Atomic Structure Atomic Notation Atomic Notation represents the



- Slides: 17

Atomic Structure

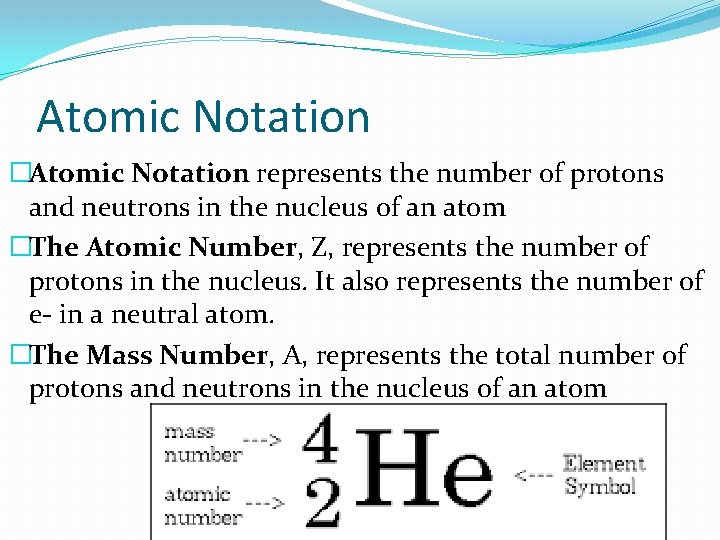
Atomic Notation �Atomic Notation represents the number of protons and neutrons in the nucleus of an atom �The Atomic Number, Z, represents the number of protons in the nucleus. It also represents the number of e- in a neutral atom. �The Mass Number, A, represents the total number of protons and neutrons in the nucleus of an atom

Atomic Notation �Atomic Number is 11 �# of p+ = 22 protons; # e- = 11 electrons �The Mass number is 23 �#n= 23 -11=12 neutrons

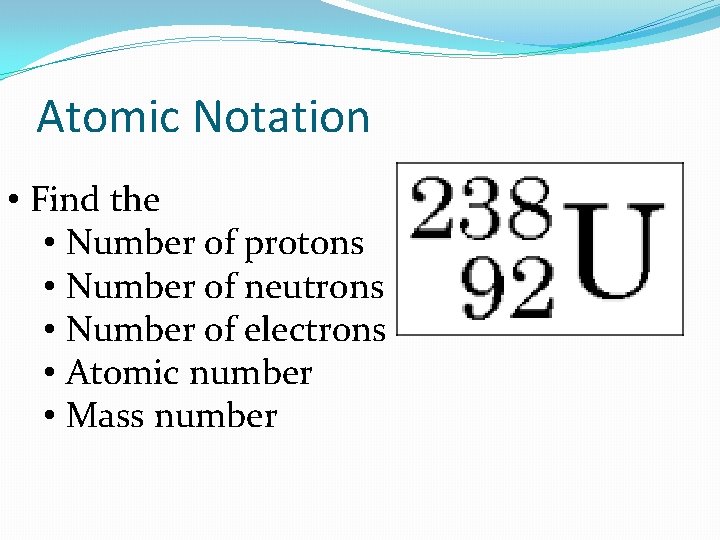
Atomic Notation • Find the • Number of protons • Number of neutrons • Number of electrons • Atomic number • Mass number

Atomic Notation �If an element has an atomic number of 34 and a mass number of 78 what is the �Number of protons �Number of neutrons �Number of electrons �Complete symbol?

Isotopes �Atoms of the same element can have different numbers of neutrons (Dalton was wrong) �These atoms of the same element would have different mass numbers �These “cousins” of the same element are called isotopes �Most elements occur naturally with varying number of neutrons

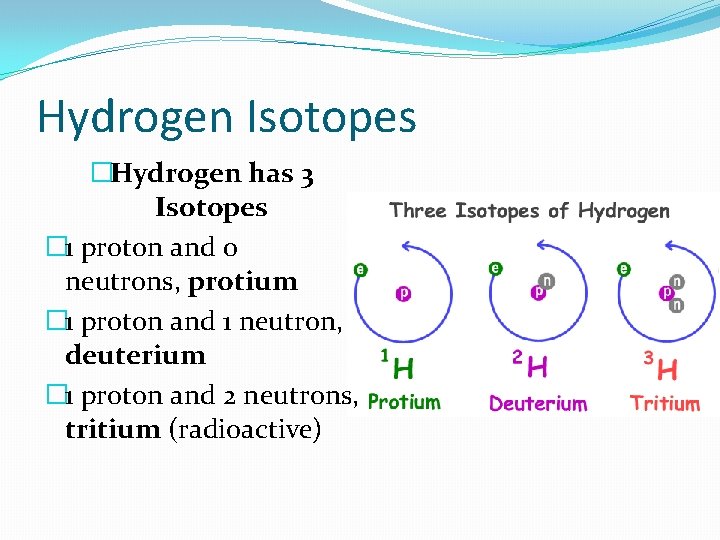
Hydrogen Isotopes �Hydrogen has 3 Isotopes � 1 proton and 0 neutrons, protium � 1 proton and 1 neutron, deuterium � 1 proton and 2 neutrons, tritium (radioactive)

Naming Isotopes �To name an isotope properly, we put the mass number after the name of the element �Carbon-12 (6 protons and 6 neutrons) �Carbon-14 (6 protons and 8 neutrons) �Uranium-235 (92 protons and 143 neutrons)

Practice �Write the symbol for Cobalt-60 �How many protons and neutrons does an atom of mercury-202 have? �How many electrons are present in an atom of Copper-63?

