Energy basics Temperature and Heat Temperature and heat

Energy basics

Temperature and Heat Temperature and heat are NOT THE SAME.
Temperature How hot or cold it is. Measured in degrees Celsius o Fahrenheit.
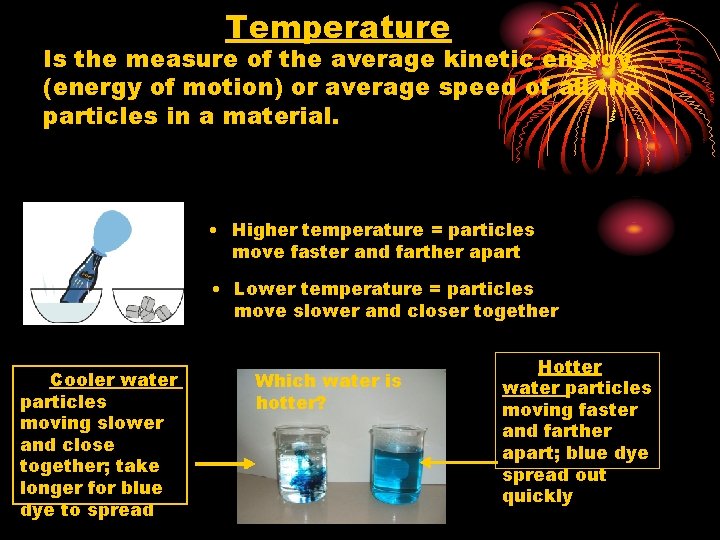
Temperature Is the measure of the average kinetic energy (energy of motion) or average speed of all the particles in a material. • Higher temperature = particles move faster and farther apart • Lower temperature = particles move slower and closer together Cooler water particles moving slower and close together; take longer for blue dye to spread Which water is hotter? Hotter water particles moving faster and farther apart; blue dye spread out quickly
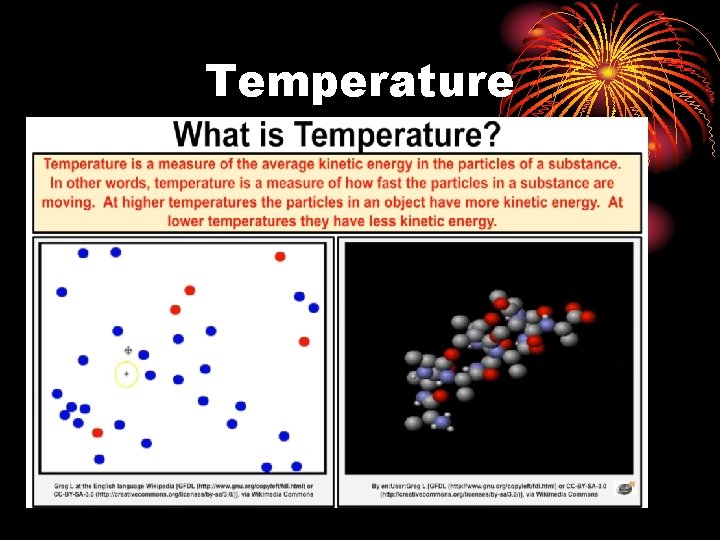
Temperature

Temperature Simulation • file: ///C: /Users/Delgado/Download s/states-of-matterbasics_en. html

