HEAT TEMPERATURE AND HEAT Temperature Measure of the











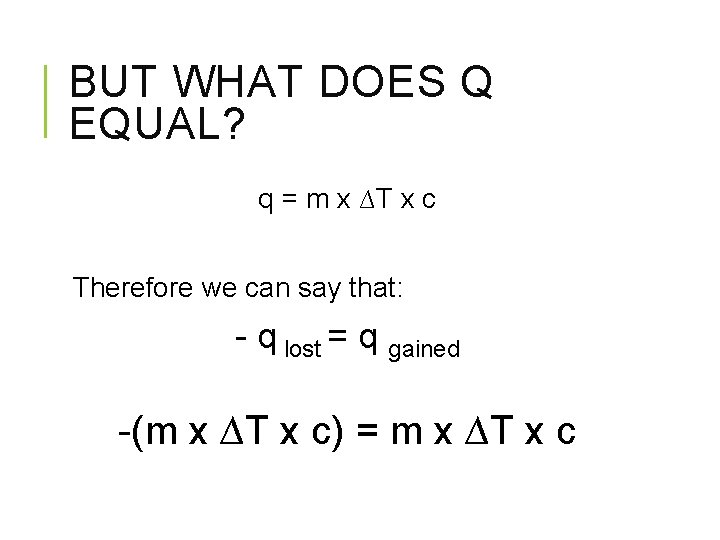



- Slides: 25

HEAT

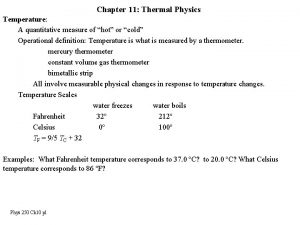
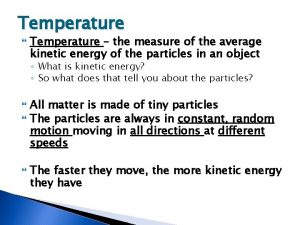
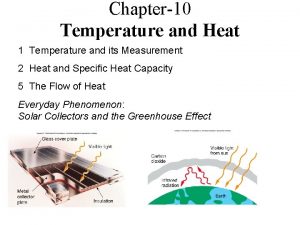
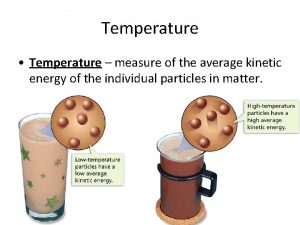
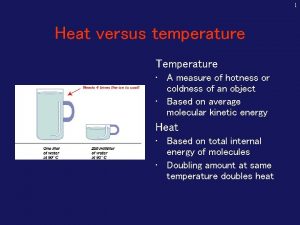
TEMPERATURE AND HEAT Temperature Measure of the average kinetic energy of the particles in a substance Heat The transfer of energy from one substance to another due to temperature differences Always from high E to low E

THE NATURE OF ENERGY Energy The ability to do work or produce heat 2 types: potential and kinetic

POTENTIAL ENERGY Potential energy - is the energy stored in a body or in a system

KINETIC ENERGY Kinetic energy - energy which it possesses due to its motion Depends on the mass of the object and its velocity

MORE KINETIC ENERGY

MORE ABOUT ENERGY Law of Conservation of Energy can be converted from one form to another but cannot be created or destroyed