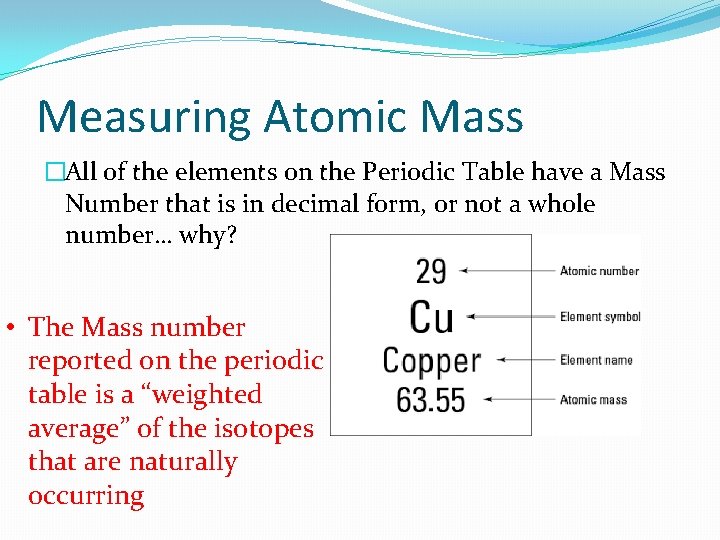
Measuring Atomic Mass �All of the elements on the Periodic Table have a Mass Number that is in decimal form, or not a whole number… why? • The Mass number reported on the periodic table is a “weighted average” of the isotopes that are naturally occurring

Measuring Atomic Mass �The Mass Number= the Atomic Mass, or Atomic Weight �The mass of 1 mole of an element (unit 4) �The number of neutrons and protons present �The mass of one atom of an element relative to an atom of another element �Unit: Atomic Mass Unit (amu) �The standard for the amu scale is Carbon: �Carbon= 6 p+ and 6 n 0= mass standard of 12 amu �All other atoms are designated relative to Carbon. I. e. Hydrogen is 1/12 th the mass of carbon (or Atomic Mass 1 amu

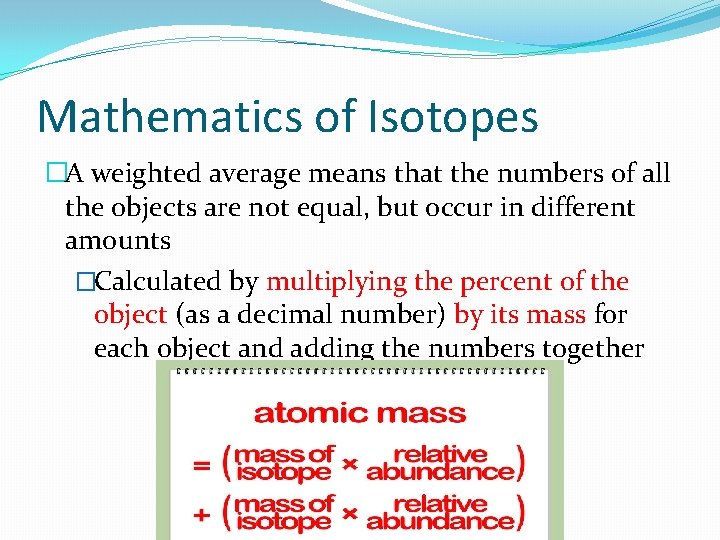
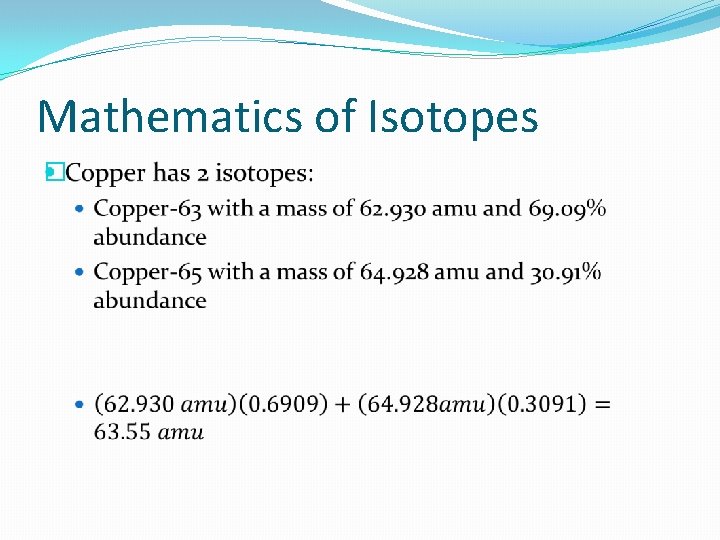
Mathematics of Isotopes �A weighted average means that the numbers of all the objects are not equal, but occur in different amounts �Calculated by multiplying the percent of the object (as a decimal number) by its mass for each object and adding the numbers together

