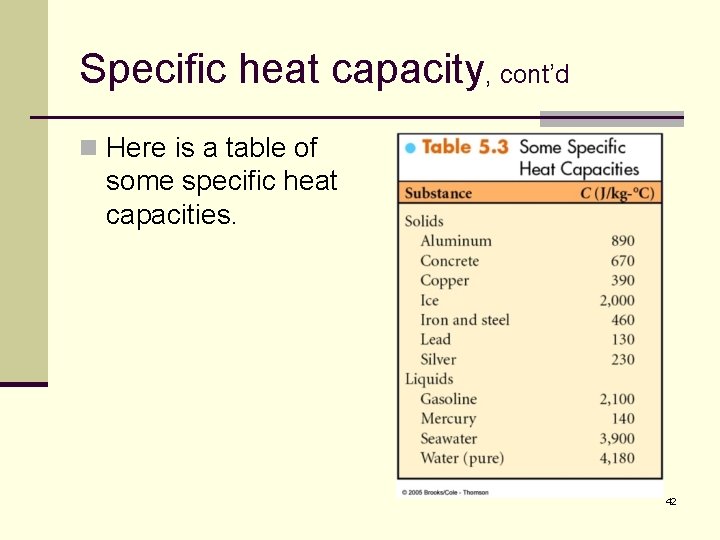
Specific heat capacity, cont’d n Here is a table of some specific heat capacities. 42

Specific heat capacity, cont’d n Recall that heat is a transfer of energy. n So we use joules as a unit for heat. n Historically, the unit of a calorie was used for heat. n It was a revolutionary idea that heat and energy are equivalent. n The conversion between joules are calories: 43

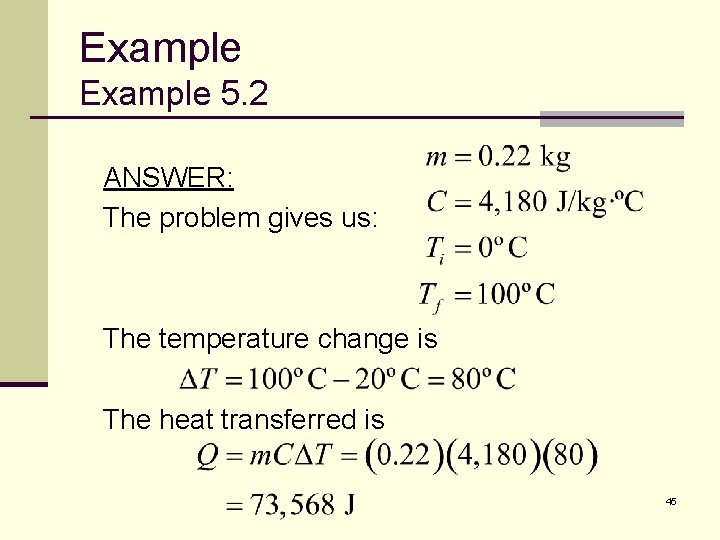
Example 5. 2 Let’s compute how much energy it takes to make a cup of coffee or tea. Eight ounces of water has a mass of about 0. 22 kilograms. How much heat must be transferred to the water to raise its temperature from 20°C to the boiling point, 100°C? 44

Example 5. 2 ANSWER: The problem gives us: The temperature change is The heat transferred is 45

Example 5. 2 DISCUSSION: This is approximately the same energy required to accelerate a pickup to a speed of nearly 30 mph. 46