SO LETS TALK ABOUT HEAT…

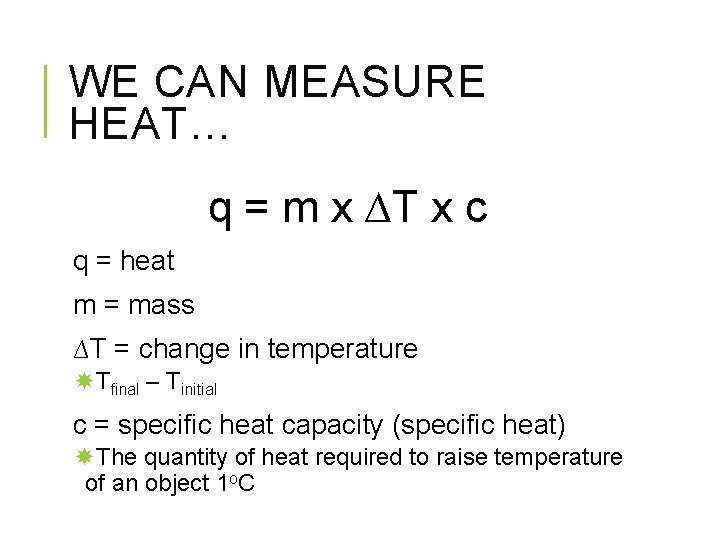
WE CAN MEASURE HEAT… q = m x ∆T x c q = heat m = mass ∆T = change in temperature Tfinal – Tinitial c = specific heat capacity (specific heat) The quantity of heat required to raise temperature of an object 1 o. C

USING THE FORMULA We need 3 out of the 4 so we can solve for the 4 th q = m x ∆T x c If have q, m, and c – what solving for? If have m, c, and ∆T – what solving for?

VALUE OF “Q” What is “q” – HEAT q can be positive or negative If q is positive – energy is added The reaction is endothermic If q is negative – energy is removed/leaving The reaction is exothermic

YOU SAID WHAT? ? Exothermic process: a change (ie. a chemical reaction) that releases heat. Ie: Burning fossil fuels Think “exit” Endothermic process: a change (ie. a chemical reaction) that absorbs heat. Photosynthesis is an endothermic reaction (requires energy input from sun) Think “into”

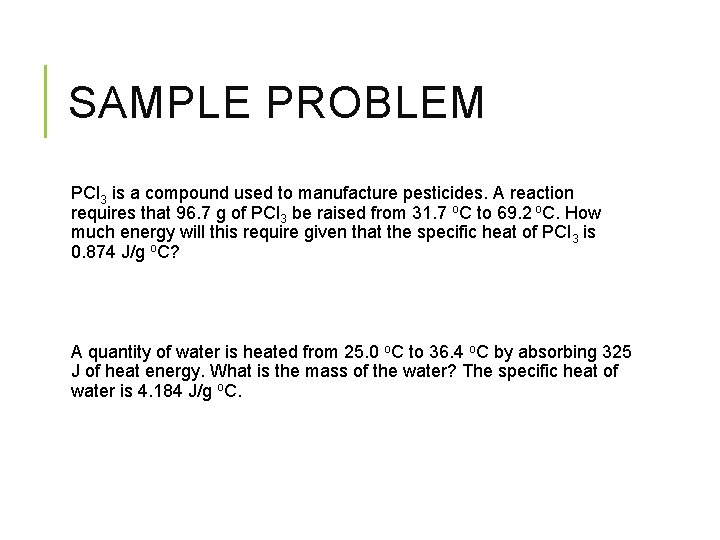
SAMPLE PROBLEM PCl 3 is a compound used to manufacture pesticides. A reaction requires that 96. 7 g of PCl 3 be raised from 31. 7 o. C to 69. 2 o. C. How much energy will this require given that the specific heat of PCl 3 is 0. 874 J/g o. C? A quantity of water is heated from 25. 0 o. C to 36. 4 o. C by absorbing 325 J of heat energy. What is the mass of the water? The specific heat of water is 4. 184 J/g o. C.

