HEAT ENERGY HEAT Heat Energy What is Heat






- Slides: 13

HEAT ENERGY HEAT

Heat Energy What is Heat? u It is the form energy of_______. u u Heat does not have mass or take up space. Hence, it is not matter a____.

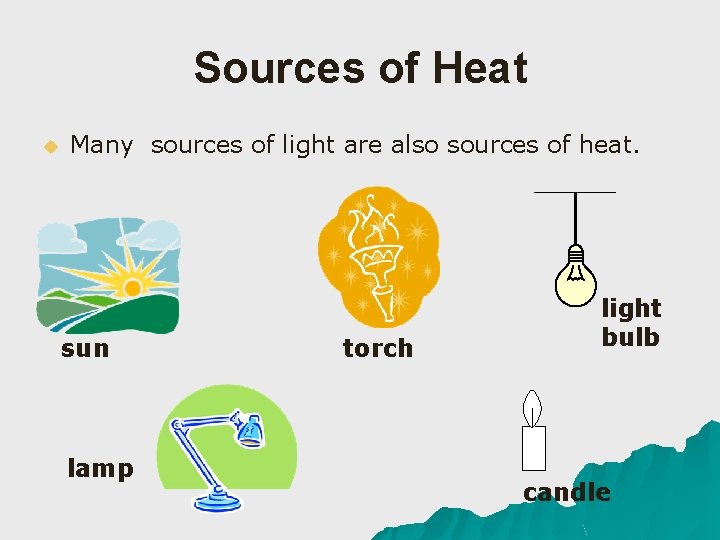
Sources of Heat u Many sources of light are also sources of heat. sun lamp torch light bulb candle

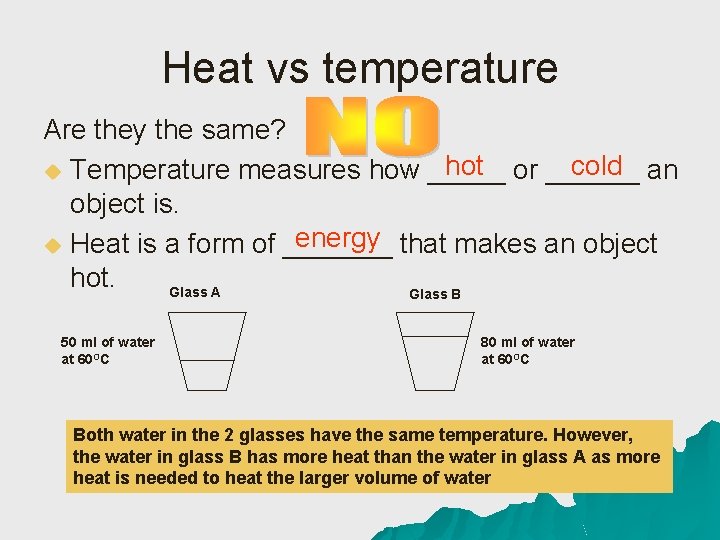
Heat vs temperature Are they the same? hot or ______ cold an u Temperature measures how _____ object is. energy that makes an object u Heat is a form of _______ hot. Glass A Glass B 50 ml of water at 60 OC 80 ml of water at 60 OC Both water in the 2 glasses have the same temperature. However, the water in glass B has more heat than the water in glass A as more heat is needed to heat the larger volume of water

