Heat and Temperature Objectives Heat Temperature Absolute Zero

Heat and Temperature

Objectives • • • Heat Temperature Absolute Zero Fahrenheit, Celsius and Kelvin Scales Methods of Energy Transfer Conductors and Insulators Specific Heating Systems Cooling Systems
Heat • The transfer of energy from the particles of one object to those of another object due to a temperature difference between the two objects
Temperature • A measure of the average kinetic energy of all the particles within an object

• Are heat and temperature the same thing?
Thermometer • A device that measures temperature • Liquid thermometers – Mercury – Alcohol

Fahrenheit Scale • Based on the numbers 12 and 8 • 32°F = water freezes • 212 °F = water boils • Most familiar scale in the United States Daniel Gabriel Fahrenheit (1686 -1736) German Physicist

Celsius Scale • Based on the number ten • 0 °C = water freezes • 100 °C = water boils • Used in many foreign countries and by most scientists Anders Celsius (1701 -1744) Swedish Astronomer

Kelvin Scale • Based on the metric scale and the number ten • Developed to eliminate negative temperatures • Used by many scientists, especially if they work with extremely low temperatures • 0 K = -273 °C • 273 K = water freezes • 373 K = water boils • NO degree sign!

Absolute Zero • The temperature at which an object’s energy is minimal. • -273. 15 °C • 0 Kelvin But Mom, why can’t I wear shorts today? It’s supposed to be 254 Kelvin! …and we think the Artic Circle is cold!!!
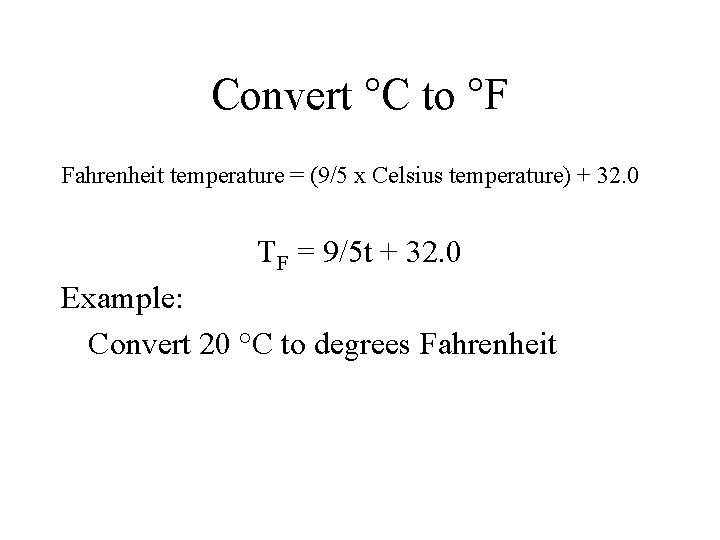
Convert °C to °F Fahrenheit temperature = (9/5 x Celsius temperature) + 32. 0 TF = 9/5 t + 32. 0 Example: Convert 20 °C to degrees Fahrenheit
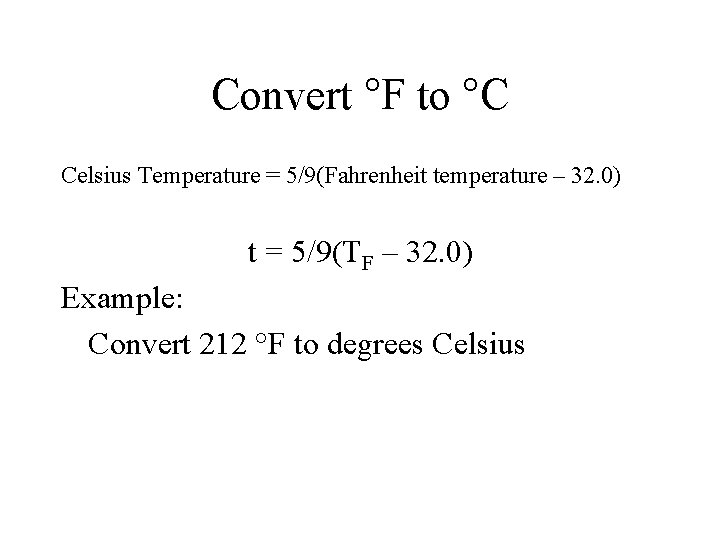
Convert °F to °C Celsius Temperature = 5/9(Fahrenheit temperature – 32. 0) t = 5/9(TF – 32. 0) Example: Convert 212 °F to degrees Celsius

Convert °C to Kelvin temperature = Celsius temperature + 273 T = t + 273 Example: Convert 10 °C to Kelvin

