Ferromagneti sm Ferromagnetism The atomic moments in these
![Origin of Ferromagnetism Hund’s rule Fe: [Ar]3 d 64 s 2 Origin of Ferromagnetism Hund’s rule Fe: [Ar]3 d 64 s 2](https://slidetodoc.com/presentation_image_h/cb6604365f96150ccd420f1863113d89/image-5.jpg)

- Slides: 48

Ferromagneti sm

Ferromagnetism The atomic moments in these materials exhibit very strong interactions, resulting in a parallel or antiparallel alignment of atomic moments. Exchange forces are very large, equivalent to a field on the order of 1000 Tesla, or approximately a 100 million times the strength of the earth's field. The exchange force is a quantum mechanical phenomenon due to the relative orientation of the spins of two electron.

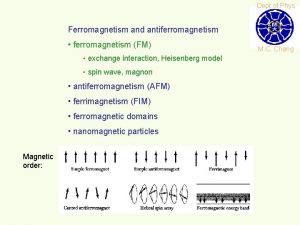
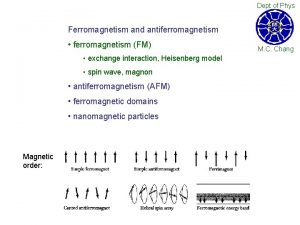
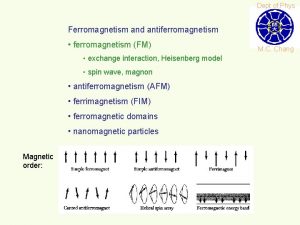
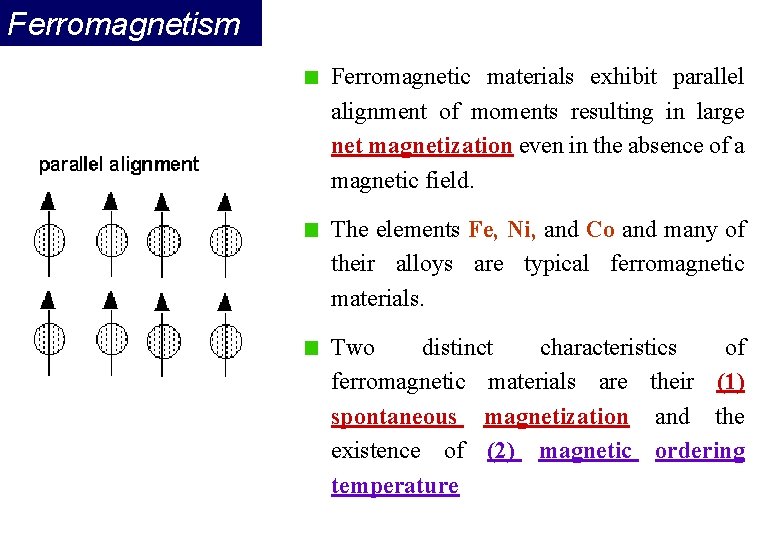
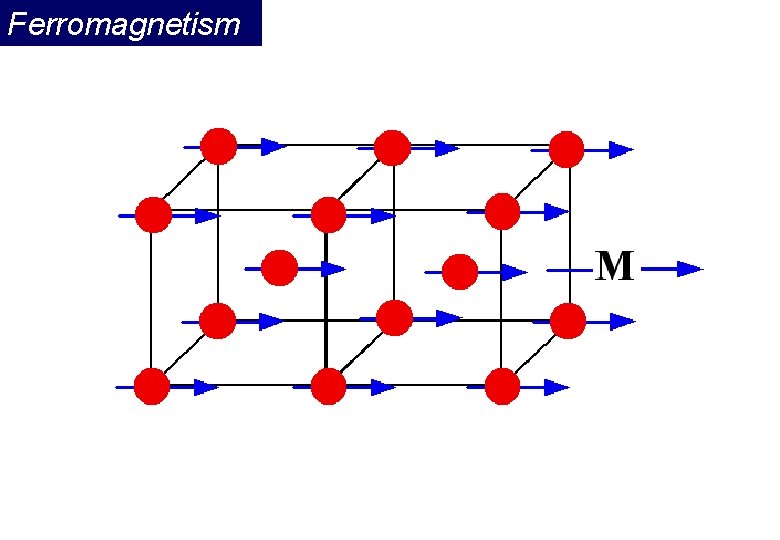
Ferromagnetism Ferromagnetic materials exhibit parallel alignment of moments resulting in large net magnetization even in the absence of a magnetic field. The elements Fe, Ni, and Co and many of their alloys are typical ferromagnetic materials. Two distinct characteristics of ferromagnetic materials are their (1) spontaneous magnetization and the existence of (2) magnetic ordering temperature

Ferromagnetism
![Origin of Ferromagnetism Hunds rule Fe Ar3 d 64 s 2 Origin of Ferromagnetism Hund’s rule Fe: [Ar]3 d 64 s 2](https://slidetodoc.com/presentation_image_h/cb6604365f96150ccd420f1863113d89/image-5.jpg)
Origin of Ferromagnetism Hund’s rule Fe: [Ar]3 d 64 s 2

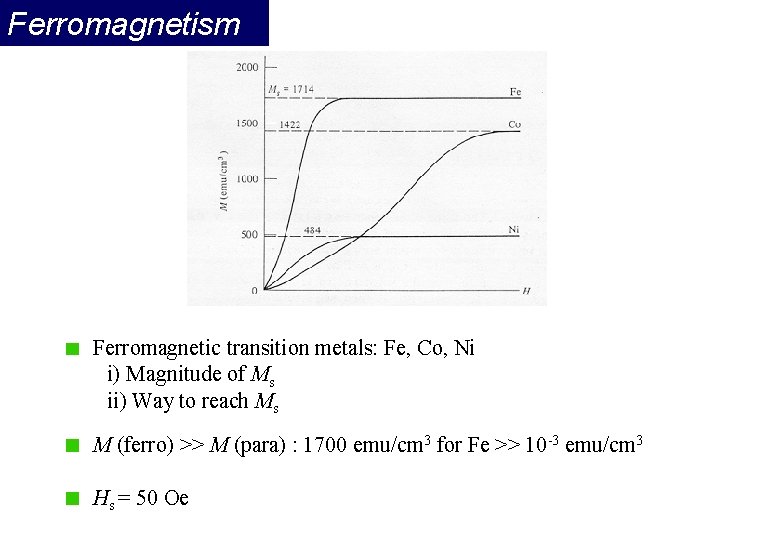
Ferromagnetism Ferromagnetic transition metals: Fe, Co, Ni i) Magnitude of Ms ii) Way to reach Ms M (ferro) >> M (para) : 1700 emu/cm 3 for Fe >> 10 -3 emu/cm 3 Hs = 50 Oe