Mathematics of Isotopes �

Isotope Examples �Magnesium has 3 isotopes. 78. 99% Magnesium-24 with a mass of 23. 9850 amu, Magnesium-25 with a mass of 24. 9858 amu, and the rest Magnesium-26 with a mass of 25. 9826 amu. What is the atomic mass of Magnesium? �Boron is 20% B-10 and 80% B-11. What is the Atomic Mass of Boron?

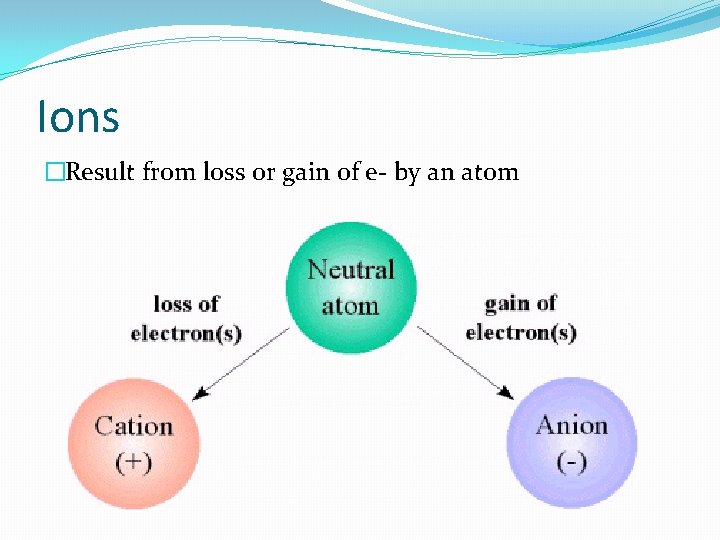
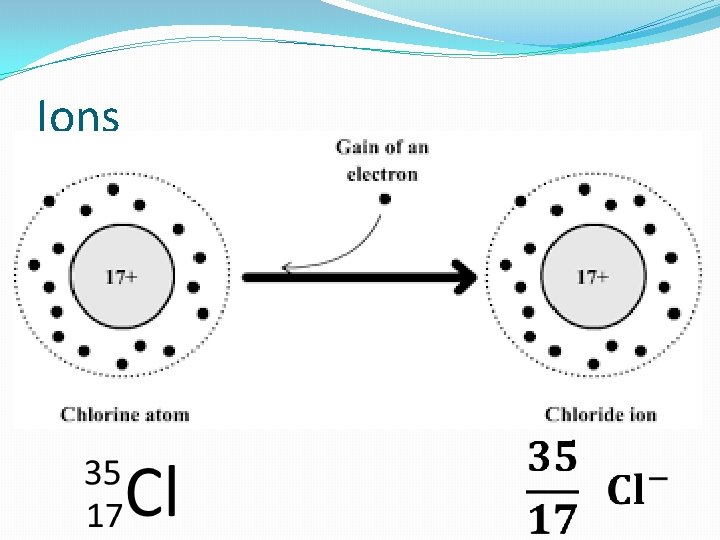
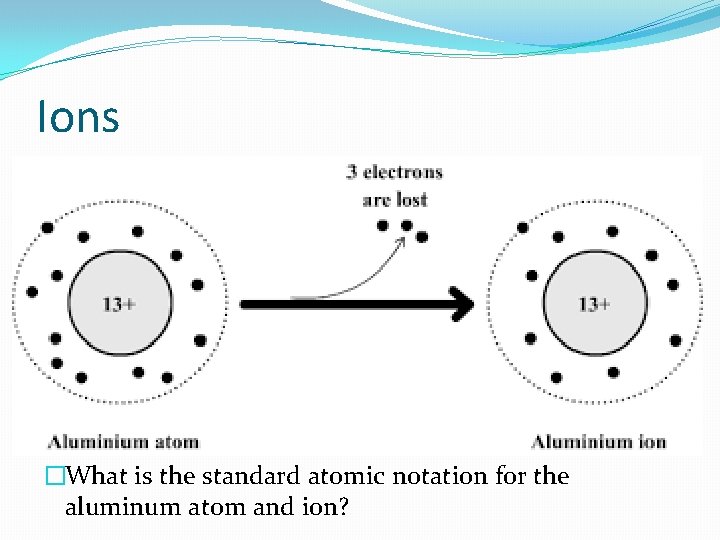
Ions �Result from loss or gain of e- by an atom

Ions

Ions �What is the standard atomic notation for the aluminum atom and ion?