Heat is thermal energy transferred from a hot object to a cold object. Heat is measured in energy units -Joules or calories.
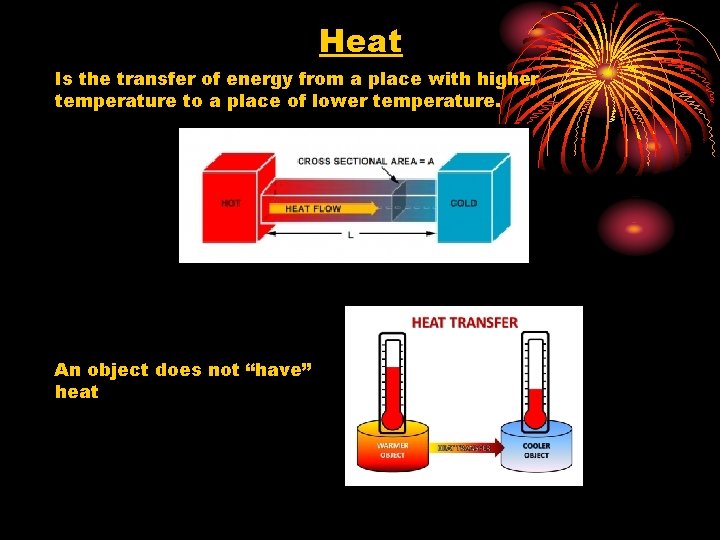
Heat Is the transfer of energy from a place with higher temperature to a place of lower temperature. An object does not “have” heat

Heat Transfer • Heat always moves from a warmer place to a cooler place. • Hot objects in a cooler room will cool to room temperature. • Cold objects in a warmer room will heat up to room temperature.

Measuring Heat • When temperature increases, heat is being added • When temperature decreases, heat is being removed • Heat is measured in calories

Calorie/Joules • 1 calorie is the amount of heat needed to raise 1 gram of water 1°C • Example – 1 calorie is needed to raise the temperature of 1 g H 2 O from 24°C to 25°C • 1 cal = 4. 18 J • The amount of heat needed to increase the temperature of an object depends on the mass of the object

The heat transferred is proportional to the mass of the object, the specific heat capacity of the object and the temperature change
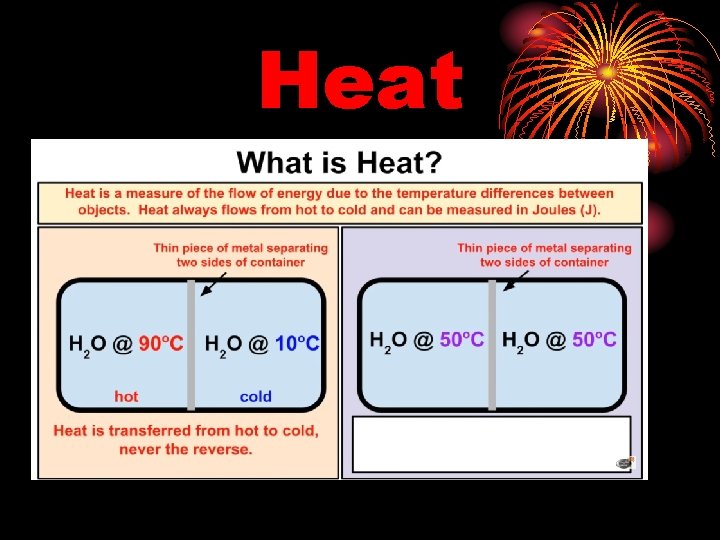
Heat

Heat • https: //phet. colorado. edu/en/sim ulation/legacy/energy-forms-andchanges
Specific Heat Capacity •

A swimming pool at 30°C is at a lower temperature than a cup of tea at 80°C. BUT the swimming pool contains more water, so it stores more thermal energy or heat.
Specific Heat Capacity
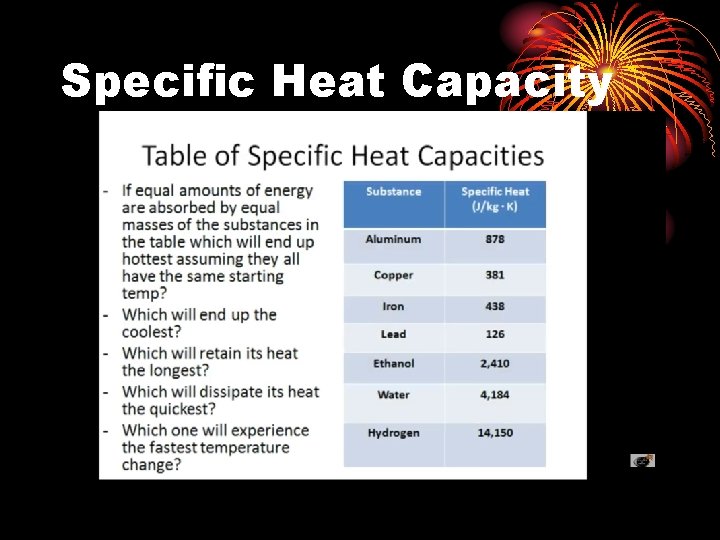
Specific Heat Capacity