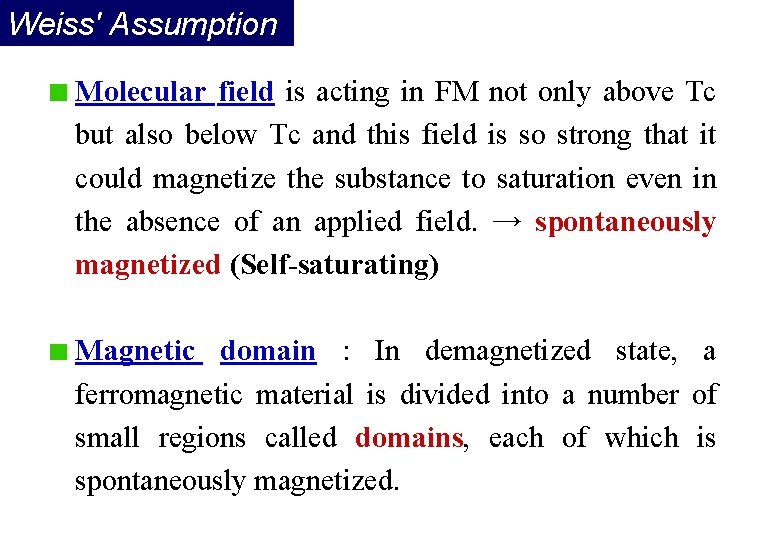
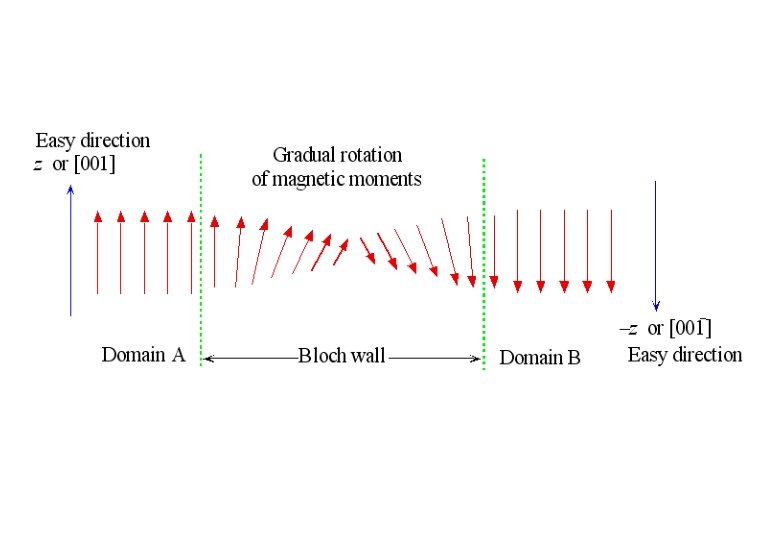
Weiss' Assumption Molecular field is acting in FM not only above Tc but also below Tc and this field is so strong that it could magnetize the substance to saturation even in the absence of an applied field. → spontaneously magnetized (Self-saturating) Magnetic domain : In demagnetized state, a ferromagnetic material is divided into a number of small regions called domains, each of which is spontaneously magnetized.

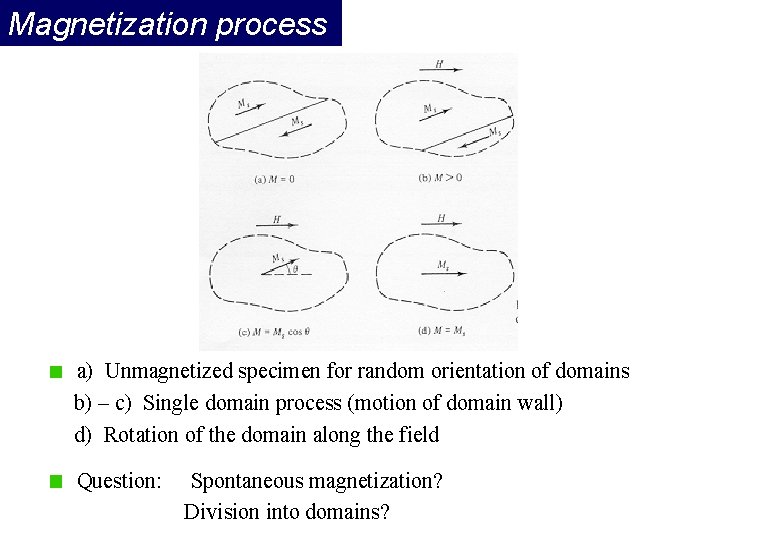
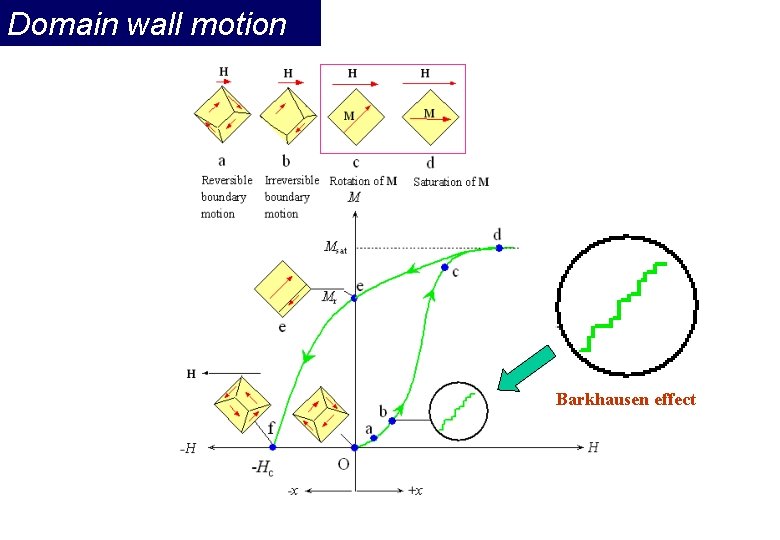
Magnetization process a) Unmagnetized specimen for random orientation of domains b) – c) Single domain process (motion of domain wall) d) Rotation of the domain along the field Question: Spontaneous magnetization? Division into domains?

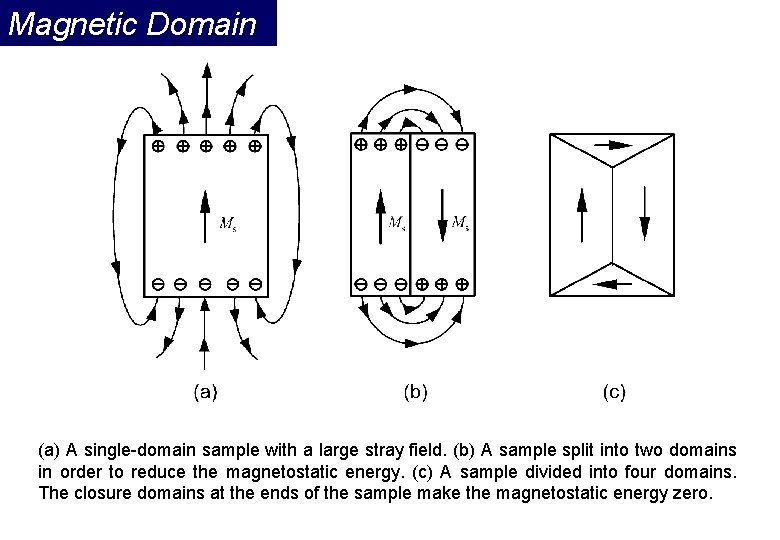
Magnetic Domain (a) A single-domain sample with a large stray field. (b) A sample split into two domains in order to reduce the magnetostatic energy. (c) A sample divided into four domains. The closure domains at the ends of the sample make the magnetostatic energy zero.

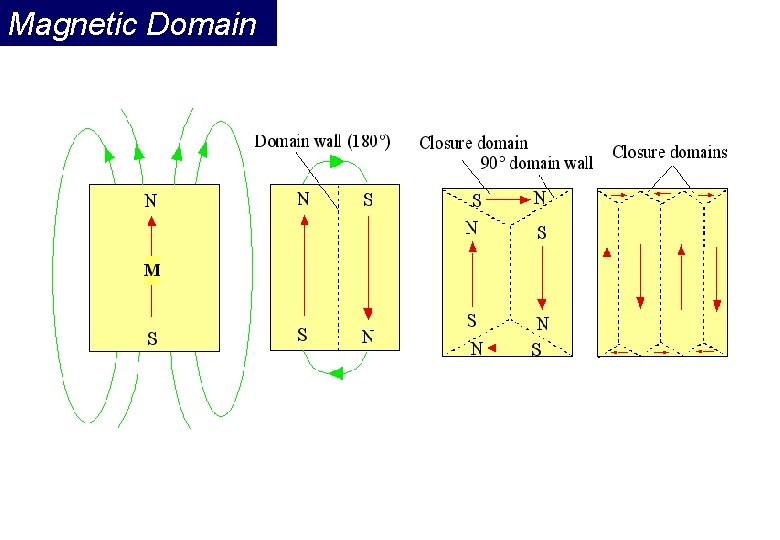
Magnetic Domain


Domain wall motion Barkhausen effect

Magnetic Order Are ferromagnets already in an ordered state before a magnetic field is applied or is the order by the field?

