TEMPERATURE AND HEAT TEMPERATURE q Temperature is the






- Slides: 10

TEMPERATURE AND HEAT

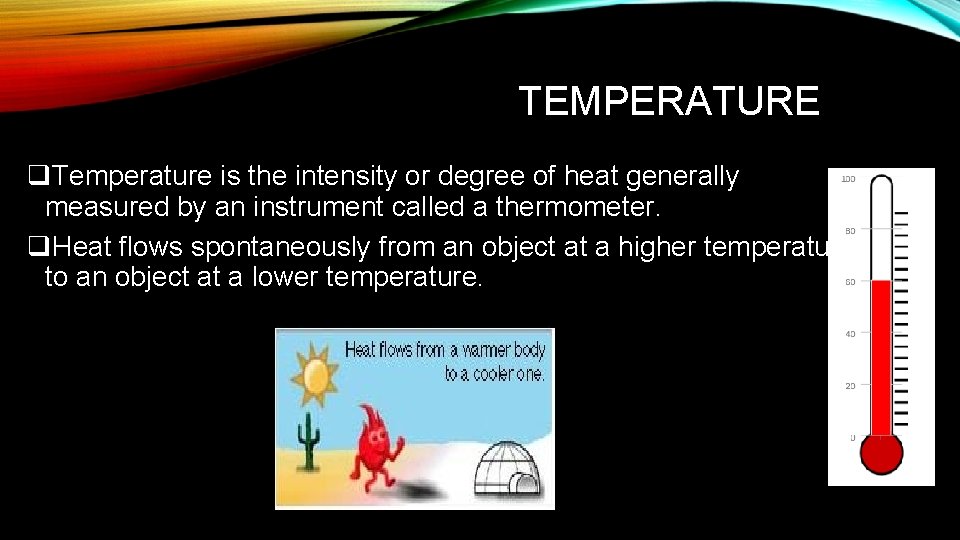
TEMPERATURE q. Temperature is the intensity or degree of heat generally measured by an instrument called a thermometer. q. Heat flows spontaneously from an object at a higher temperature to an object at a lower temperature.

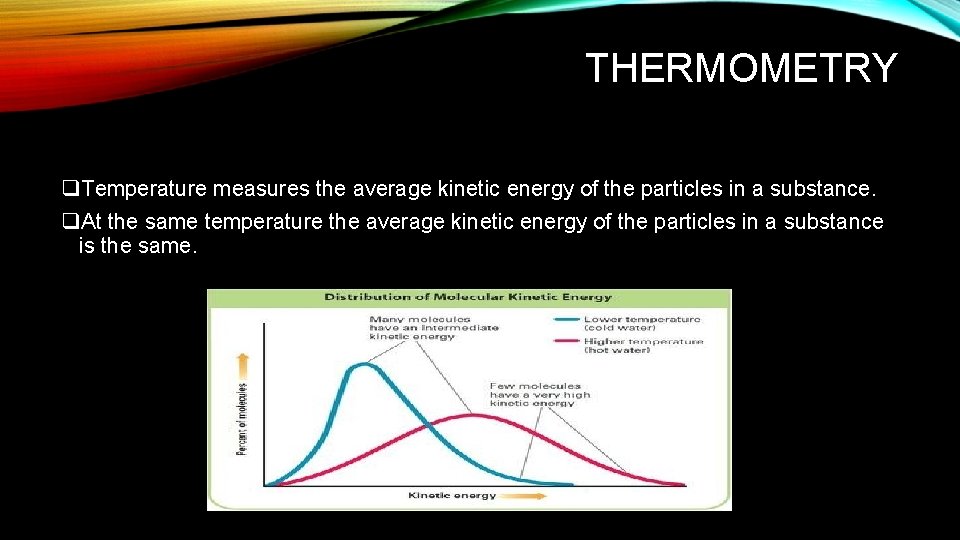
THERMOMETRY q. Temperature measures the average kinetic energy of the particles in a substance. q. At the same temperature the average kinetic energy of the particles in a substance is the same.

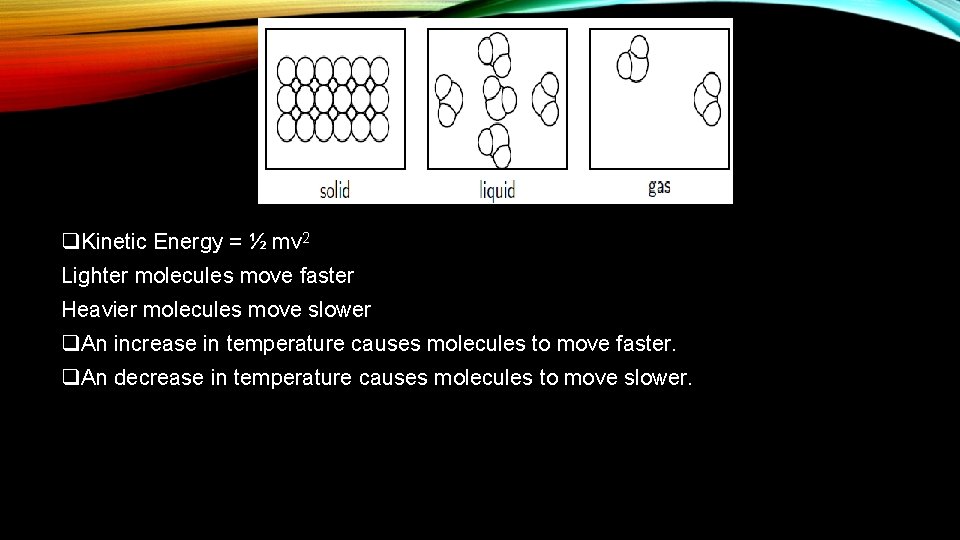
q. Kinetic Energy = ½ mv 2 Lighter molecules move faster Heavier molecules move slower q. An increase in temperature causes molecules to move faster. q. An decrease in temperature causes molecules to move slower.