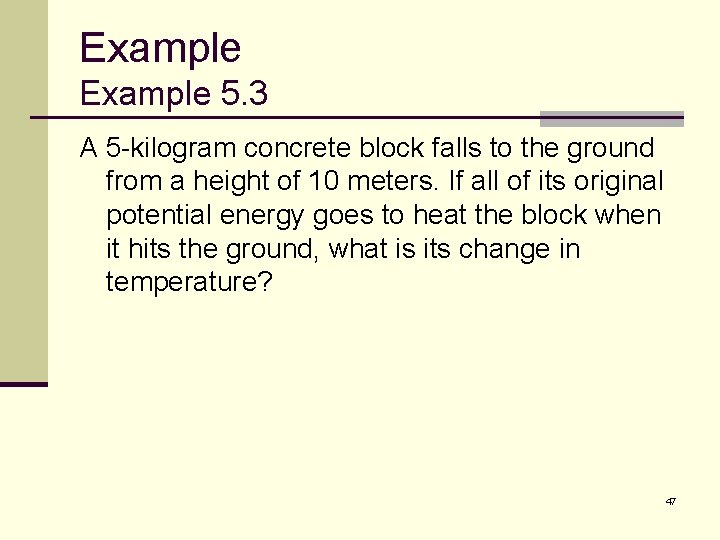
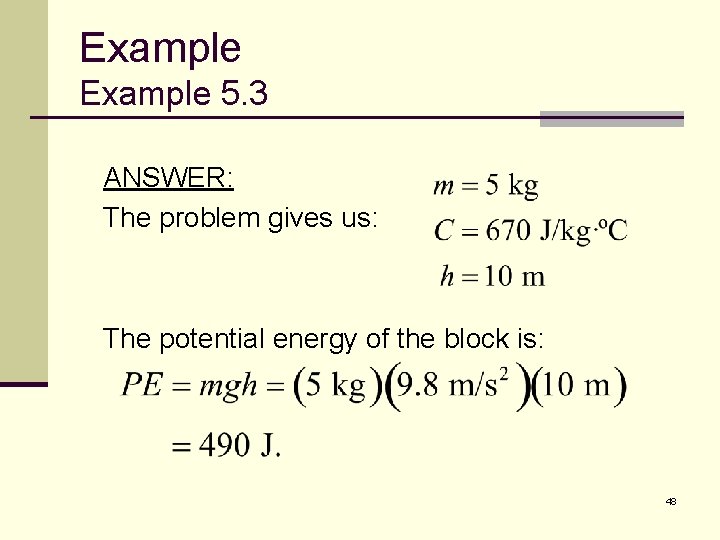
Example 5. 3 A 5 -kilogram concrete block falls to the ground from a height of 10 meters. If all of its original potential energy goes to heat the block when it hits the ground, what is its change in temperature? 47

Example 5. 3 ANSWER: The problem gives us: The potential energy of the block is: 48

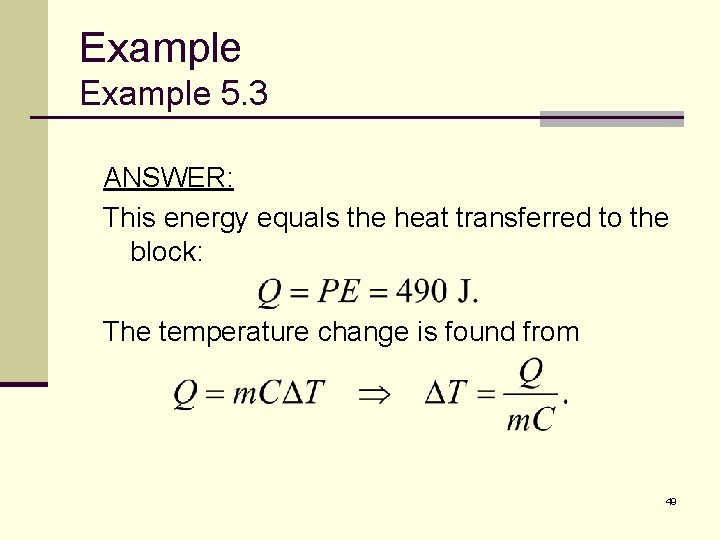
Example 5. 3 ANSWER: This energy equals the heat transferred to the block: The temperature change is found from 49

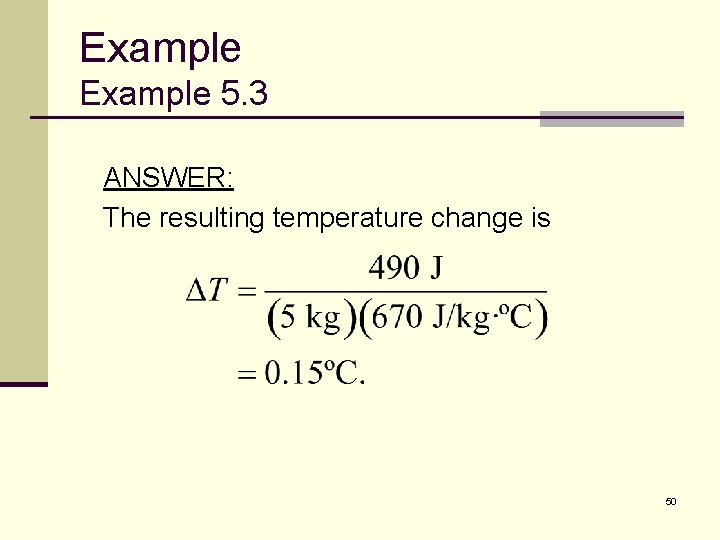
Example 5. 3 ANSWER: The resulting temperature change is 50