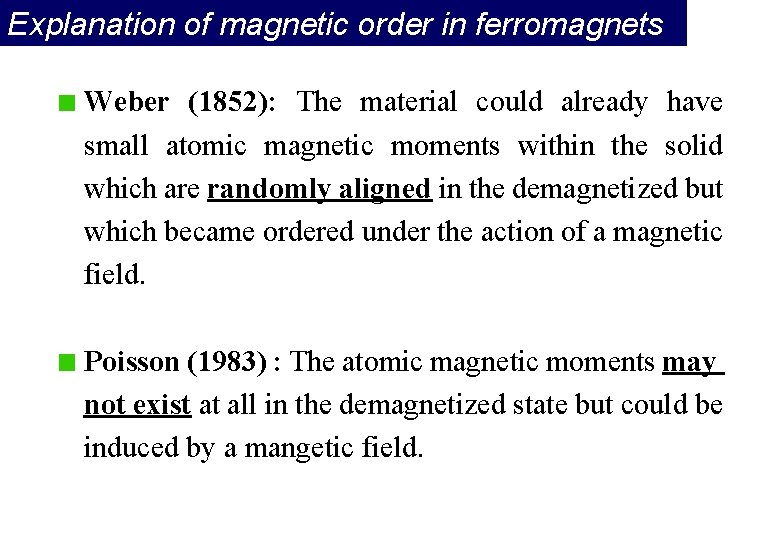
Explanation of magnetic order in ferromagnets Weber (1852): The material could already have small atomic magnetic moments within the solid which are randomly aligned in the demagnetized but which became ordered under the action of a magnetic field. Poisson (1983) : The atomic magnetic moments may not exist at all in the demagnetized state but could be induced by a mangetic field.

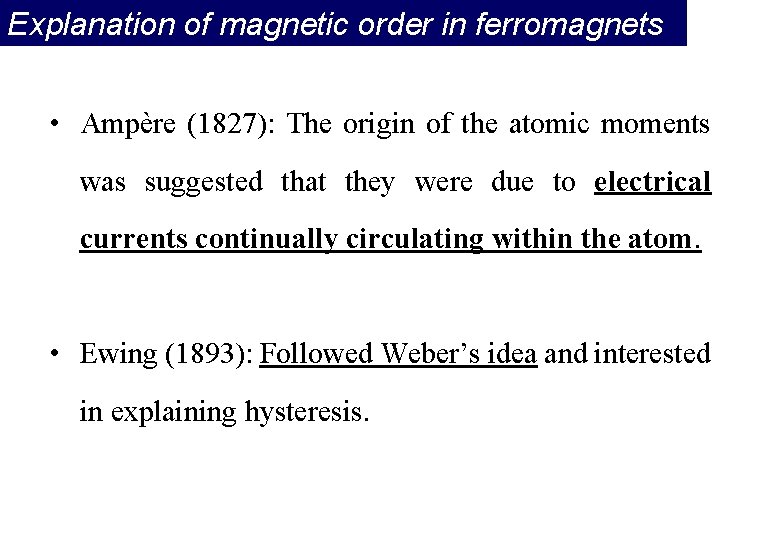
Explanation of magnetic order in ferromagnets • Ampère (1827): The origin of the atomic moments was suggested that they were due to electrical currents continually circulating within the atom. • Ewing (1893): Followed Weber’s idea and interested in explaining hysteresis.

Weiss domain theory Atomic magnetic moments were in permanent existence (Weber’s hypothesis) Atomic magnetic moments were ordered even in the demagnetized state. It was the domains only which were randomly aligned in the demagnetized state. The magnetization process consisting of reorienting the domains so that more domains were aligned with field.

Magnetic Domain In order to minimize its magnetostatic energy, the magnetic material divides up into magnetic domains. Weiss (1907): concept of magnetic domains. A magnetic material consisted of a number of distinct regions termed ‘domains’ each of which was saturated in a different direction. The concept of domains is able to explain why ferromagnetic materials can be demagnetized even below their Curie temperature.

Weiss Mean Field Theory What is the origin of the alignment of the atomic magnetic moments? It is the Weiss mean field (later the “molecular field”, further later exchange coupling from quantum mechanics)

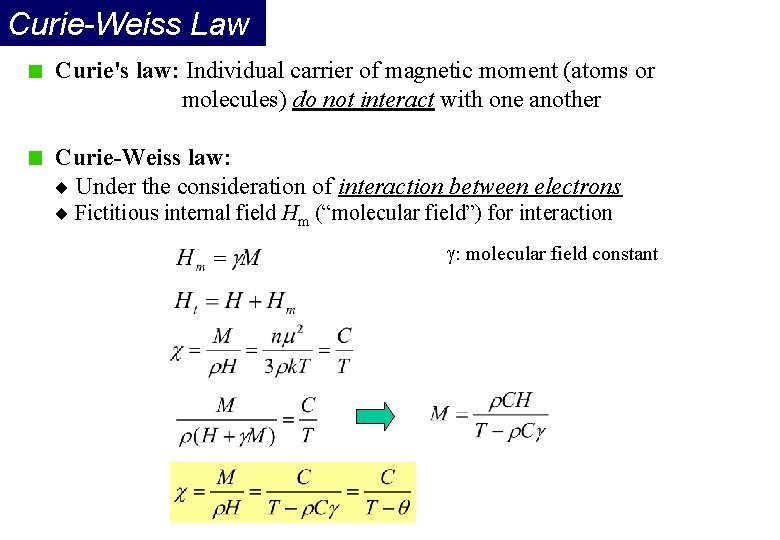
Curie-Weiss Law Curie's law: Individual carrier of magnetic moment (atoms or molecules) do not interact with one another Curie-Weiss law: Under the consideration of interaction between electrons Fictitious internal field Hm (“molecular field”) for interaction : molecular field constant

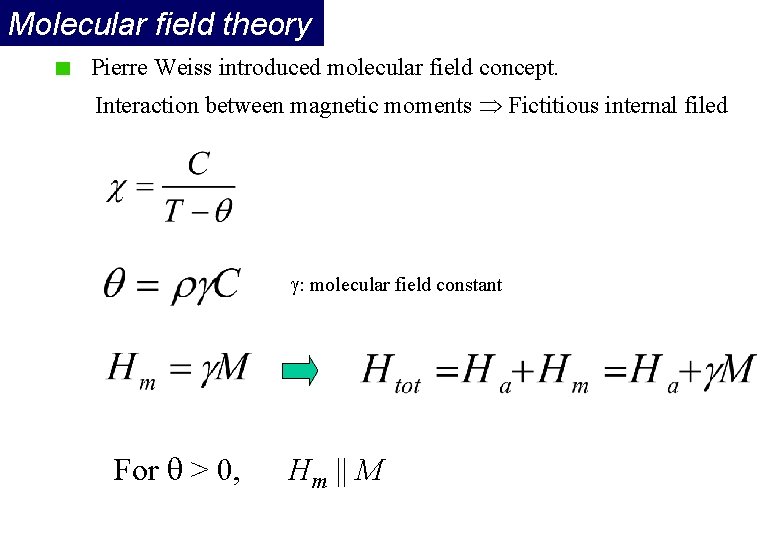
Molecular field theory Pierre Weiss introduced molecular field concept. Interaction between magnetic moments Fictitious internal filed : molecular field constant For > 0, Hm || M

