Temperature Heat and Thermal Energy Thermal Energy The






- Slides: 13

Temperature, Heat, and Thermal Energy

Thermal Energy The measure of the TOTAL amount of energy in ALL of the particles in an object or substance. n Objects with the same Temperature do NOT necessarily have the same total energy. n Ex: 2 L of Water @ 75 C > 1 L @ 75 C n

Temperature n Measure of the average kinetic energy in the particles of a substance – a measure of how fast the particles are moving around in the substance

Temperature Scales n Fahrenheit (F) - Commonly used in the United States. 32°F is assigned as the temp. water FREEZES while 212°F is assigned as the temp. water BOILS. n Celsius (C. ) - Commonly used in most of the World (as well as in science). 0°C is assigned as the temp. water FREEZES while 100°C is assigned as the temp. water BOILS.

Kelvin (K)- Commonly used in Physical Science. 273 K is assigned as the temp. water FREEZES while 373 K is assigned as the temp. water BOILS. Absolute Zero (0 K) means no movement of particles n CONVERSIONS: n Fahrenheit to Celsius: n • 5/9 x (F-32) = C n Celsius to Fahrenheit: • 9/5 C + 32 = F n Celsius to Kelvin: • C + 273 = K

Heat n The movement of Thermal Energy from a substance with Higher temp. to another with Lower Temp. n 3 types of Movement: n -Conduction n -Convection n -Radiation

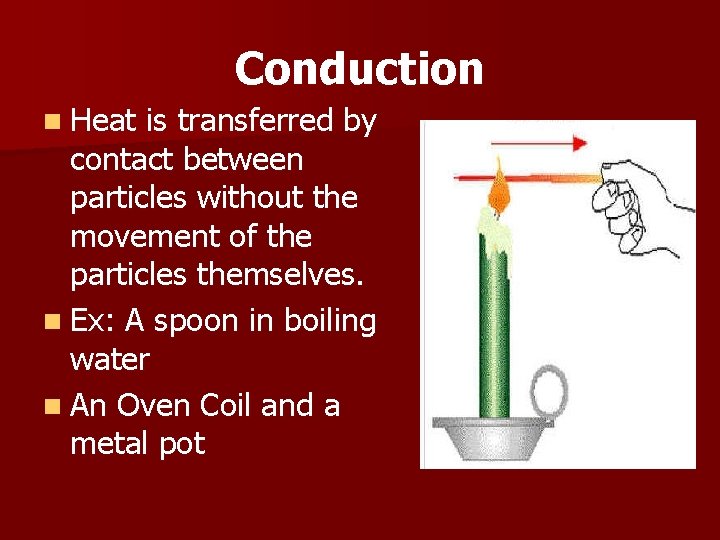
Conduction n Heat is transferred by contact between particles without the movement of the particles themselves. n Ex: A spoon in boiling water n An Oven Coil and a metal pot

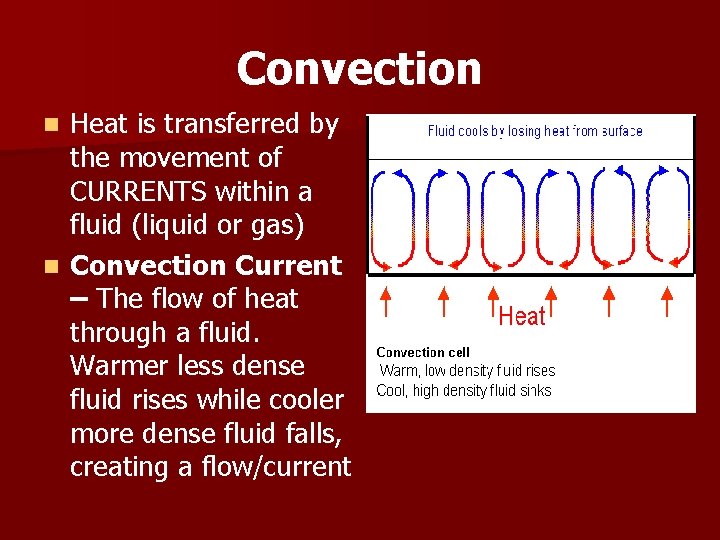
Convection Heat is transferred by the movement of CURRENTS within a fluid (liquid or gas) n Convection Current – The flow of heat through a fluid. Warmer less dense fluid rises while cooler more dense fluid falls, creating a flow/current n

Radiation Heat is transferred through Electromagnetic waves n Ex: Warmth from a Bonfire or Heat Lamp n Does not require matter to transfer (can transfer heat through “outer space”) n Ex: Sun’s radiation travels through empty space to reach Earth n

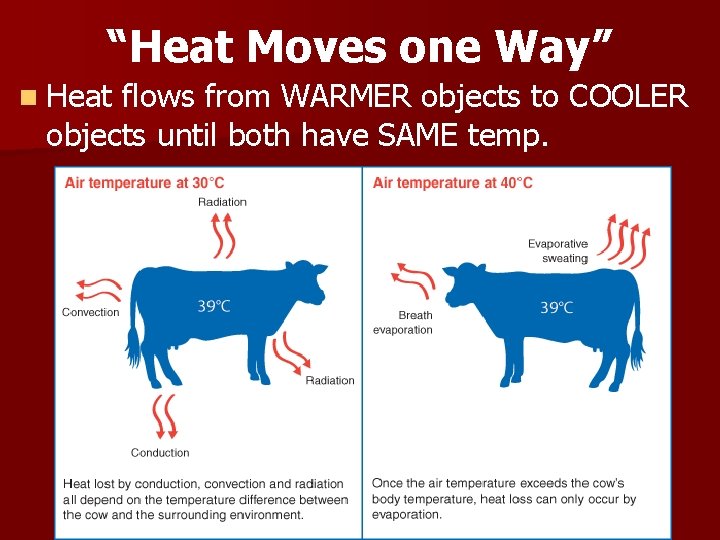
“Heat Moves one Way” n Heat flows from WARMER objects to COOLER objects until both have SAME temp.

Which direction will heat flow? n Your pillow and your head – Head Pillow n Your seat and your body – Body Seat n Hot food and the room – Food Room

Conductors n Conductor – Any material in which thermal energy is transferred quickly – Particles tend to be close together n Examples: – Common metals: § Copper § Iron (skillet) – Diamonds

Insulators n Insulator – Any material in which thermal energy is transferred slowly – Particles tend to be farther apart n Examples: – – – Cotton Cardboard Wood Rubber Plastic