Heat Temperature Heat Transfer Thermal Expansion Thermodynamics Heat








- Slides: 18

Heat, Temperature, Heat Transfer, Thermal Expansion & Thermodynamics

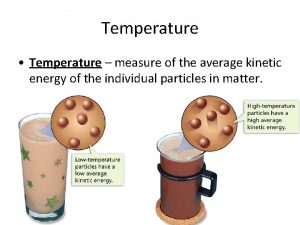
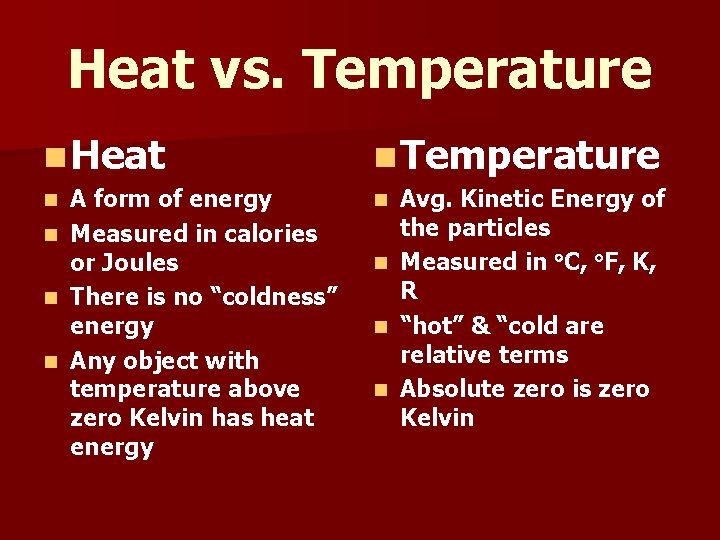
Heat vs. Temperature n Heat n Temperature A form of energy n Measured in calories or Joules n There is no “coldness” energy n Any object with temperature above zero Kelvin has heat energy n n Avg. Kinetic Energy of the particles n Measured in C, F, K, R n “hot” & “cold are relative terms n Absolute zero is zero Kelvin

Heat Transfer (3 methods) 1. Conduction - requires direct contact or particle to particle transfer of energy; usually occurs in solids 2. Convection - heat moves in currents; only happens in fluid states of matter 3. Radiation - heat waves travel through empty space, no matter needed; IR

Thermal Equilibrium n. A system is in thermal equilibrium when all of its parts are at the same temperature. n Heat transfers only from high to low temperatures and only until thermal equilibrium is reached.

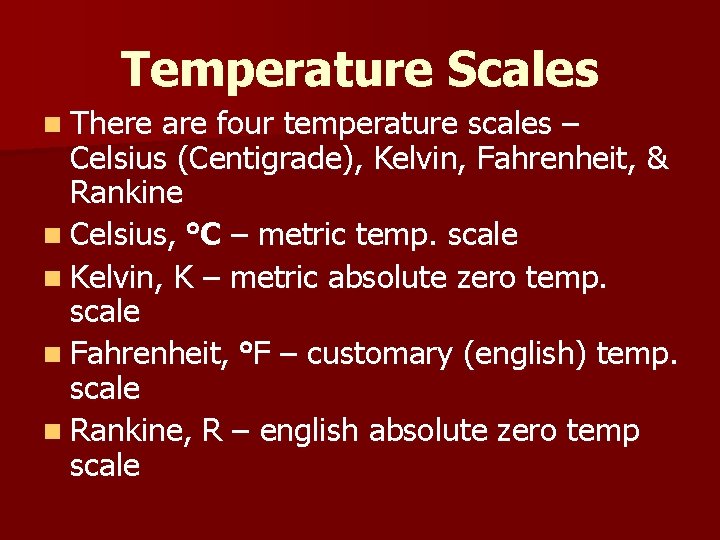
Temperature Scales n There are four temperature scales – Celsius (Centigrade), Kelvin, Fahrenheit, & Rankine n Celsius, C – metric temp. scale n Kelvin, K – metric absolute zero temp. scale n Fahrenheit, F – customary (english) temp. scale n Rankine, R – english absolute zero temp scale