Curie Temperature

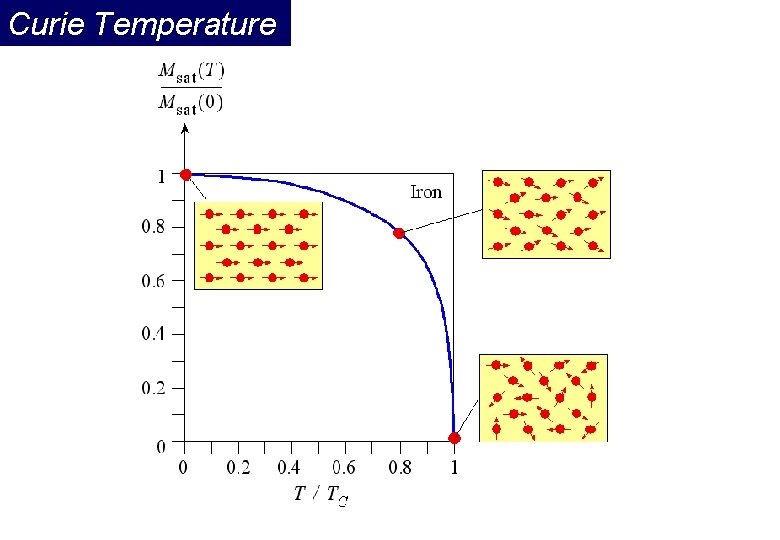
Curie Temperature Even though electronic exchange forces in ferromagnets are very large, thermal energy eventually overcomes the exchange and produces a randomizing effect. This occurs at a particular temperature called the Curie temperature (TC). Below the Curie temperature, the ferromagnet is ordered and above it, disordered. The saturation magnetization goes to zero at the Curie temperature.

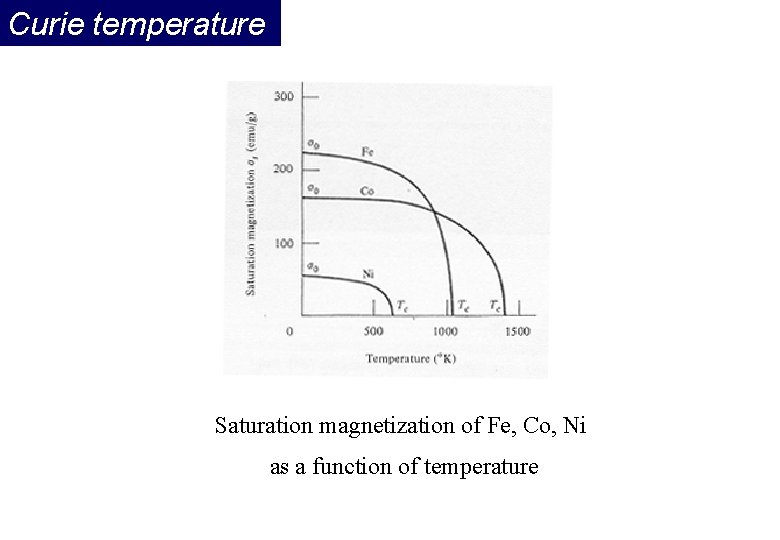
Curie temperature Saturation magnetization of Fe, Co, Ni as a function of temperature

Exchange Energy Exchange force depends on relative orientation of spins of two electrons due to Pauli's exclusion principle When two atoms, such as hydrogen atoms, are coming together, there are electrostatic attractive (e-↔p+) and repulsive (e-↔e-, p+↔p+) forces and exchange force. The internal field is produced by interactions between nearestneighbor dipole moments. The interaction arises from the electrostatic electron-electron interaction, and is called the ”exchange interaction” or exchange force.

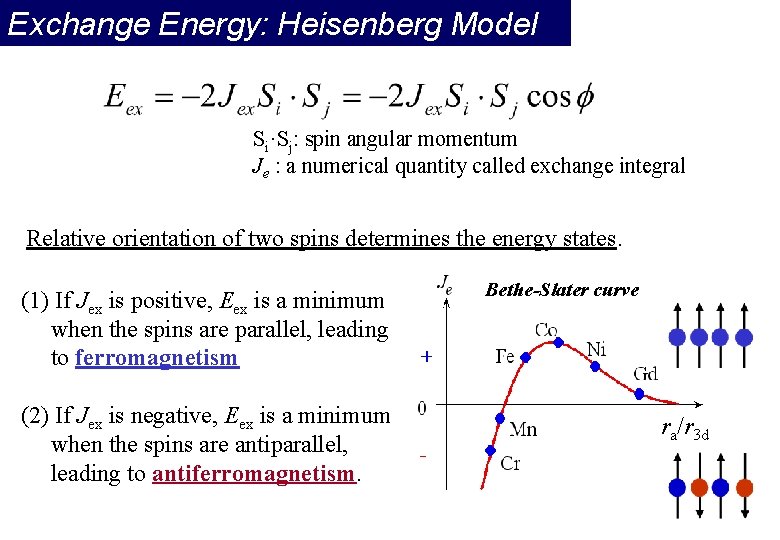
Exchange Energy: Heisenberg Model Si·Sj: spin angular momentum Je : a numerical quantity called exchange integral Relative orientation of two spins determines the energy states. (1) If Jex is positive, Eex is a minimum when the spins are parallel, leading to ferromagnetism (2) If Jex is negative, Eex is a minimum when the spins are antiparallel, leading to antiferromagnetism. Bethe-Slater curve ra/r 3 d

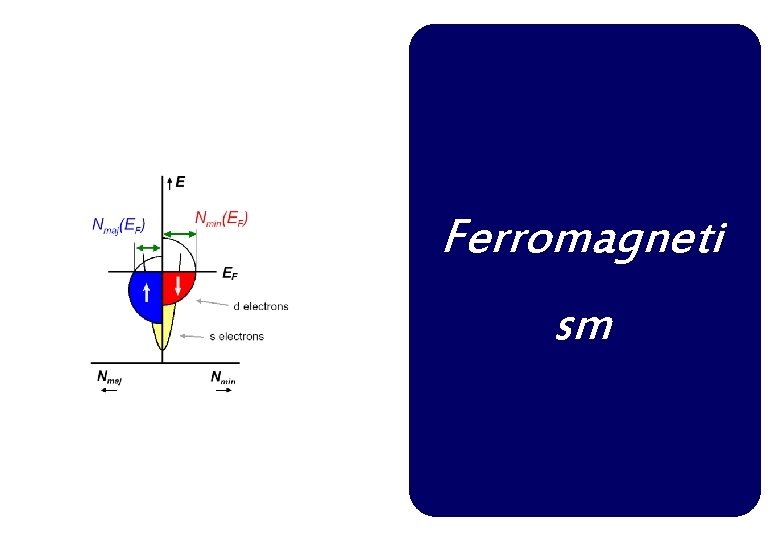
Band Theory of Ferromagnetism A simple extension of the band theory of paramagnetism by the introduction of an exchange coupling between the electrons. Source of magnetic moments: unpaired electrons In partially filled energy band, an imbalance of spins leads to a net magnetic moment per atom.