Example 5. 2 DISCUSSION: You typically do no notice the temperature changes associated with everyday actions. These temperature changes are usually too small to be noticed. But not always… 51

Example 5. 4 A satellite in low Earth orbit experiences slight air resistance and eventually reenters the Earth’s atmosphere. As it moves downward through the increasingly dense air, the friction force of air resistance converts the satellite’s kinetic energy into internal energy. If the satellite is mostly aluminum and all of its kinetic energy is converted into internal energy, what would be its temperature increase. (Take its speed to initially be 7, 900 m/s. ) 52

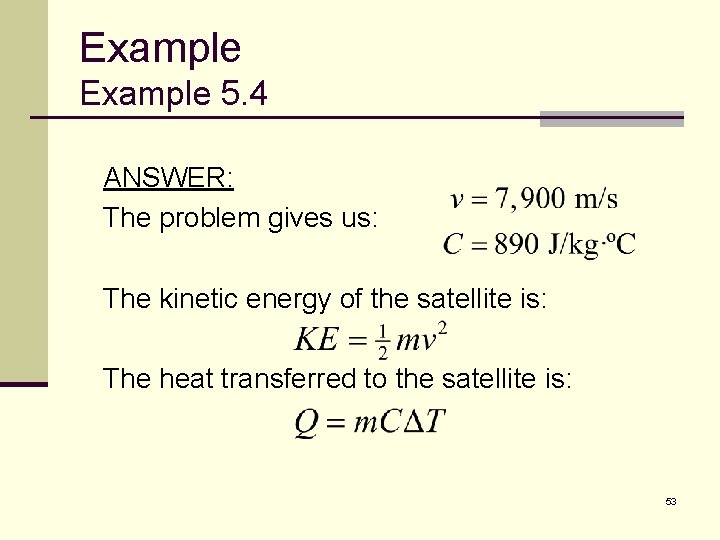
Example 5. 4 ANSWER: The problem gives us: The kinetic energy of the satellite is: The heat transferred to the satellite is: 53

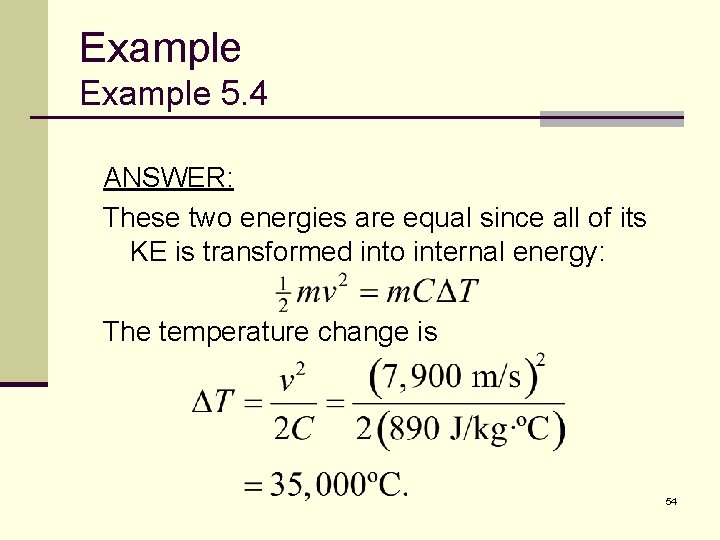
Example 5. 4 ANSWER: These two energies are equal since all of its KE is transformed into internal energy: The temperature change is 54

Example 5. 4 DISCUSSION: Note that the internal energy would not increase this much — the aluminum would melt. Even it 90% of the energy was lost to the air, the remaining 10% would be enough to melt the satellite — at least fry the electronics. 55

Specific heat capacity, cont’d n The “dietary calorie” is related to the “physics calorie. ” A “food calorie” is actually a kilocalorie. n It is typically denoted as a Calorie as opposed to the calorie we use in physics. n 56