FIXED POINTS ON A THERMOMETER q. Freezing point of water: temperature at which water changes from a liquid to a solid. (ice-water equilibrium) at 1 atmosphere of pressure (standard pressure) q. Boiling point of water: temperature at which water changes to a gas. (steamwater equilibrium) at 1 atmosphere of pressure. q. The space between these reference points is divided evenly into degrees. q. Absolute zero: temperature at which all molecular motion ceases. Theoretically the lowest possible temperature.

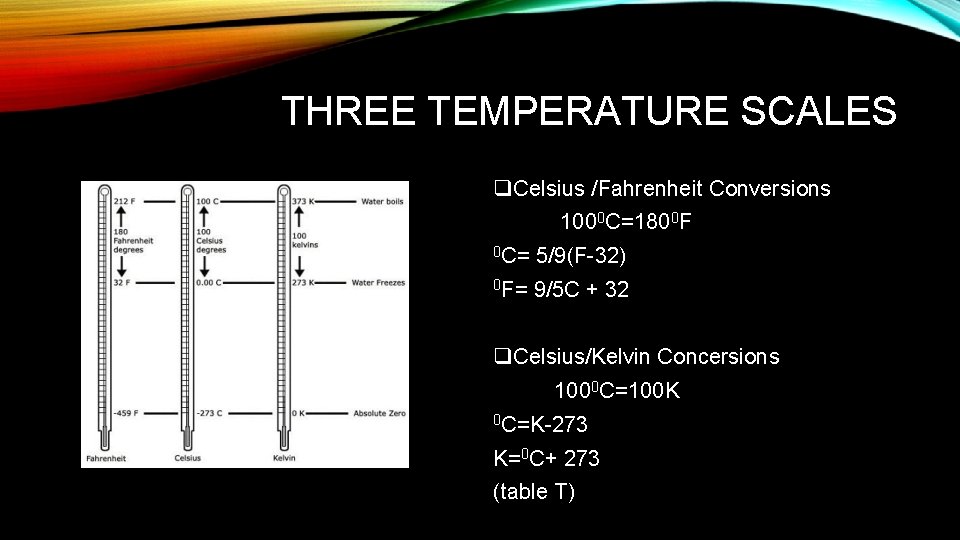
THREE TEMPERATURE SCALES q. Celsius /Fahrenheit Conversions 1000 C=1800 F 0 C= 5/9(F-32) 0 F= 9/5 C + 32 q. Celsius/Kelvin Concersions 1000 C=100 K 0 C=K-273 K=0 C+ 273 (table T)

PRACTICE TIME Try These Answers: 1. 59 0 F = ? 0 C 1. 2. 60 0 C= ? 0 F 2. 140 3. 10 0 C = ? K 3. 283 4. -23 0 C = ? K 4. 250 5. 300 K= ? 0 C 5. 27 15

HEAT q. Heat is measured quantitatively by the change in temperature it can produce in a given mass of water. q. Non-SI unit for heat is the calorie. q. Calorie is defined as the amount of heat needed to raise the temperature of 1. 0 gram of water by 1. 0 degree Celsius, 1 kcal =1000 calories (food calorie) q. SI unit for heat is the joule. 1 calorie = 4. 18 joules q Calorimeter is used to determine the quantity of heat given off or absorbed in a chemical reaction.

SPECIFIC HEAT q. Specific heat of a substance is the number of calories/joules required to raise the temperature of one gram of a substance by one degree Celsius. q. Specific heat of water is 1. 0 calorie/gram 0 C = 4. 18 Joules/gram 0 C (table B) q. Specific heat of aluminum is. 214 calories/gram 0 C =. 895 Joules/gram 0 C q. Heat gained or lost by a substance= (mass of substance)(specific heat for substance)(change in temperature) Table T

HEAT EQUATION: Q= M C ▲T q Try this: How many calories are released when 50. 0 grams of water are cooled from 75. 0 to 35. 0 degrees Celcius? q. Solution: Q= m c ▲T Q= (50. 0 grams) (4. 18 Joules/gram 0 C)(40. 0 0 C) Q= 8360 Joules