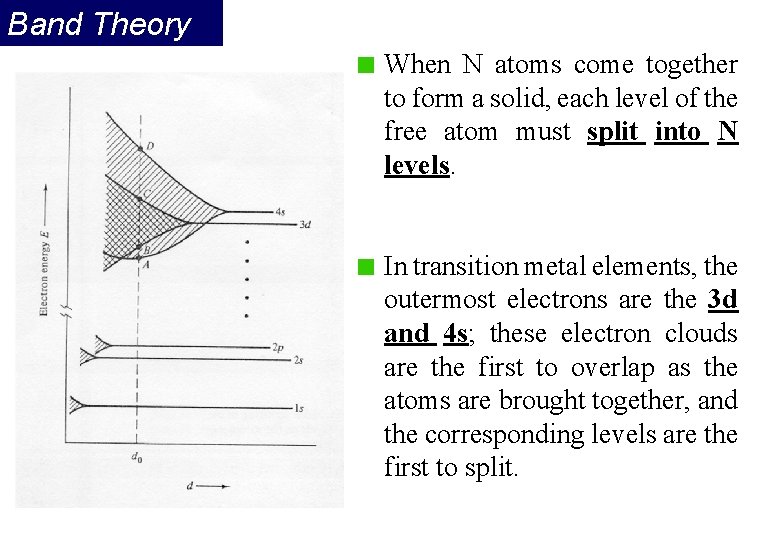
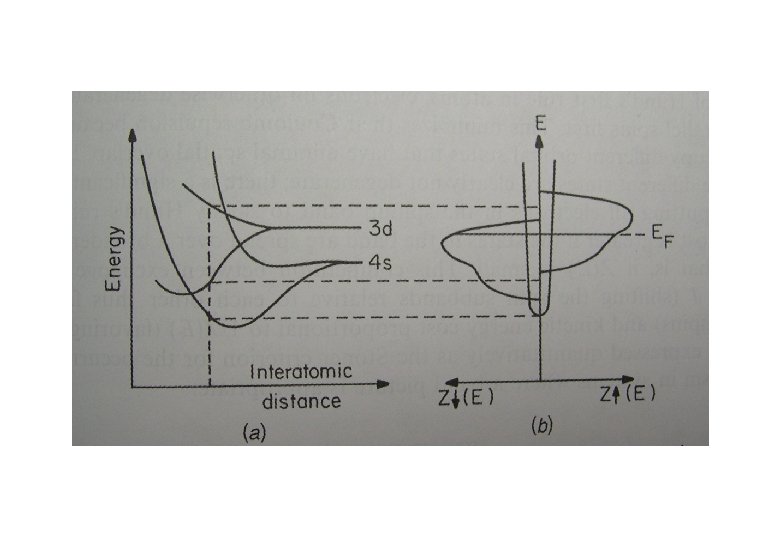
Band Theory When N atoms come together to form a solid, each level of the free atom must split into N levels. In transition metal elements, the outermost electrons are the 3 d and 4 s; these electron clouds are the first to overlap as the atoms are brought together, and the corresponding levels are the first to split.

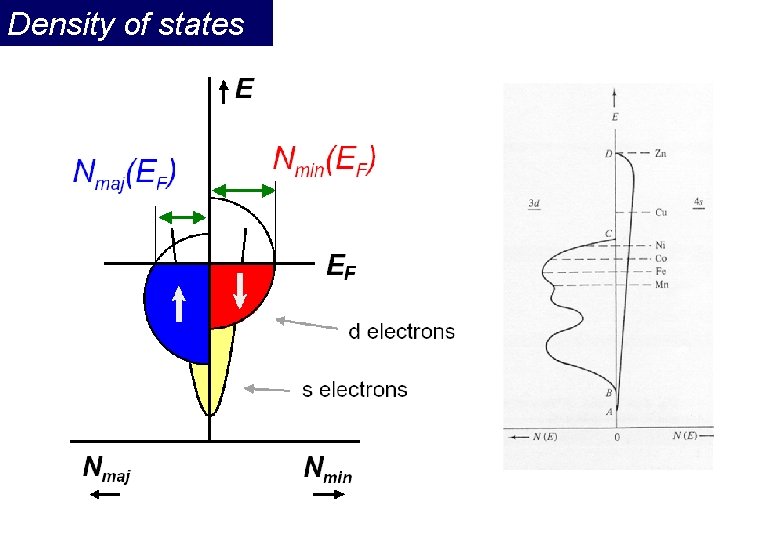
Density of states


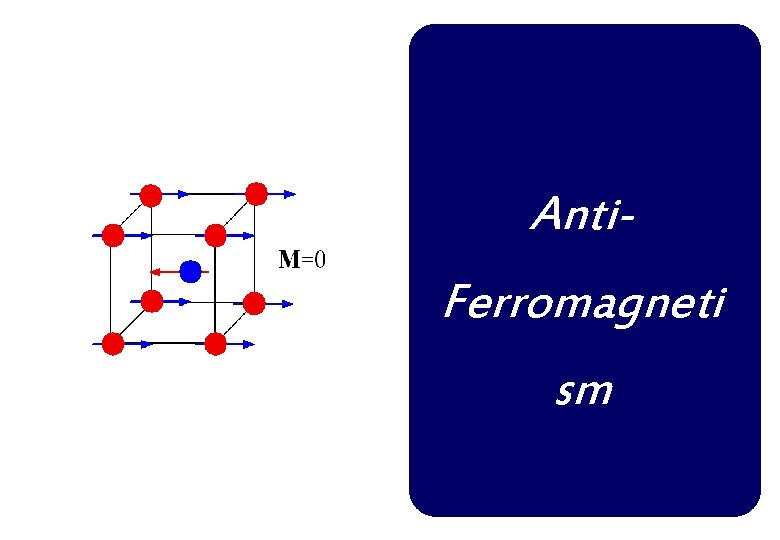
Anti. Ferromagneti sm

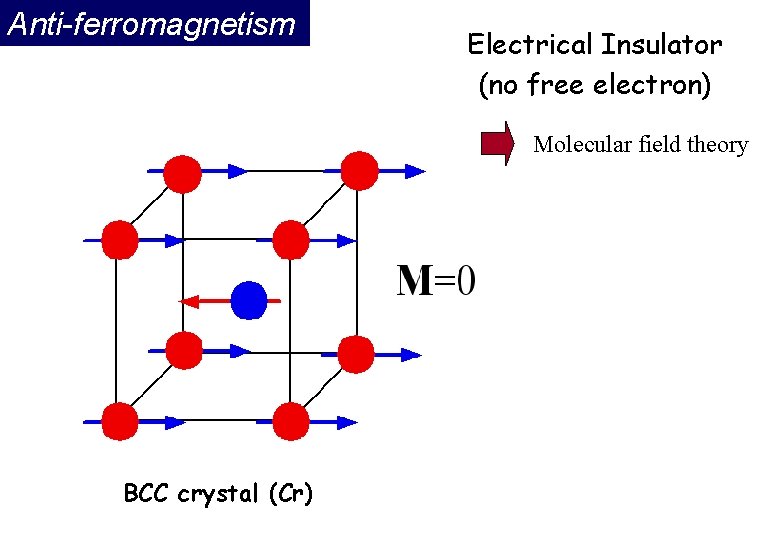
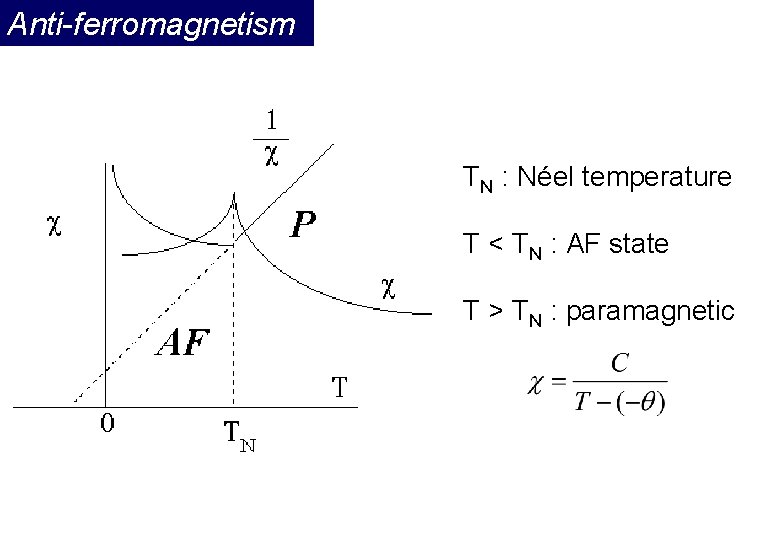
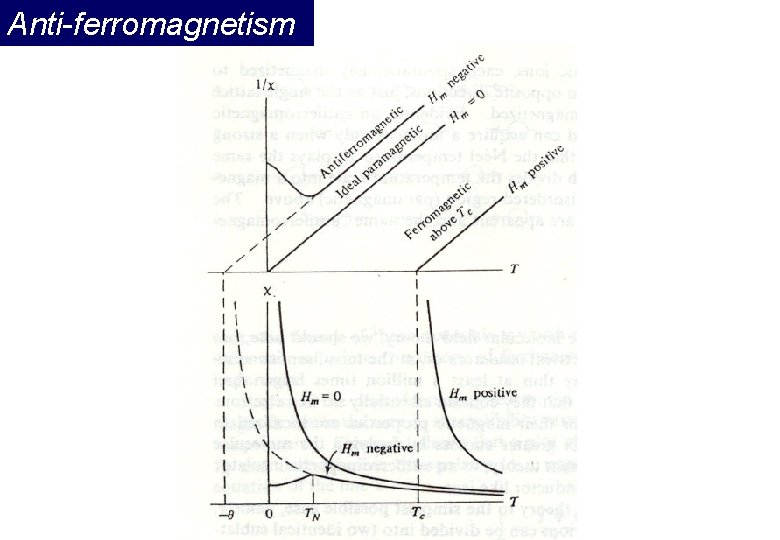
Anti-ferromagnetism If the A and B sublattice moments are exactly equal but opposite, the net moment is zero. This type of magnetic ordering is called antiferromagnetism. The clue to antiferromagnetism is the behavior of susceptibility above a critical temperature, called the Néel temperature (TN). Above TN, the susceptibility obeys the Curie-Weiss law for paramagnets but with a negative intercept indicating negative exchange interactions.