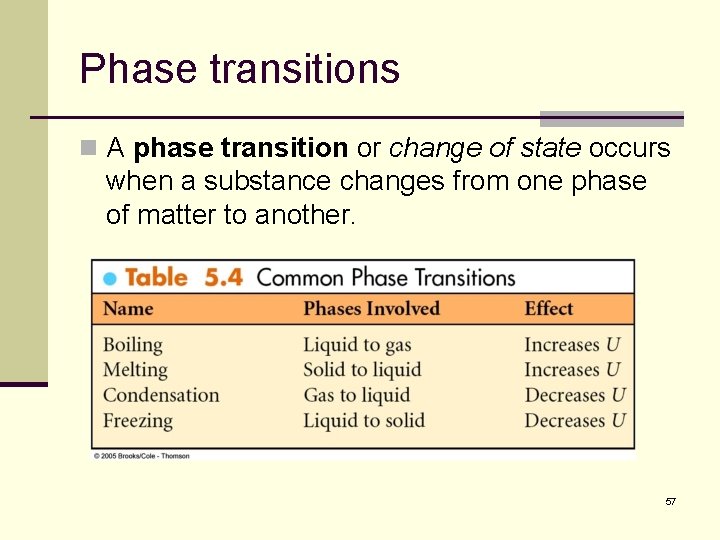
Phase transitions n A phase transition or change of state occurs when a substance changes from one phase of matter to another. 57

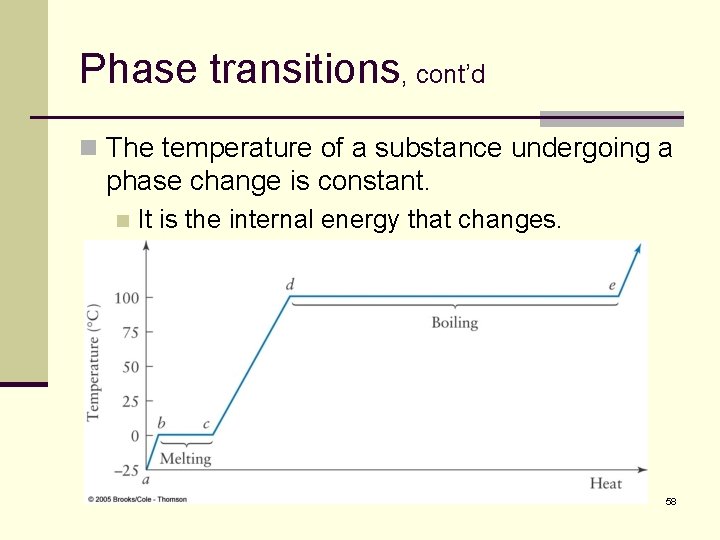
Phase transitions, cont’d n The temperature of a substance undergoing a phase change is constant. n It is the internal energy that changes. 58

Phase transitions, cont’d n To melt or freeze 1 kg of ice (2. 2 lb) requires that 334, 00 J (90 kcal) of energy be transferred. n This amount of energy is called the latent heat of fusion of water. n To boil or condense 1 kg of water requires that 2, 260, 000 J (540 kcal) of energy be transferred. n This amount of energy is called the latent heat of vaporization of water. 59

Phase transitions, cont’d n This explains why we use ice to keep drinks cold. It requires a large amount of energy to melt ice. n The energy to melt the ice is removed from your drink. n As long as there is ice in the drink, the temperature remains near 0°C. n 60

Example 5. 5 Ice at 0ºC is used to cool water from room temperature (20ºC) to 0ºC. How much water can be cooled by using 1 kilogram of ice? 61

Example 5. 5 ANSWER: The problem gives us: The amount of water is: 62

Example 5. 4 DISCUSSION: So ice at 0ºC can cool about four times its own mass of water from 20ºC to 0ºC. Note that we assumed no energy is lost to the environment — the cup, the air, the table, … 63

Humidity n At temperature below their boiling point, liquids gradually go into the gas phase through a process called evaporization. Some liquid molecules escape the liquid’s surface because of their large KE. n During this same time, some vapor molecules are absorbed by the liquid’s surface since they have low KE. n How much liquid evaporates depends upon how many vapor molecules are in the air? n 64

Humidity, cont’d n Humidity is the mass of water vapor in the air per unit volume. It is the density of the water vapor in the air. n It has the same unit as mass density (kg/m 3). n n n Low humidity is around 0. 001 kg/m 3 (cold day in a dry climate). High humidity is about 0. 03 kg/m 3 (hot, humid day). § Normal air density is around 1. 29 kg/m 3. 65