Question • If a cup of coffee and a red popsickle were left on the table in this room what would happen to them? Why? • The cup of coffee will cool until it reaches room temperature. The popsickle will melt and then the liquid will warm to room temperature.

The small beaker of water boils first The large beaker contains more water and needs more thermal energy or heat to reach 100°C.

Transfer of Heat
Thermal Energy and Heat • Temperature – a measure of the AVERAGE kinetic energy of the individual particles of a substance. • Thermal energy – TOTAL energy of all of the particles • Heat – thermal energy moving from a warmer object to a cooler object, trying to reach thermodynamic equilibrium

Heat • A form of energy associated with the motion of atoms or molecules. • Transferred from higher temperature objects to objects at a lower temperature.
Heat Energy • Heat is energy caused by the internal motion of molecules of matter • Heat energy moves from warmer objects to cooler objects • Some objects transfer heat better than others
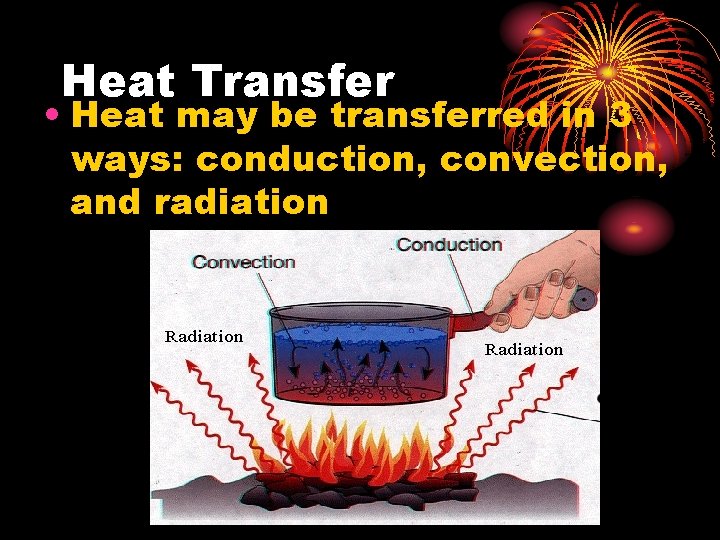
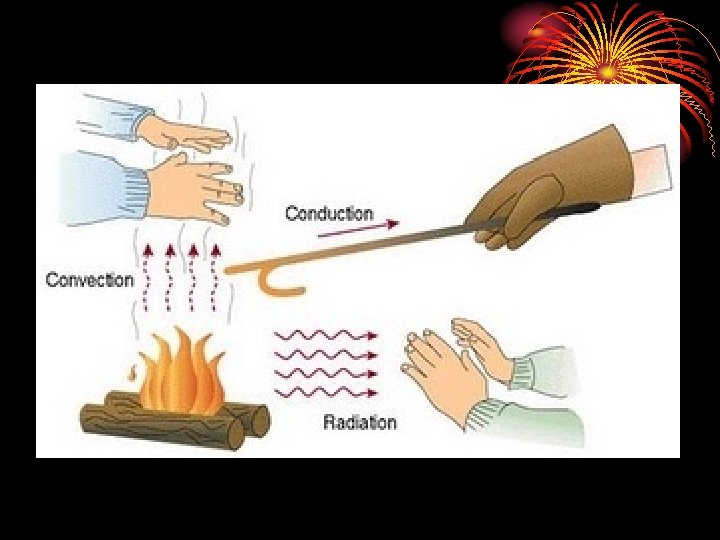
Heat Transfer • Heat may be transferred in 3 ways: conduction, convection, and radiation
Conduction • Transfer of heat through direct contact. • Occurs anytime objects at different temperatures are touching each other. • As long as the objects are in contact, transfer of heat will continue until the temperature of the objects is the same.
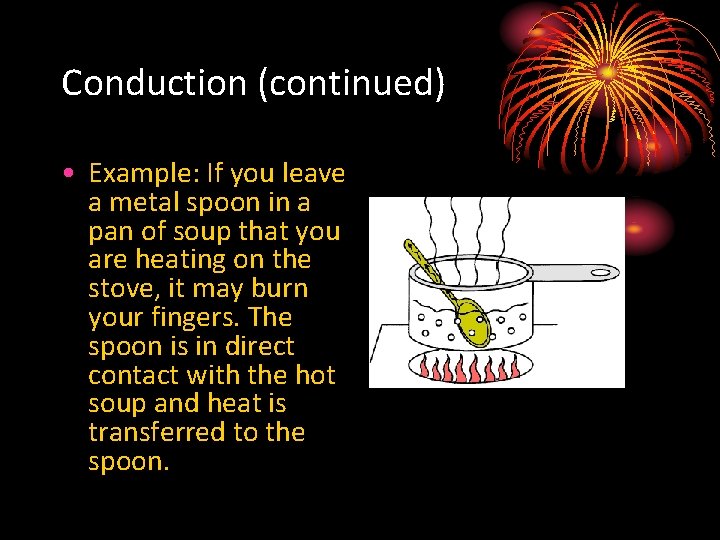
Conduction (continued) • Example: If you leave a metal spoon in a pan of soup that you are heating on the stove, it may burn your fingers. The spoon is in direct contact with the hot soup and heat is transferred to the spoon.