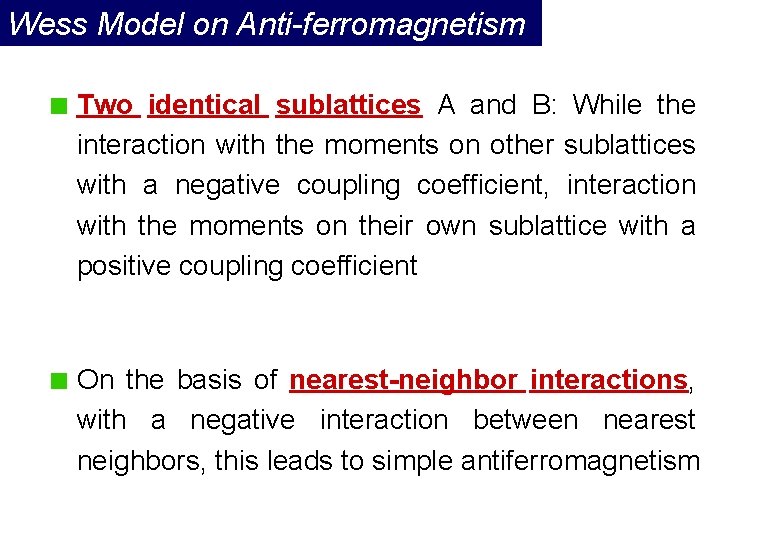
Wess Model on Anti-ferromagnetism Two identical sublattices A and B: While the interaction with the moments on other sublattices with a negative coupling coefficient, interaction with the moments on their own sublattice with a positive coupling coefficient On the basis of nearest-neighbor interactions, with a negative interaction between nearest neighbors, this leads to simple antiferromagnetism

Anti-ferromagnetism Electrical Insulator (no free electron) Molecular field theory BCC crystal (Cr)

Anti-ferromagnetism TN : Néel temperature T < TN : AF state T > TN : paramagnetic

Anti-ferromagnetism

Ferrimagnetis m

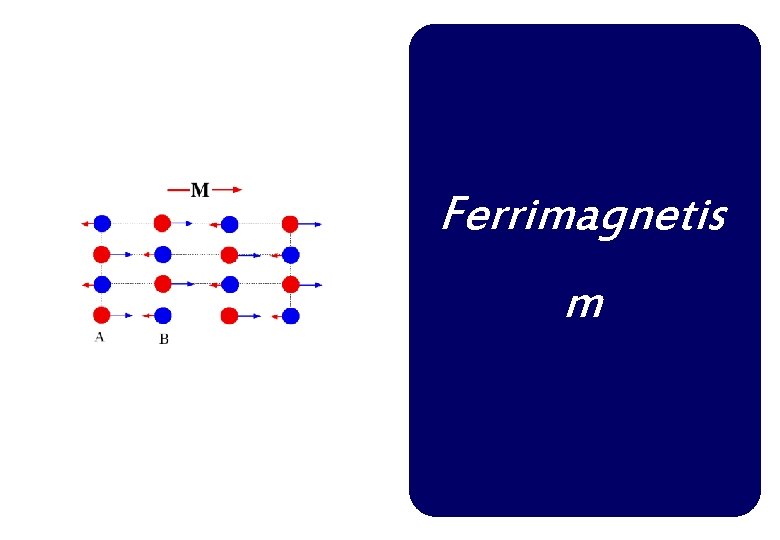
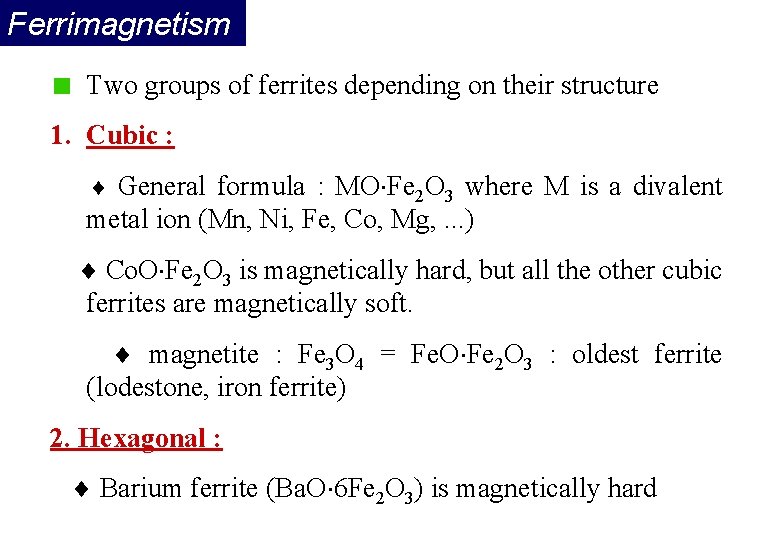
Ferrimagnetism In ferrimagnets, the magnetic moments of the A and B sublattices are not equal and result in a net magnetic moment. Ferrimagnetism is therefore similar to ferromagnetism. It exhibits all the hallmarks of ferromagnetic behavior- spontaneous magnetization, Curie temperatures, hysteresis, and remanence. However, ferro- and ferrimagnets have very different magnetic ordering.