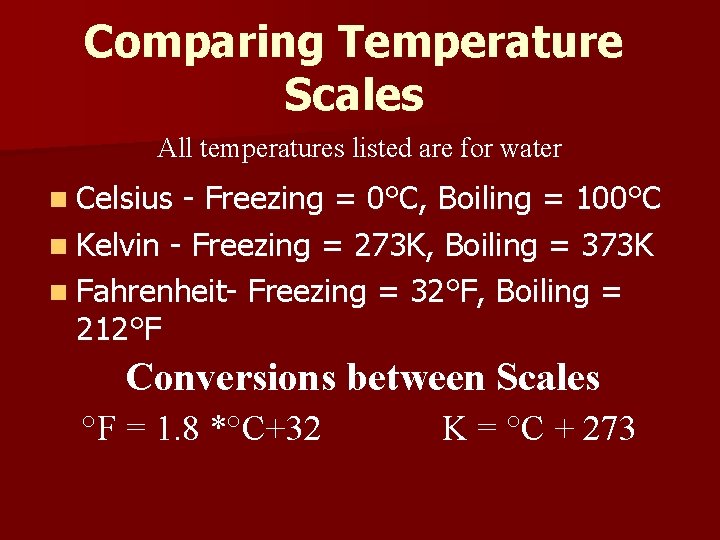
Comparing Temperature Scales All temperatures listed are for water n Celsius - Freezing = 0°C, Boiling = 100°C n Kelvin - Freezing = 273 K, Boiling = 373 K n Fahrenheit- Freezing = 32°F, Boiling = 212°F Conversions between Scales °F = 1. 8 *°C+32 K = °C + 273

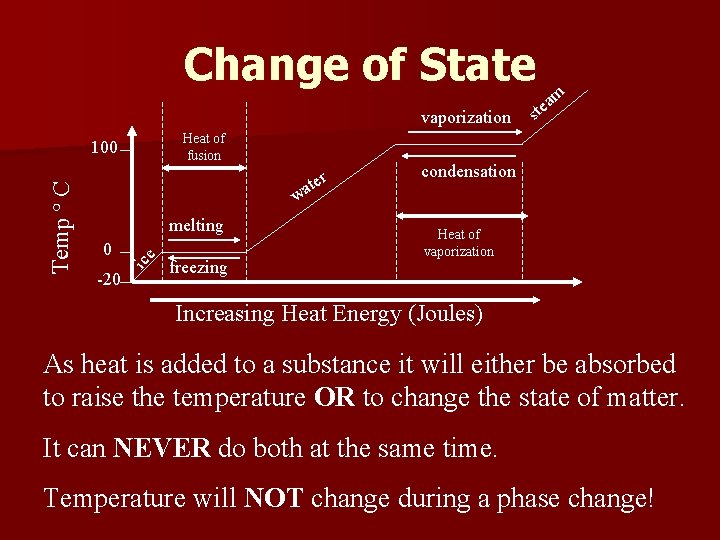
Change of State vaporization Heat of fusion w melting 0 -20 ice Temp ° C 100 freezing r ate m ts ea condensation Heat of vaporization Increasing Heat Energy (Joules) As heat is added to a substance it will either be absorbed to raise the temperature OR to change the state of matter. It can NEVER do both at the same time. Temperature will NOT change during a phase change!

Specific Heat The amount of heat energy needed to raise the temperature of 1 gram of substance by 1°C. Substances with lower specific heats change temperature faster. Symbol : c units : cal/g°C or J/kg°C For water: c = 1 cal/g°C = 4. 18 J/g°C = 4180 J/kg°C

Latent Heat The amount of heat energy required to change the state of 1 gram of substance. Heat of fusion - latent heat for changes between the solid and liquid phases. Lf =80 cal/g for water Heat of vaporization - latent heat for changesbetween the solid and liquid phases. Lv =540 cal/g for water

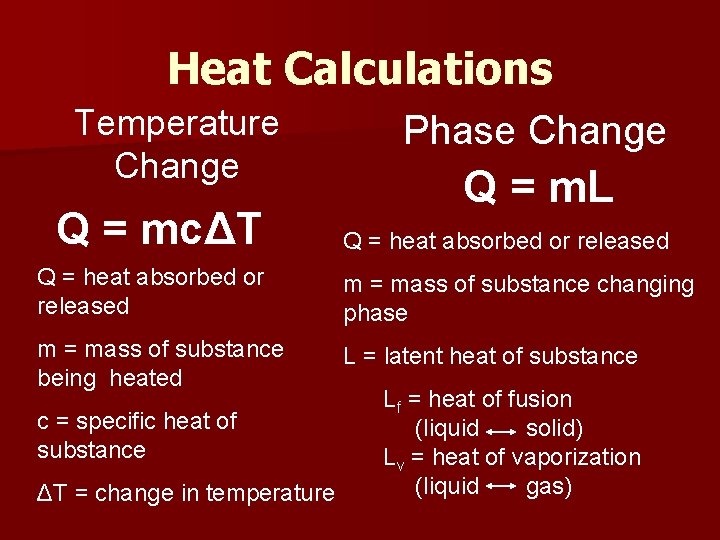
Heat Calculations Temperature Change Q = mcΔT Phase Change Q = m. L Q = heat absorbed or released m = mass of substance changing phase m = mass of substance being heated L = latent heat of substance c = specific heat of substance ΔT = change in temperature Lf = heat of fusion (liquid solid) Lv = heat of vaporization (liquid gas)