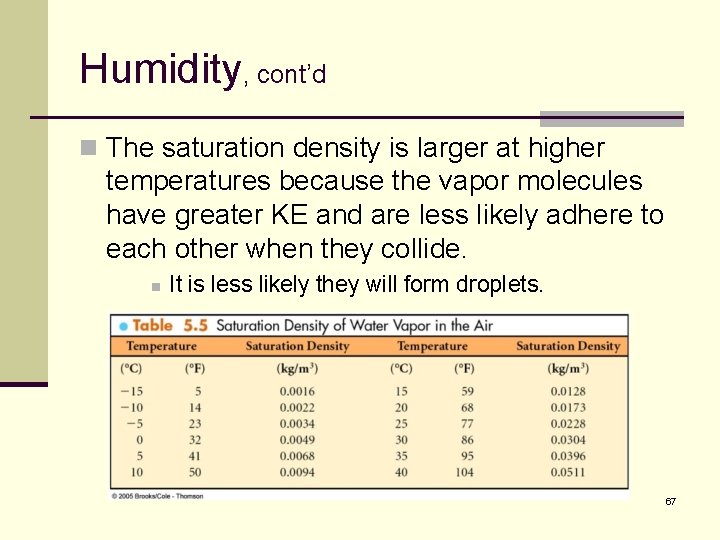
Humidity, cont’d n At any given temperature, there is a maximum possible humidity called the saturation density. n At the saturation density, the water vapor readily transitions to the liquid phase. n Water condenses in the air and on any available surfaces. § This is what happens during the formation of for and dew. 66

Humidity, cont’d n The saturation density is larger at higher temperatures because the vapor molecules have greater KE and are less likely adhere to each other when they collide. n It is less likely they will form droplets. 67

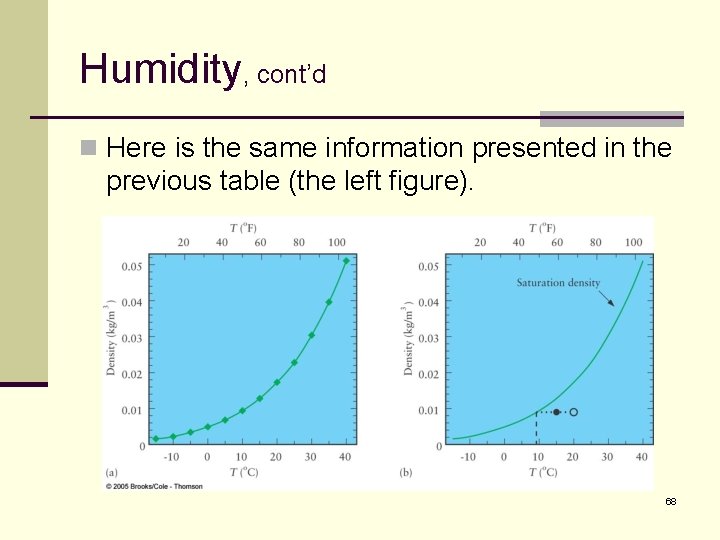
Humidity, cont’d n Here is the same information presented in the previous table (the left figure). 68

Humidity, cont’d n So the humidity alone does not determine how readily water condenses. n The saturation density is also relevant. n These two are related through the relative humidity. 69

Humidity, cont’d n Relative humidity is expressed as a percentage of the humidity to the saturation density: n The temperature at which the condensation appears for a constant humidity is called the dew point. 70

Humidity, cont’d n The dew point is therefore the temperature at which a given humidity equals the saturation density. 71