Conductors and Insulators • Some materials conduct heat better than others. • Materials that transfer heat well are called conductors. • Metals are usually good conductors. • Wood, paper and plastic are not. • Materials that stop the transfer of heat are called insulators (styrofoam, wool, fiberglass).

Conduction Animation: http: //www. passmyexams. co. uk/GCSE/physics/conductionheat-transfer. html Eureka Video clip:

Convection • The transfer of energy in a liquid or gas. • When part of a gas or liquid is heated, the particles it is made up of move faster and spread out more. • The moving particles bump into other particles, causing them to move faster and spread out more.
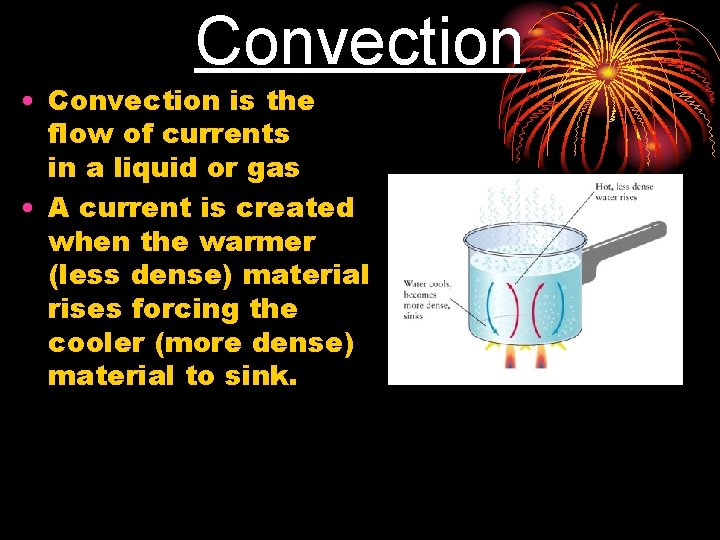
Convection • Convection is the flow of currents in a liquid or gas • A current is created when the warmer (less dense) material rises forcing the cooler (more dense) material to sink.