n Thermodynamics study of changes in thermal properties of matter n Follows Law of Conservation of Energy n 1 st Law – the total increase in thermal energy of a system is the sum of the work done on it and the heat added to it n 2 nd Law – natural processes tend to increase the total entropy (disorder) of the universe.

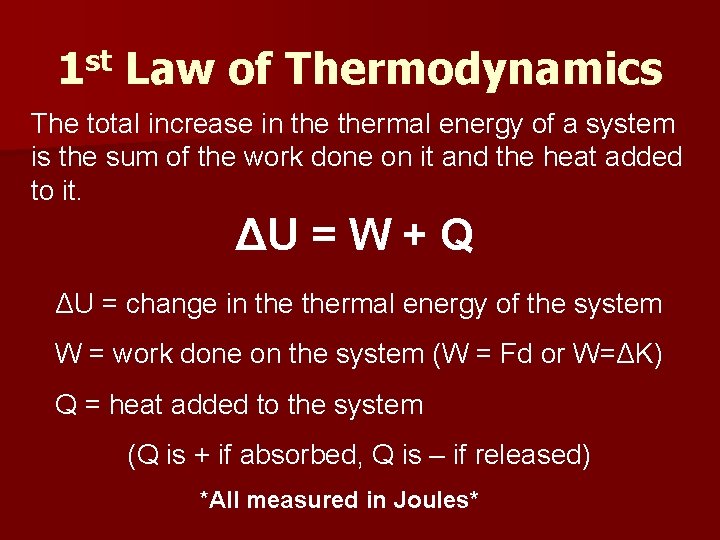
1 st Law of Thermodynamics The total increase in thermal energy of a system is the sum of the work done on it and the heat added to it. ΔU = W + Q ΔU = change in thermal energy of the system W = work done on the system (W = Fd or W=ΔK) Q = heat added to the system (Q is + if absorbed, Q is – if released) *All measured in Joules*

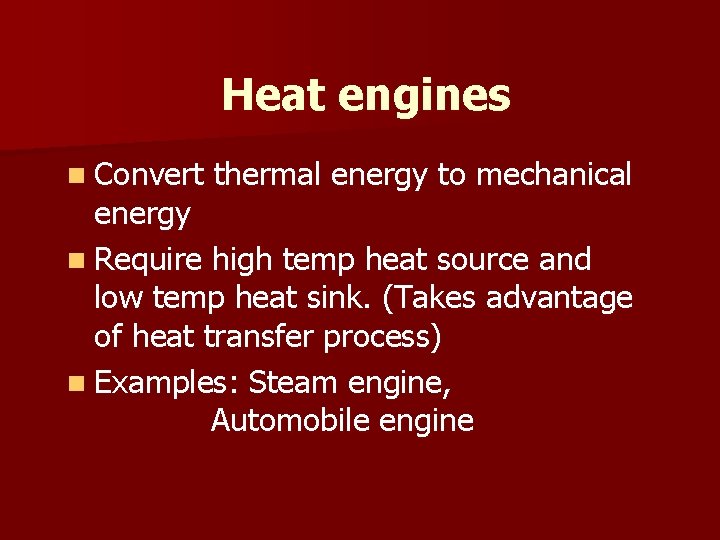
Heat engines n Convert thermal energy to mechanical energy n Require high temp heat source and low temp heat sink. (Takes advantage of heat transfer process) n Examples: Steam engine, Automobile engine

Refrigerators and Heat Pumps n It is possible to remove heat from a cold environment and deposit it into a warmer environment. n This requires an outside source of energy. n Examples: Refrigerators, Air conditioning units n Heat pumps are refrigeration units that work in either direction.

2 nd Law of Thermodynamics All natural processes go in a direction that increases the total entropy of the universe. Entropy is a measure of the disorder of a system. If heat is added, entropy is increased. If heat is removed, entropy is decreased. Work with no ΔT, entropy is unchanged

Thermal Expansion n Substances expand as they heat and contract as they cool. n The rate of expansion depends on the substance’s coefficient of expansion (α) n The exception to this rule is water. As water is cooled from 4°C to 0°C, it expands which explains why ice floats (it is less dense than water.