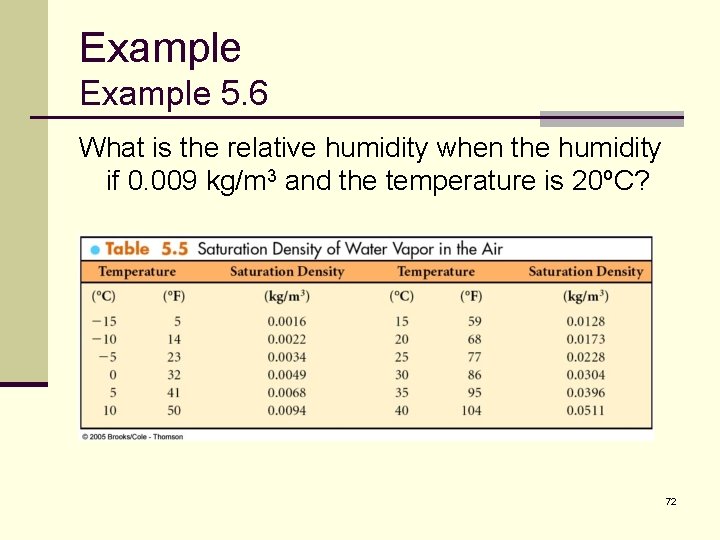
Example 5. 6 What is the relative humidity when the humidity if 0. 009 kg/m 3 and the temperature is 20ºC? 72

Example 5. 6 ANSWER: The relative humidity is: 73

Heat engines and the 2 nd law of thermodynamics n A heat engine is a device that transforms heat into mechanical energy or work. It absorbs heat from a hot source (such as burning fuel), n Converts some of this energy into usable mechanical energy or work, and n Outputs the remaining energy as heat to some lower-temperature reservoir. n 74

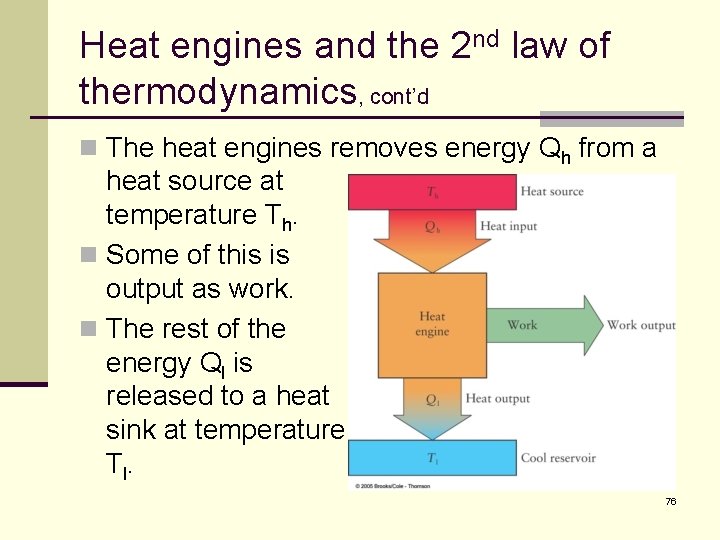
Heat engines and the 2 nd law of thermodynamics, cont’d n Gasoline, diesel and jet engines are all heat engines. n Each converts the heat released from burning a fuel into mechanical energy. n Gasoline and diesel engines release the rest of the heat to the air (exhaust pipe, radiator, etc). n Although the details of heat engines are complicated, we can represent them with a simple diagram. 75

Heat engines and the 2 nd law of thermodynamics, cont’d n The heat engines removes energy Qh from a heat source at temperature Th. n Some of this is output as work. n The rest of the energy Ql is released to a heat sink at temperature T l. 76

Heat engines and the 2 nd law of thermodynamics, cont’d n The Second Law of Thermodynamics states that no device can be built that will repeatedly extract heat from a source and deliver mechanical work or energy without ejecting some heat to a lower-temperature reservoir. n This basically means that you cannot create a device of perfect efficiency. n You must have some wasted energy that is released as heat. 77

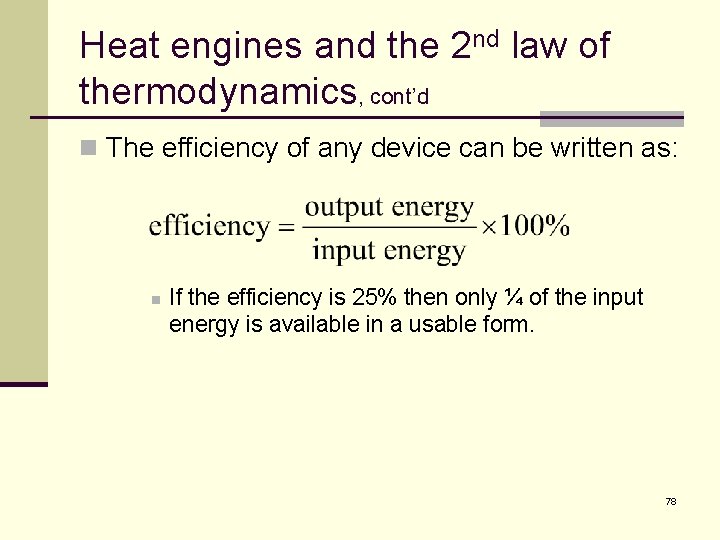
Heat engines and the 2 nd law of thermodynamics, cont’d n The efficiency of any device can be written as: n If the efficiency is 25% then only ¼ of the input energy is available in a usable form. 78