Assignment • Page 328 Practice Problems – Write the questions – Show your work!
Energy Transfer • • • Conduction Convection Current Radiation Conductor Insulator
Conduction • The transfer of energy as heat between particles as they collide within a substance or between two objects in contact. – Objects must have direct contact
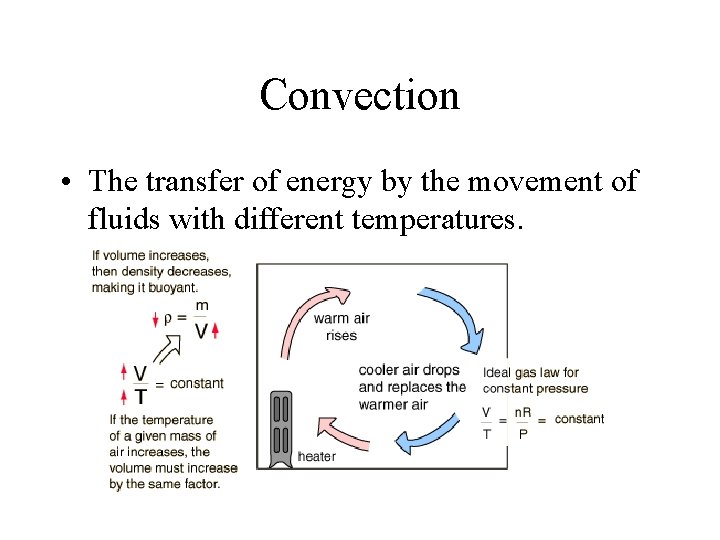
Convection • The transfer of energy by the movement of fluids with different temperatures.
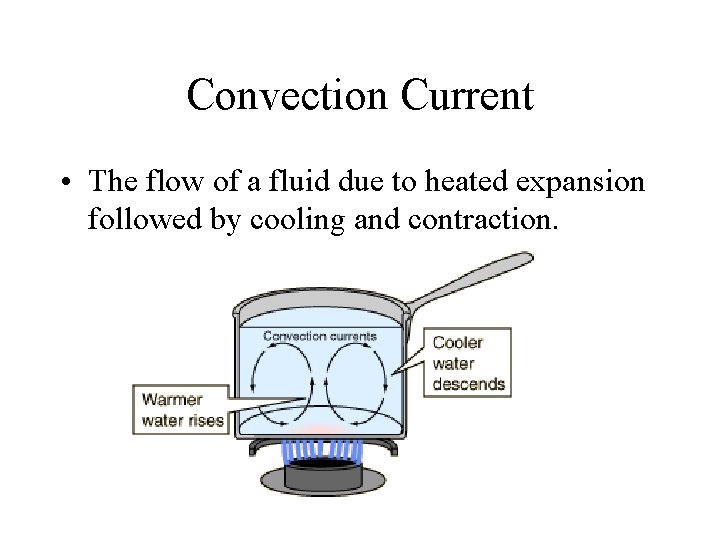
Convection Current • The flow of a fluid due to heated expansion followed by cooling and contraction.

Convection Currents • Vital to the earth – Weather – Ocean currents – Magma currents – Tectonic Plate movement
Radiation • The transfer of energy by electromagnetic waves.
Conductor • A material through which energy can be easily transferred as heat.

Insulator • A material that is a poor energy conductor.
Using Heat • Heating systems • Cooling systems • Refrigerant
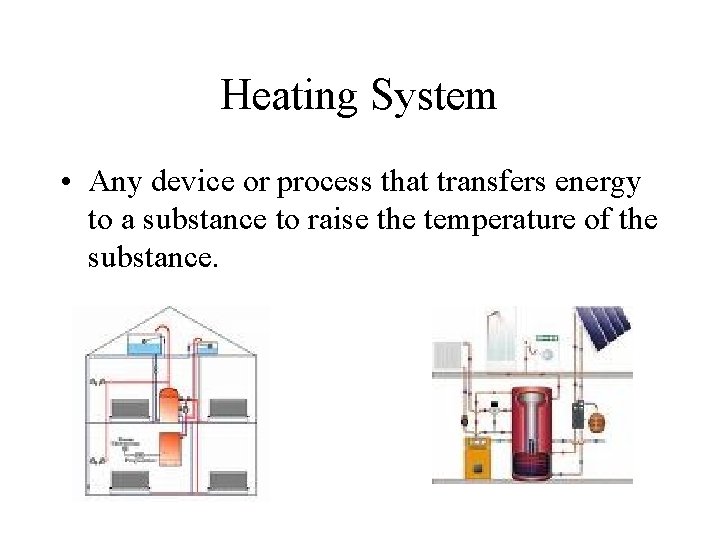
Heating System • Any device or process that transfers energy to a substance to raise the temperature of the substance.
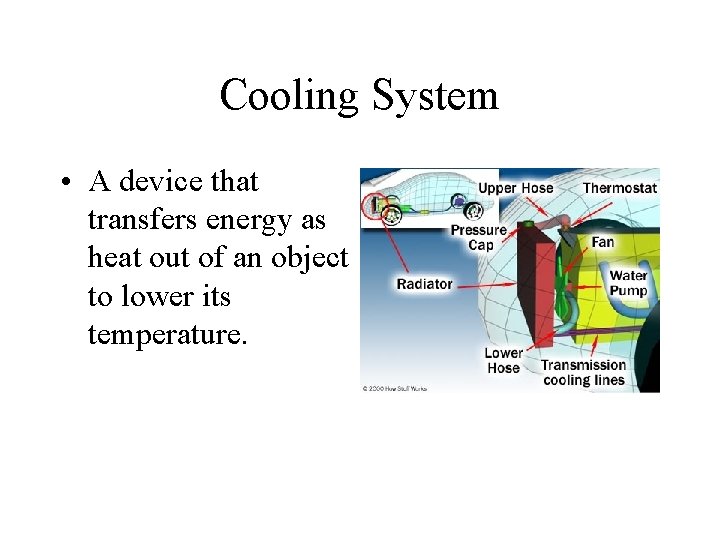
Cooling System • A device that transfers energy as heat out of an object to lower its temperature.
Refrigerant • A substance used in cooling systems that transfers large amounts of energy as it changes state.

Review • • Conduction Convection current Radiation Conductor Insulator Heating System Cooling System
- Slides: 27