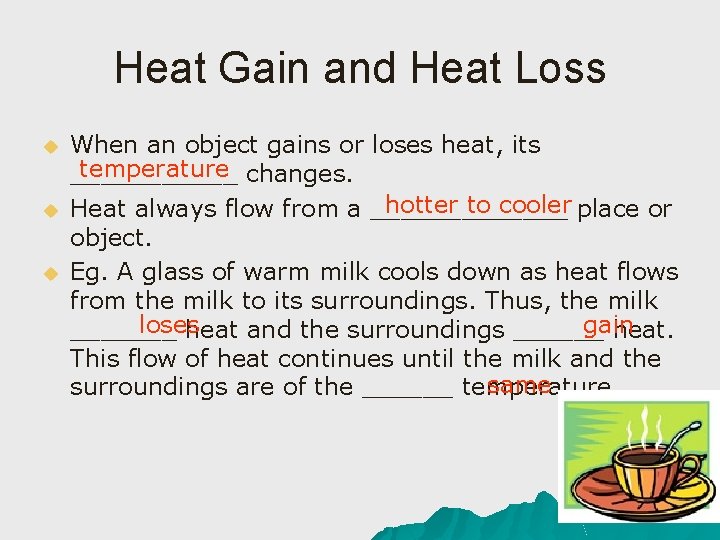
Heat Gain and Heat Loss u u u When an object gains or loses heat, its temperature changes. ______ hotter to cooler place or Heat always flow from a _______ object. Eg. A glass of warm milk cools down as heat flows from the milk to its surroundings. Thus, the milk loses gain _______ heat and the surroundings ______ heat. This flow of heat continues until the milk and the same surroundings are of the ______ temperature.

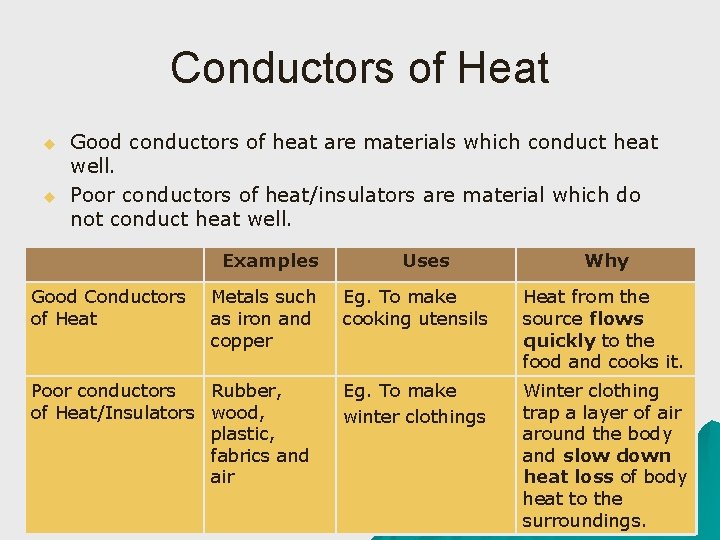
Conductors of Heat u u Good conductors of heat are materials which conduct heat well. Poor conductors of heat/insulators are material which do not conduct heat well. Examples Good Conductors of Heat Metals such as iron and copper Poor conductors Rubber, of Heat/Insulators wood, plastic, fabrics and air Uses Why Eg. To make cooking utensils Heat from the source flows quickly to the food and cooks it. Eg. To make winter clothings Winter clothing trap a layer of air around the body and slow down heat loss of body heat to the surroundings.

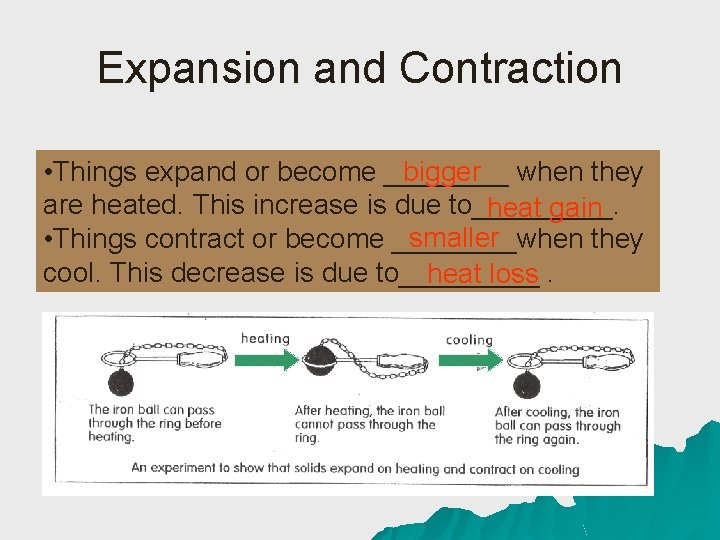
Expansion and Contraction • Things expand or become ____ bigger when they are heated. This increase is due to_____. heat gain smaller • Things contract or become ____when they cool. This decrease is due to_____ heat loss.