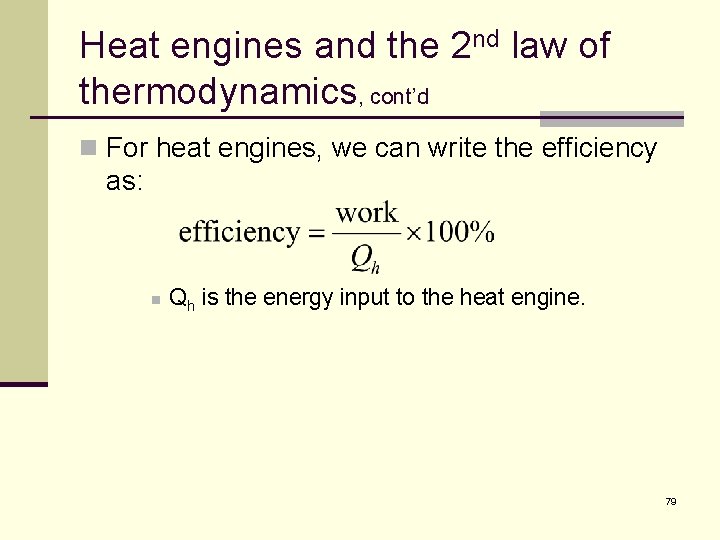
Heat engines and the 2 nd law of thermodynamics, cont’d n For heat engines, we can write the efficiency as: n Qh is the energy input to the heat engine. 79

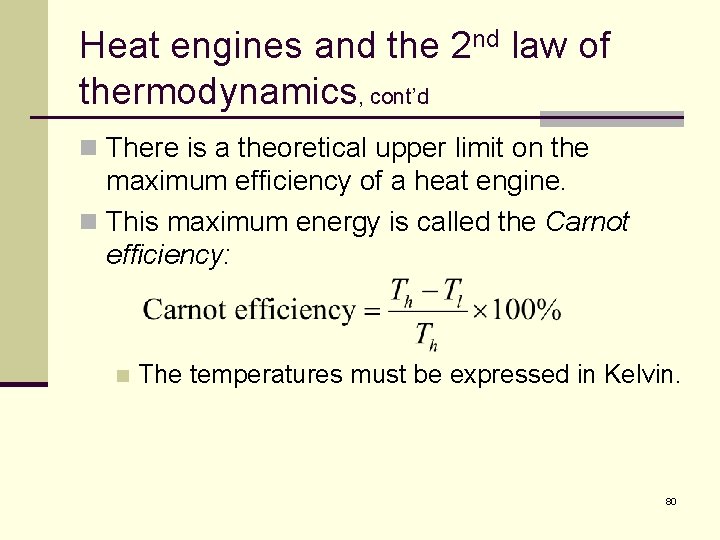
Heat engines and the 2 nd law of thermodynamics, cont’d n There is a theoretical upper limit on the maximum efficiency of a heat engine. n This maximum energy is called the Carnot efficiency: n The temperatures must be expressed in Kelvin. 80

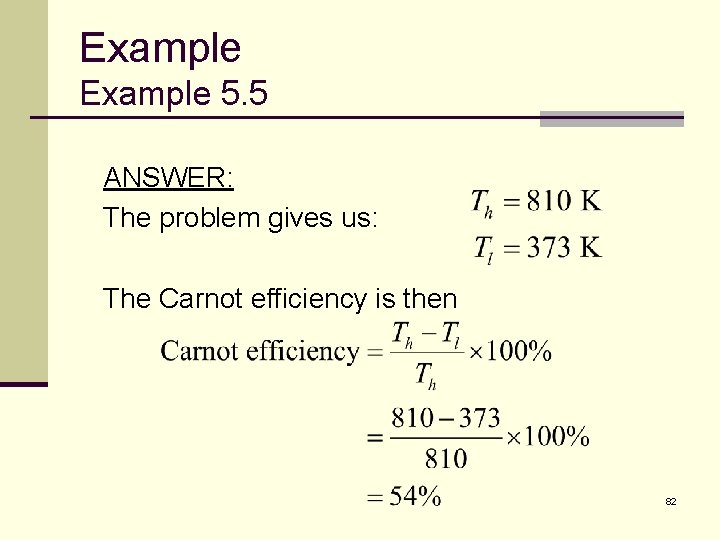
Example 5. 7 A typical coal-fired power plant uses steam at a temperature of 1, 000ºF (810 K). The steam leaves the turbine at a temperature of about 212ºF (373 K). What is theoretical maximum efficiency of the power plant? 81