HEAT TRANSFER

HEAT TRANSFER Heat will transfer from one object to something else This is a transfer of energy from a place of high E to a place of low E The transfer of E will change the temperatures

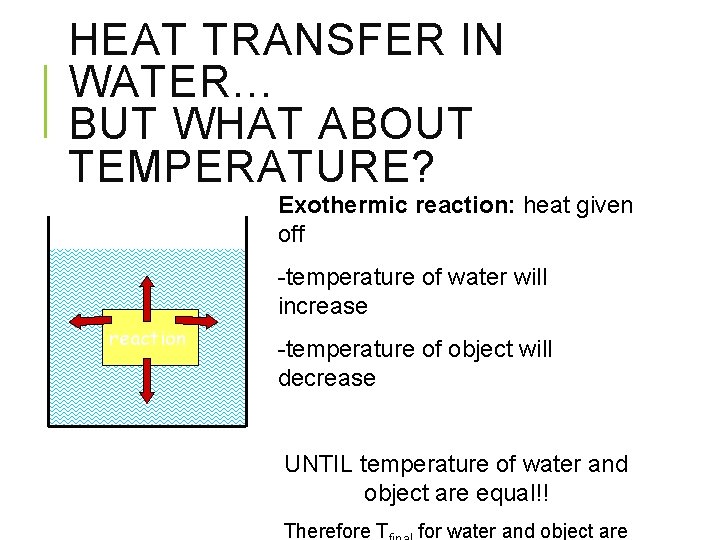
HEAT TRANSFER IN WATER… BUT WHAT ABOUT TEMPERATURE? Exothermic reaction: heat given off -temperature of water will increase reaction -temperature of object will decrease UNTIL temperature of water and object are equal!! Therefore T for water and object are

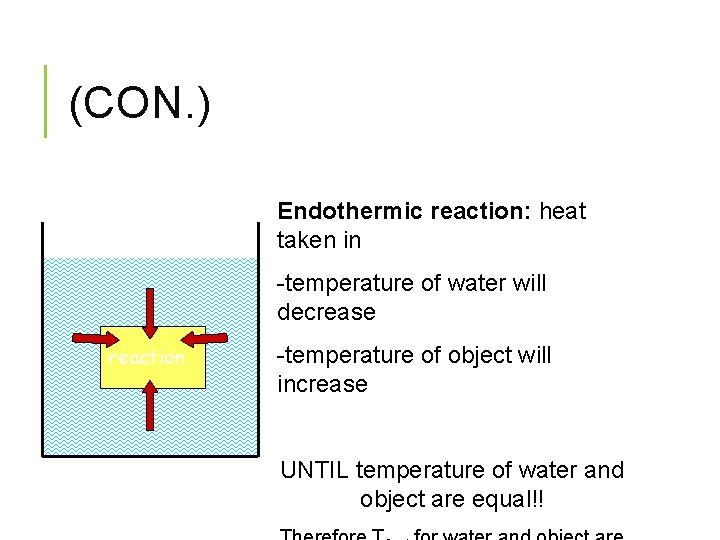
(CON. ) Endothermic reaction: heat taken in -temperature of water will decrease reaction -temperature of object will increase UNTIL temperature of water and object are equal!!