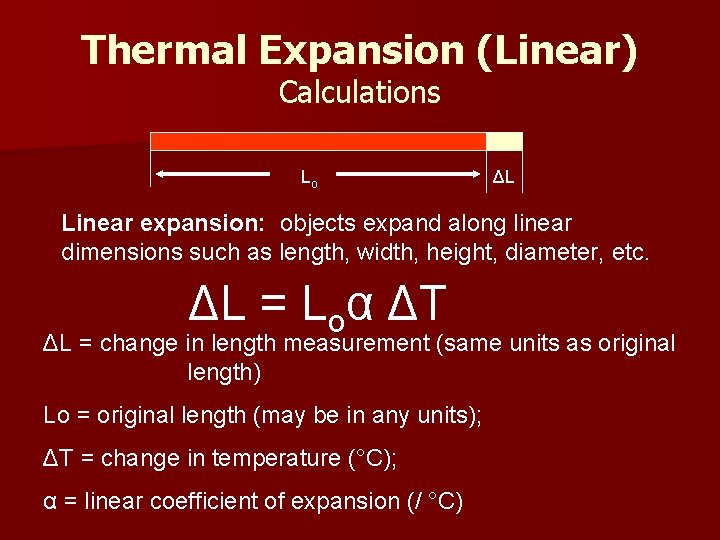
Thermal Expansion (Linear) Calculations Lo ΔL Linear expansion: objects expand along linear dimensions such as length, width, height, diameter, etc. ΔL = Loα ΔT ΔL = change in length measurement (same units as original length) Lo = original length (may be in any units); ΔT = change in temperature (°C); α = linear coefficient of expansion (/ °C)

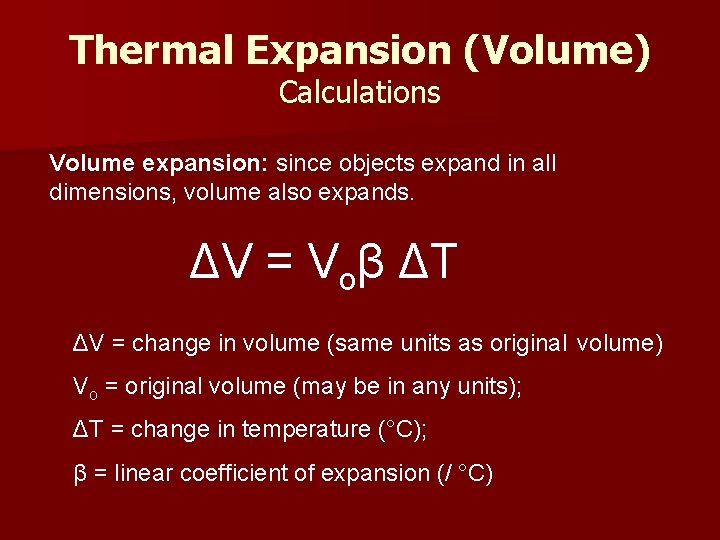
Thermal Expansion (Volume) Calculations Volume expansion: since objects expand in all dimensions, volume also expands. ΔV = Voβ ΔT ΔV = change in volume (same units as original volume) Vo = original volume (may be in any units); ΔT = change in temperature (°C); β = linear coefficient of expansion (/ °C)
 Thermal transfer vs direct thermal printing
Thermal transfer vs direct thermal printing Chapter 21 temperature heat and expansion answer key
Chapter 21 temperature heat and expansion answer key Chapter 15 temperature heat and expansion
Chapter 15 temperature heat and expansion Chapter 21 temperature, heat and expansion answer key
Chapter 21 temperature, heat and expansion answer key Microscopic slush in water tends
Microscopic slush in water tends Chapter 21 temperature heat and expansion
Chapter 21 temperature heat and expansion Chapter 21 temperature heat and expansion
Chapter 21 temperature heat and expansion Thermal energy in states of matter
Thermal energy in states of matter Heat thermal energy and temperature
Heat thermal energy and temperature Temperature and heat
Temperature and heat Heat vs thermal energy vs temperature
Heat vs thermal energy vs temperature Thermal energy vs temperature
Thermal energy vs temperature Thermal equilibrium formula
Thermal equilibrium formula Thermal expansion examples
Thermal expansion examples Thermal expansion examples
Thermal expansion examples Thermometer and thermal expansion
Thermometer and thermal expansion Thermometer thermal expansion
Thermometer thermal expansion Why does water behave in this unusual fashion?
Why does water behave in this unusual fashion? Thermal expansion notes
Thermal expansion notes