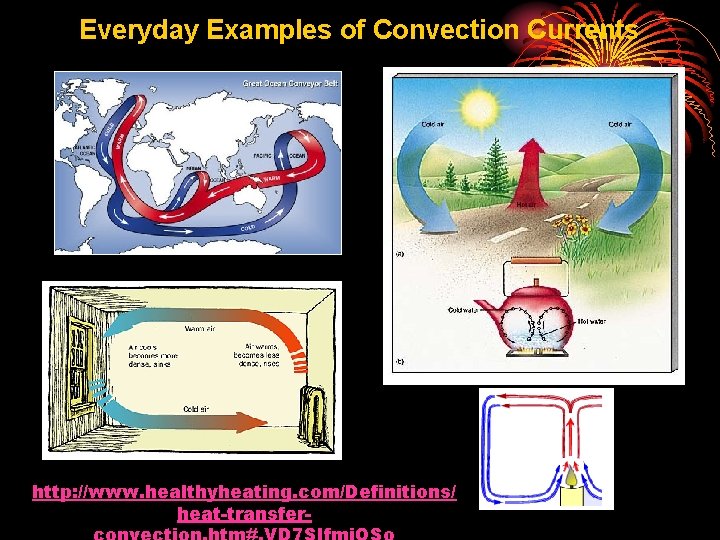
Everyday Examples of Convection Currents http: //www. healthyheating. com/Definitions/ heat-transfer-

Convection Animations and Video Clips http: //www. passmyexams. co. uk/GCSE/physics/convectionheat-transfer. html Eureka Video clip:

Radiation • Energy transferred in the form of rays or waves or particles. • Acceleration of charged particles releases electromagnetic radiation. • More acceleration the more the electromagnetic radiation. • You observe electromagnetic radiation in wavelengths that your eye considers to be visible light.
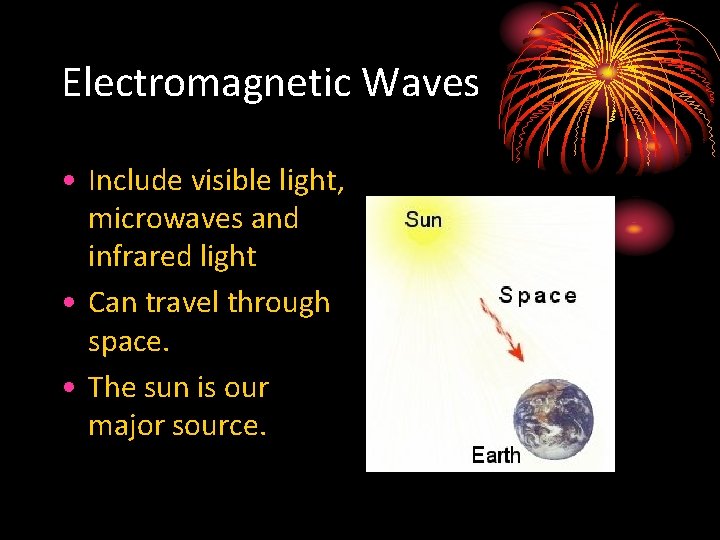
Electromagnetic Waves • Include visible light, microwaves and infrared light • Can travel through space. • The sun is our major source.

Heat From the Sun • You can feel the sun warm your skin on a sunny day. • This is because the energy causes the particles in your skin to move faster = more heat energy.