Ferrimagnetism Two groups of ferrites depending on their structure 1. Cubic : General formula : MO Fe 2 O 3 where M is a divalent metal ion (Mn, Ni, Fe, Co, Mg, . . . ) Co. O Fe 2 O 3 is magnetically hard, but all the other cubic ferrites are magnetically soft. magnetite : Fe 3 O 4 = Fe. O Fe 2 O 3 : oldest ferrite (lodestone, iron ferrite) 2. Hexagonal : Barium ferrite (Ba. O 6 Fe 2 O 3) is magnetically hard

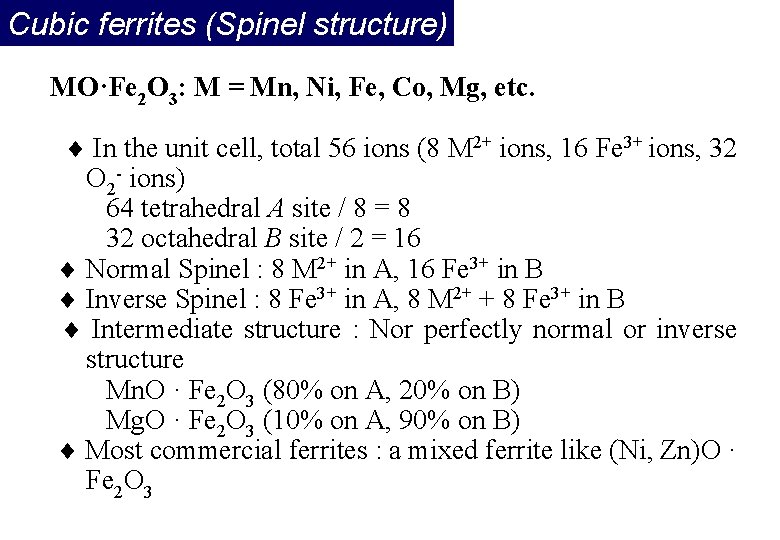
Cubic ferrites (Spinel structure) MO·Fe 2 O 3: M = Mn, Ni, Fe, Co, Mg, etc. In the unit cell, total 56 ions (8 M 2+ ions, 16 Fe 3+ ions, 32 O 2 - ions) 64 tetrahedral A site / 8 = 8 32 octahedral B site / 2 = 16 Normal Spinel : 8 M 2+ in A, 16 Fe 3+ in B Inverse Spinel : 8 Fe 3+ in A, 8 M 2+ + 8 Fe 3+ in B Intermediate structure : Nor perfectly normal or inverse structure Mn. O · Fe 2 O 3 (80% on A, 20% on B) Mg. O · Fe 2 O 3 (10% on A, 90% on B) Most commercial ferrites : a mixed ferrite like (Ni, Zn)O · Fe 2 O 3

Hexagonal Ferrites MO· 6 Fe 2 O 3(= Ba. Fe 12 O 19) where M = Ba, Sr Calculated saturation magnetization = 20μB/molecule (experimental) Other oxides Ba. O· 2 MO· 8 Fe 2 O 3 W 2(Ba. O· 2 MO· 3 Fe 2 O 3) Y 3 Ba. O· 2 MO· 12 Fe 2 O 3) Z where, M is a divalent ion

Other Ferrites γ-Fe 2 O 3 : tetragonal (calculated net moment/molecule = 2. 5μB ↔ 2. 39μB experimental) Garnets : 3 M 2 O 3・ 5 Fe 2 O 3 (M = Y or RE) Alloys : Mn 2 Sb, Mn 3 Ga, Mn 3 Ge 2, Mn 3 In, Fe. Ge 2, Fe. Se, Cr 3 As 2, Cr. Pt 3, RECo 5 (RE: Gd, Tb, Dy, Ho, Eu, or Tm)

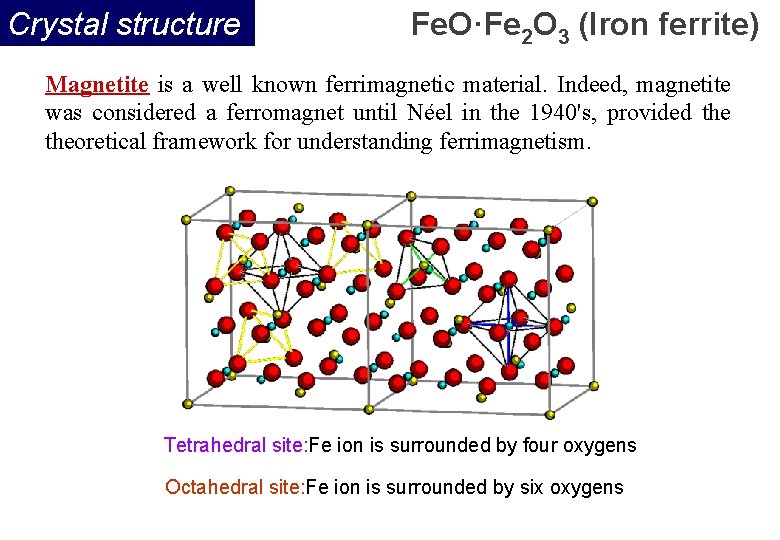
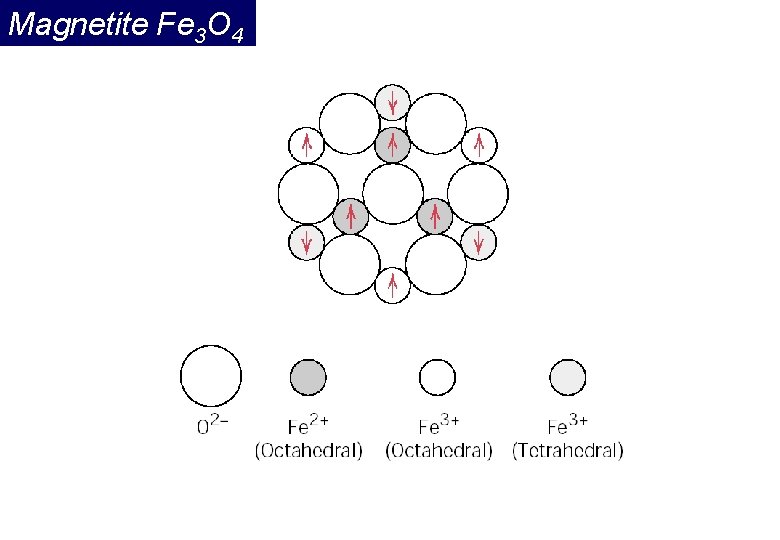
Crystal structure Fe. O·Fe 2 O 3 (Iron ferrite) Magnetite is a well known ferrimagnetic material. Indeed, magnetite was considered a ferromagnet until Néel in the 1940's, provided theoretical framework for understanding ferrimagnetism. Tetrahedral site: Fe ion is surrounded by four oxygens Octahedral site: Fe ion is surrounded by six oxygens

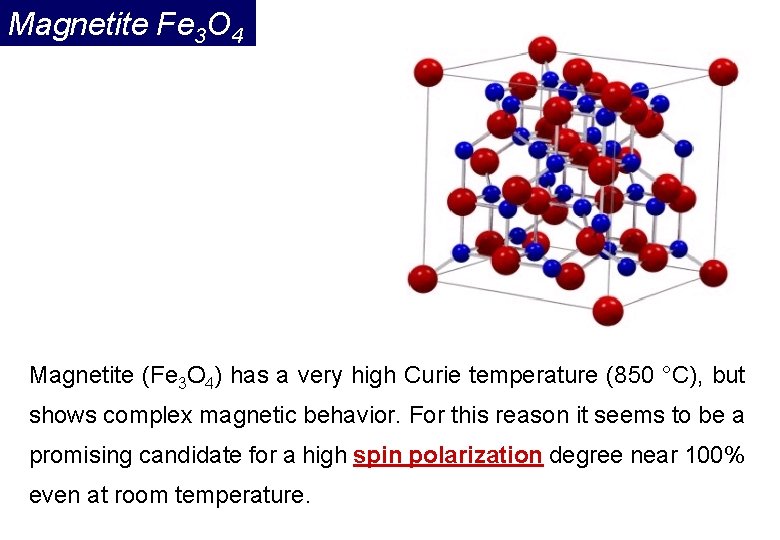
Magnetite Fe 3 O 4 Magnetite (Fe 3 O 4) has a very high Curie temperature (850 °C), but shows complex magnetic behavior. For this reason it seems to be a promising candidate for a high spin polarization degree near 100% even at room temperature.