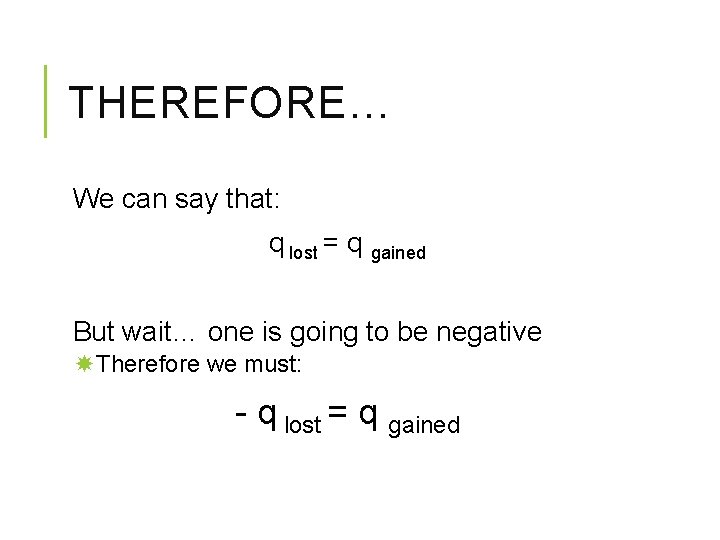
THEREFORE… We can say that: q lost = q gained But wait… one is going to be negative Therefore we must: - q lost = q gained
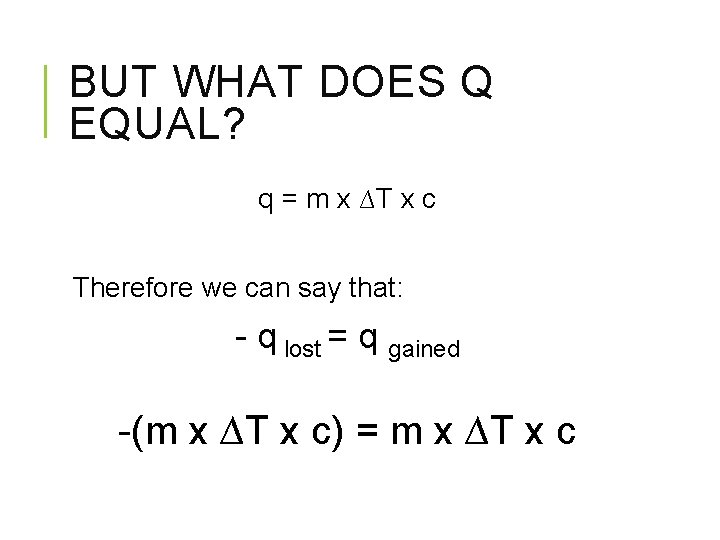
BUT WHAT DOES Q EQUAL? q = m x ∆T x c Therefore we can say that: - q lost = q gained -(m x ∆T x c) = m x ∆T x c

ENTHALPY OF FUSION

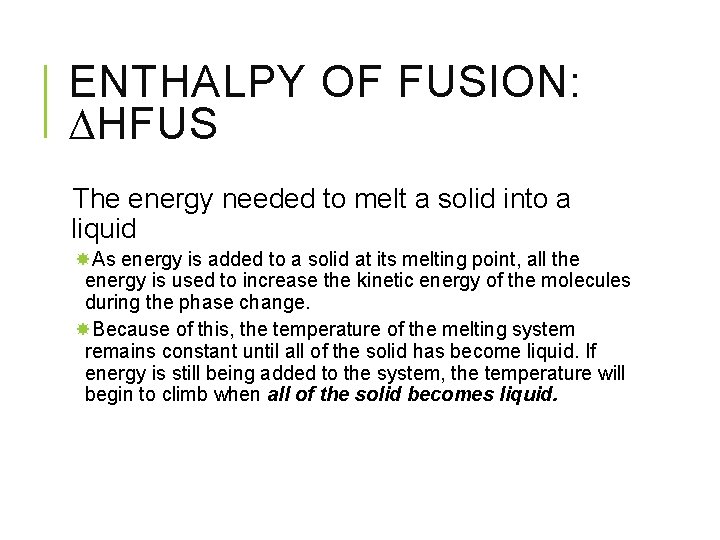
ENTHALPY OF FUSION: HFUS The energy needed to melt a solid into a liquid As energy is added to a solid at its melting point, all the energy is used to increase the kinetic energy of the molecules during the phase change. Because of this, the temperature of the melting system remains constant until all of the solid has become liquid. If energy is still being added to the system, the temperature will begin to climb when all of the solid becomes liquid.

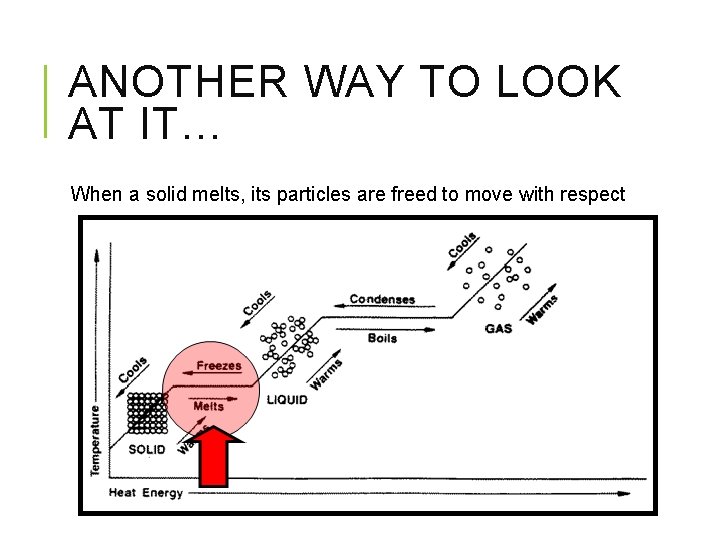
ANOTHER WAY TO LOOK AT IT… When a solid melts, its particles are freed to move with respect to one another.

FORMULA (SOLID LIQUID) Formula: q = m Hfus q = heat gained (+) or lost (-) m = mass Hfus = heat of fusion Formula for Heat Transfer with Enthalpy of Fusion m Hfus = m Tc

ENTHALPY OF SOLUTION

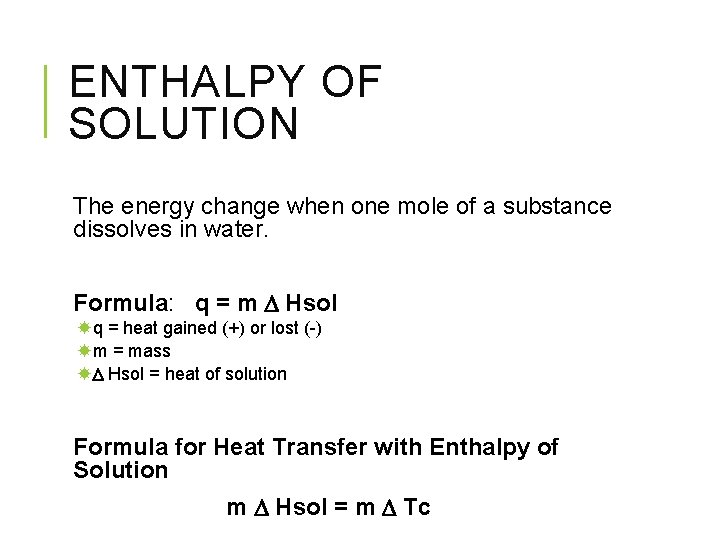
ENTHALPY OF SOLUTION The energy change when one mole of a substance dissolves in water. Formula: q = m Hsol q = heat gained (+) or lost (-) m = mass Hsol = heat of solution Formula for Heat Transfer with Enthalpy of Solution m Hsol = m Tc
 Measures air temperature
Measures air temperature Is temperature a quantitative measure of heat
Is temperature a quantitative measure of heat Gibbons jacobean city comedy download
Gibbons jacobean city comedy download Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Ferrimagnetism
Ferrimagnetism What does temperature measure
What does temperature measure The measure of average kinetic energy
The measure of average kinetic energy Temperature is a measure of average
Temperature is a measure of average Q=mct
Q=mct Temperature is a measure of the average
Temperature is a measure of the average Heat vs thermal energy vs temperature
Heat vs thermal energy vs temperature Thermal vs heat energy
Thermal vs heat energy Specific heat of platinum
Specific heat of platinum Heat thermal energy and temperature
Heat thermal energy and temperature Difference between heat and temperature
Difference between heat and temperature Heat flow
Heat flow How to define heat
How to define heat Difference between heat and temperature
Difference between heat and temperature Chapter 15 temperature heat and expansion
Chapter 15 temperature heat and expansion Si unit of heat capacity
Si unit of heat capacity Chapter 21 temperature, heat and expansion answer key
Chapter 21 temperature, heat and expansion answer key Solid liquid gas particles
Solid liquid gas particles Relation between heat and temperature
Relation between heat and temperature Physics heat and temperature
Physics heat and temperature A double pipe parallel flow heat exchanger
A double pipe parallel flow heat exchanger