Time to put on your thinking ca!

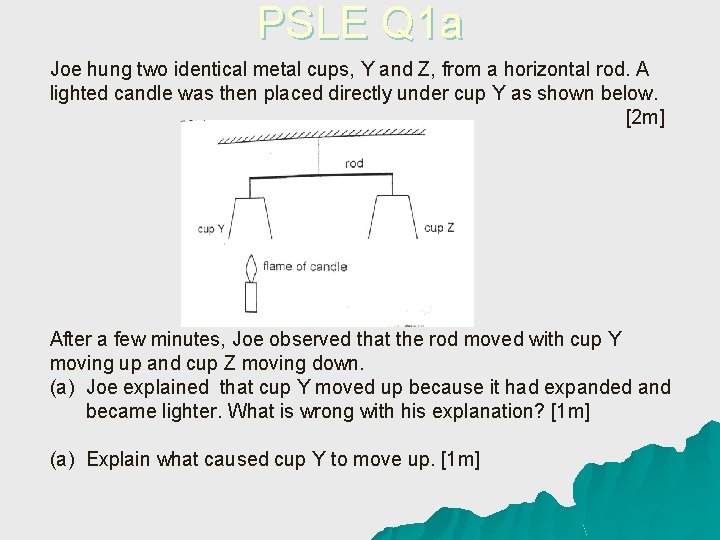
PSLE Q 1 a Joe hung two identical metal cups, Y and Z, from a horizontal rod. A lighted candle was then placed directly under cup Y as shown below. [2 m] After a few minutes, Joe observed that the rod moved with cup Y moving up and cup Z moving down. (a) Joe explained that cup Y moved up because it had expanded and became lighter. What is wrong with his explanation? [1 m] (a) Explain what caused cup Y to move up. [1 m]

Answer to PSLE Q 1 a u Joe’s explanation is wrong because even if the cup had expanded, its mass will remain the same. Thus, cup Y would not have moved up due to the cup having a lighter mass. I: Mass of the cup u C: Mass should remain the same u R: Cup Y did not move up due to it having a lighter mass. u

Answer to PSLE Q 1 b u The air above the candle flame gains heat and expands. Hot air rises and pushes cup Y to move upwards. I: Air above the candle flame u C: Air gains heat and expands in volume u R: Hot air rise and pushes cup Y to move upwards u

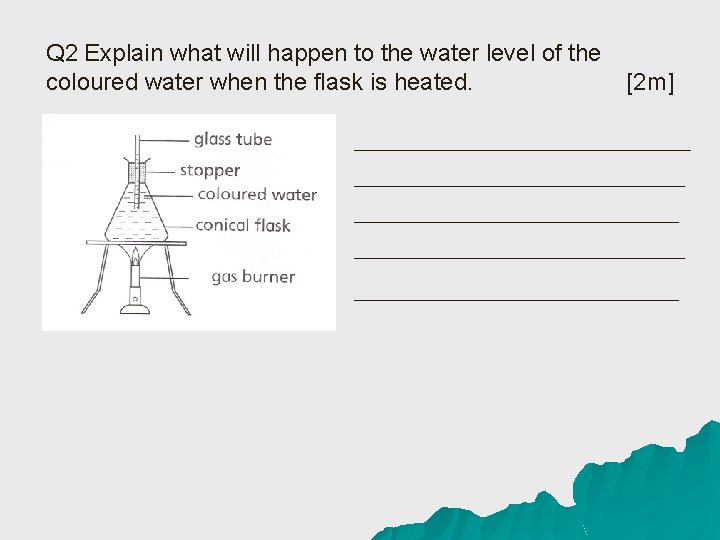
Q 2 Explain what will happen to the water level of the coloured water when the flask is heated. [2 m]

Answer to Q 2 u The flask gains heat from the bunsen burner and expands (1/2 m), hence the water level will drop slightly (1/2 m). After a while, the water in the flask gains heat and expands(1/2 m) causing the water level to increase (1/2 m). u I: Flask and Water gains heat u C: Flask and water expands in volume u R: Water level drops then rises again