Example 5. 5 ANSWER: The problem gives us: The Carnot efficiency is then 82

Example 5. 4 DISCUSSION: This is the ideal efficiency. Normal efficiencies are around 35 – 40%. 83

Heat engines and the 2 nd law of thermodynamics, cont’d n A heat mover is a device that acts like a heat engine in reverse. n It uses an external energy source to move heat to flow from a cooler substance to a warmer substance. n Examples are refrigerators, air conditioners, heat pumps, etc. 84

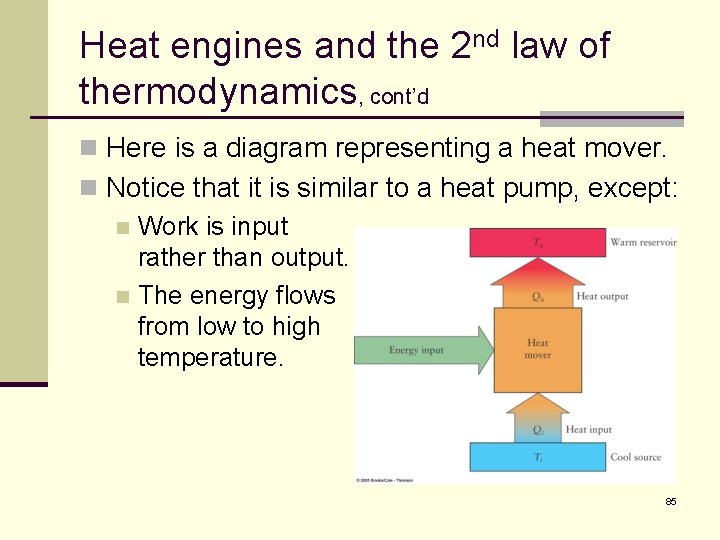
Heat engines and the 2 nd law of thermodynamics, cont’d n Here is a diagram representing a heat mover. n Notice that it is similar to a heat pump, except: n Work is input rather than output. n The energy flows from low to high temperature. 85

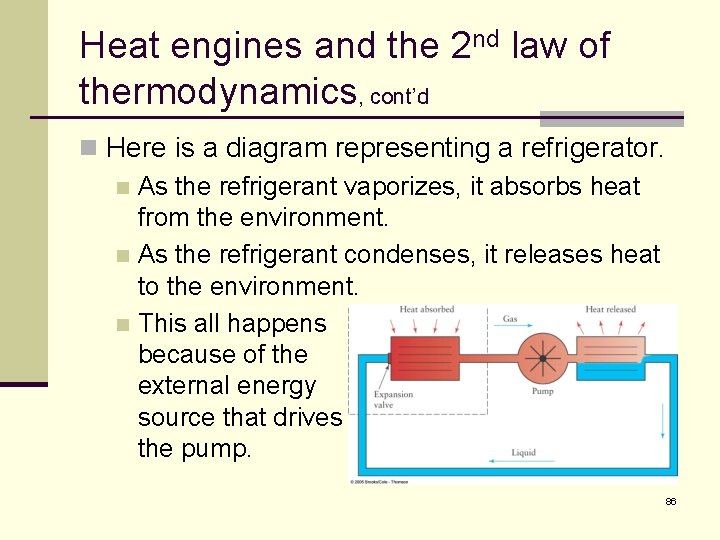
Heat engines and the 2 nd law of thermodynamics, cont’d n Here is a diagram representing a refrigerator. n As the refrigerant vaporizes, it absorbs heat from the environment. n As the refrigerant condenses, it releases heat to the environment. n This all happens because of the external energy source that drives the pump. 86