Magnetite Fe 3 O 4

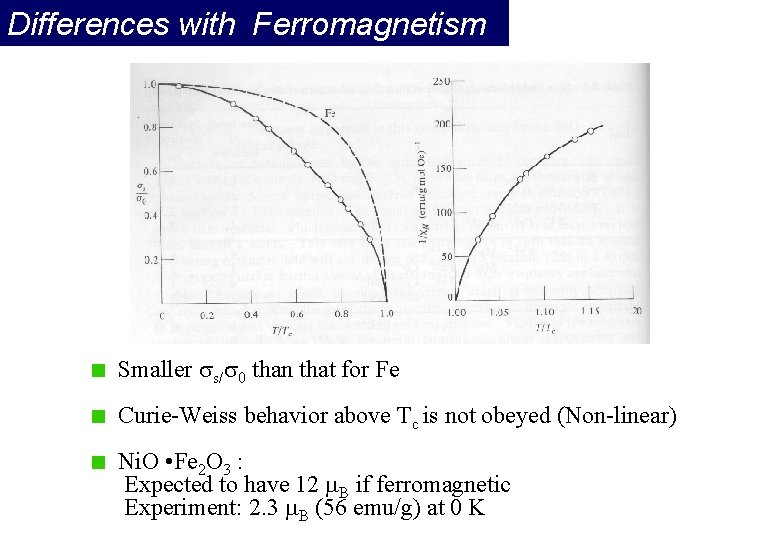
Differences with Ferromagnetism Smaller s/ 0 than that for Fe Curie-Weiss behavior above Tc is not obeyed (Non-linear) Ni. O • Fe 2 O 3 : Expected to have 12 B if ferromagnetic Experiment: 2. 3 B (56 emu/g) at 0 K

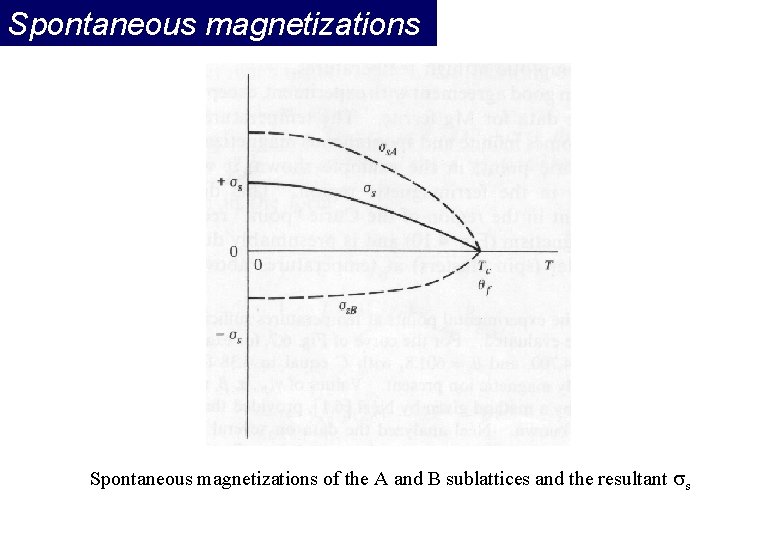
Spontaneous magnetizations of the A and B sublattices and the resultant s

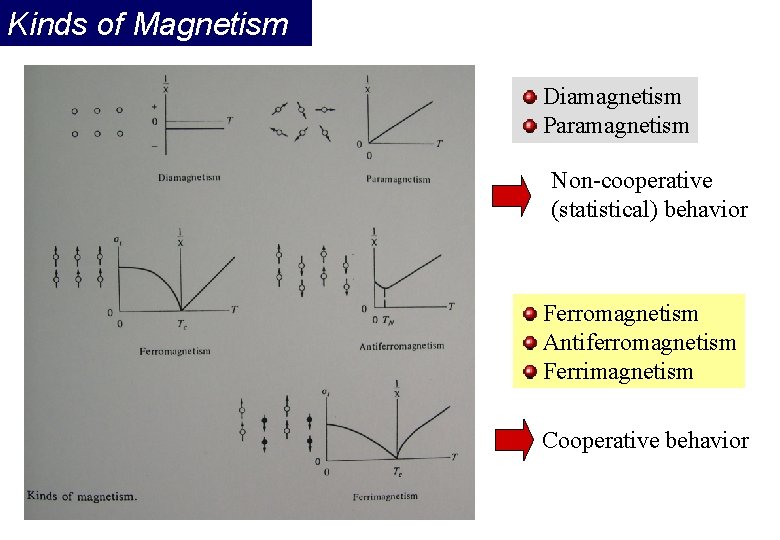
Kinds of Magnetism Diamagnetism Paramagnetism Non-cooperative (statistical) behavior Ferromagnetism Antiferromagnetism Ferrimagnetism Cooperative behavior

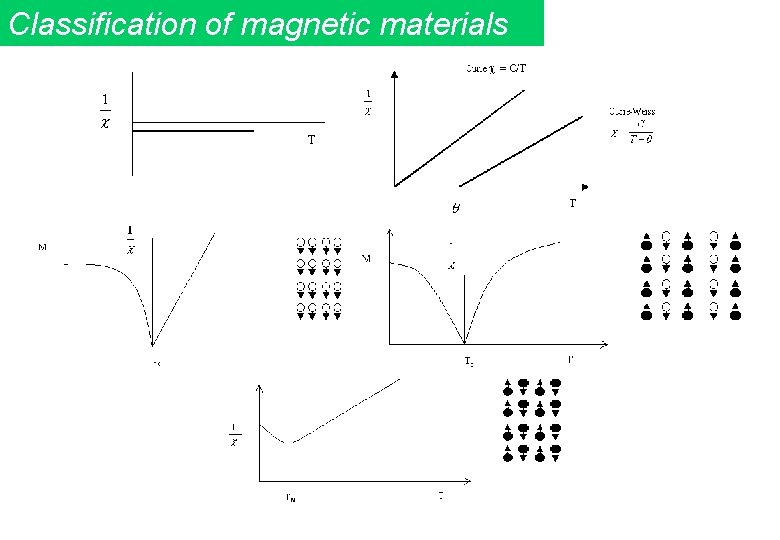
Classification of magnetic materials
 Ferromagnetis
Ferromagnetis Paramagnetic vs ferromagnetic
Paramagnetic vs ferromagnetic Ion size trend
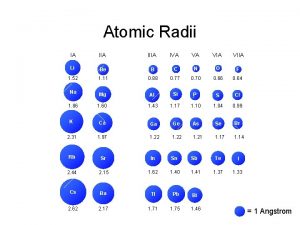
Ion size trend Atomic number vs atomic radius
Atomic number vs atomic radius Atomic radius trends
Atomic radius trends Atomic weight of oxygen
Atomic weight of oxygen Relative formula mass of hcl
Relative formula mass of hcl Whats the difference between atomic mass and atomic number
Whats the difference between atomic mass and atomic number Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phản ứng thế ankan
Phản ứng thế ankan Kể tên các môn thể thao
Kể tên các môn thể thao Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu điện thế nghỉ
điện thế nghỉ Hát lên người ơi
Hát lên người ơi Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Số nguyên tố là
Số nguyên tố là Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Fecboak
Fecboak Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất ưu thế lai là gì
ưu thế lai là gì Hệ hô hấp
Hệ hô hấp Tư thế ngồi viết
Tư thế ngồi viết Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Tư thế ngồi viết
Tư thế ngồi viết Chó sói
Chó sói Thẻ vin
Thẻ vin Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là Lp html
Lp html Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau 101012 bằng
101012 bằng Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Lời thề hippocrates
Lời thề hippocrates Chụp tư thế worms-breton
Chụp tư thế worms-breton đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công thức tính thế năng
Công thức tính thế năng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi