HEAT TEMPERATURE AND HEAT Temperature Measure of the



- Slides: 6

HEAT

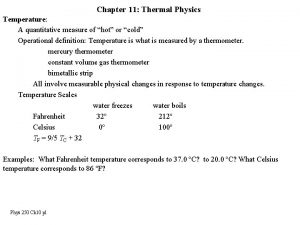
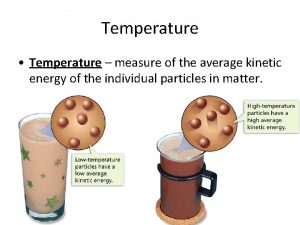
TEMPERATURE AND HEAT Temperature Measure of the average kinetic energy of the particles in a substance Heat The transfer of energy from one substance to another due to temperature differences Always from high E to low E

THE NATURE OF ENERGY Energy The ability to do work or produce heat 2 types: Potential energy Stored energy Deals with phase changes- a plateau Kinetic energy Energy of motion of an object Depends on the mass of the object and its velocity

M ORE ABOUT E NERGY Law of conservation of energy Energy can be converted from one form to another but cannot be created or destroyed

C ONCLUSIONFOR H EATING/ C OOLING L AB We looked at the melting and freezing of lauric acid. Lauric acid melts at ______ degrees Celsius. Lauric acid freezes at _____ degrees Celsius.

HEATING/COOLING CURVE Purpose: To heat and cool a substance and graph the temperature change as a function of time. Procedure: Obtain a beaker, fill it ½ full with water and place on a hot plate Place a test tube containing the substance into the beaker and melt the substance Place a thermometer into the melted substance and remove it from the beaker Record the temperature every 15 seconds until the temperature is 40 OC (gently stir the substance until the liquid freezes) Place the test tube back into the beaker of water and record the temperature again every 15 seconds until the temperature is 70 OC Remove thermometer from the melted substance and clean it off Put the test tube, with substance still in it, back in the test tube rack Make a graph of temperature vs time
 An instrument used to measure temperature
An instrument used to measure temperature Is temperature a quantitative measure of heat
Is temperature a quantitative measure of heat Gibbons jacobean city comedy download
Gibbons jacobean city comedy download Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature What does temperature measure
What does temperature measure