Radiation Eureka video clip: http: //www. youtube. co m/watch? v=2 JZci. Wt. K 6 vc
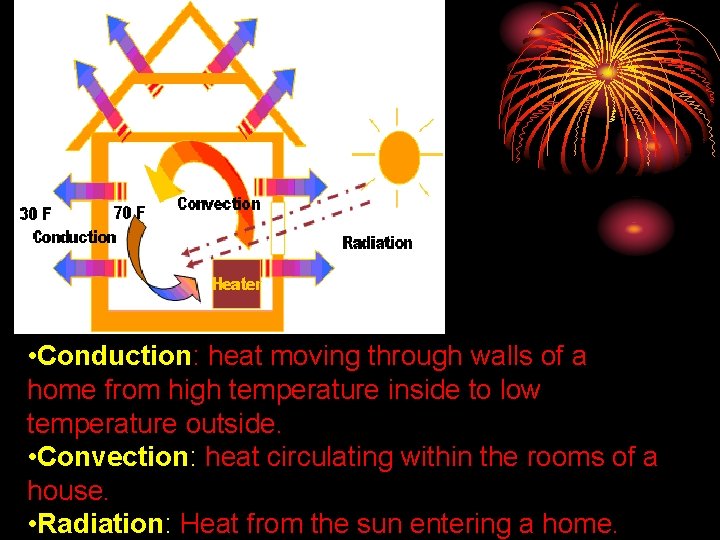
• Conduction: heat moving through walls of a home from high temperature inside to low temperature outside. • Convection: heat circulating within the rooms of a house. • Radiation: Heat from the sun entering a home.

Heat Transfer Song http: //www. youtube. c om/watch? v=wr 8 Z 4 SC ETPs

Convection questions Why does hot air rise and cold air sink? Cool air is more dense than warm air, so the cool air ‘falls through’ the warm air. Why are boilers placed beneath hot water tanks in people’s homes? Hot water rises. So when the boiler heats the water, and the hot water rises, the water tank is filled with hot water.

Radiation questions Why are houses painted white in hot countries? White reflects heat radiation and keeps the house cooler. Why are shiny foil blankets wrapped around marathon runners at the end of a race? The shiny metal reflects the heat radiation from the runner back in, this stops the runner getting cold.
1. Which of the following is not a method of heat transfer? A. Radiation B. Insulation C. Conduction D. Convection

3. How does heat energy reach the Earth from the Sun? A. Radiation B. Conduction C. Convection D. Insulation
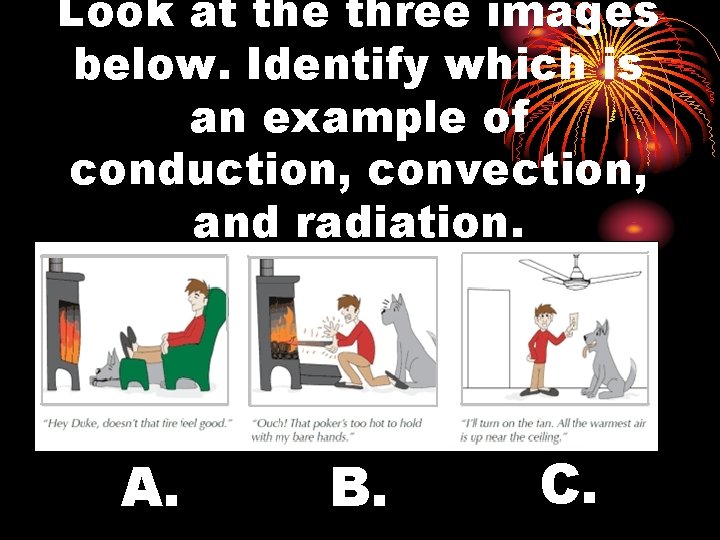
Look at the three images below. Identify which is an example of conduction, convection, and radiation. A. B. C.

Look at the three images below. Identify which is an example of conduction, convection, and radiation.

Heat Transfer Summarizer
- Slides: 48