Chapter 16 Solutions Solute Complete Solvent Na Cl









- Slides: 16

Chapter 16 Solutions

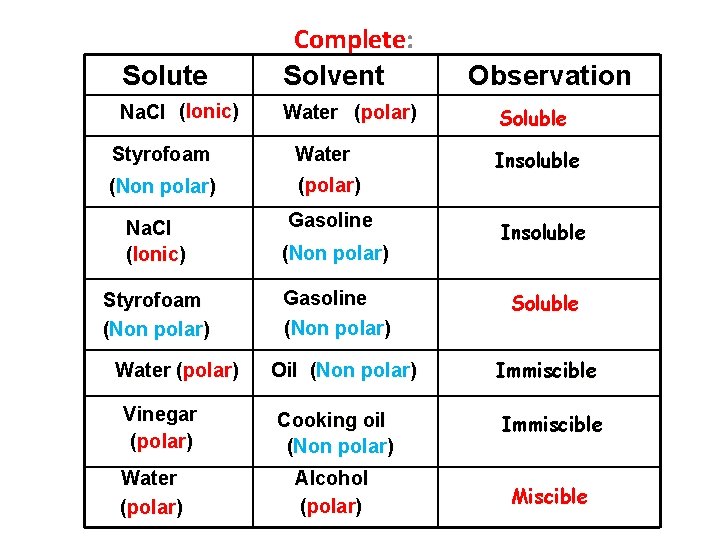
Solute Complete: Solvent Na. Cl (Ionic) Water (polar) Styrofoam Water (Non polar) (polar) Gasoline Na. Cl (Ionic) (Non polar) Styrofoam (Non polar) Gasoline (Non polar) Water (polar) Vinegar (polar) Water (polar) Observation Soluble Insoluble Soluble Oil (Non polar) Immiscible Cooking oil (Non polar) Immiscible Alcohol (polar) Miscible

Conclusion: “Likes dissolve likes”

Solutions • Solute is the dissolved substance - seems to “disappear”. - “takes on the state” of the solvent. • Solvent is the substance solute dissolves in - does not appear to change state. • when both solute and solvent have the same state, the solvent is the component present in the highest percentage. • solutions in which the solvent is water are called aqueous solutions. 4

Solubility: • When one substance (solute) dissolves in another (solvent) it is said to be soluble: • When one substance does not dissolve in another it is said to be insoluble. • When the solute & solvent have the same state (both are liquids or solids or gases) we use the terms: Miscible or immiscible. 5

Salt Dissolving in Water Tro's Introductory Chemistry, Chapter 13 6

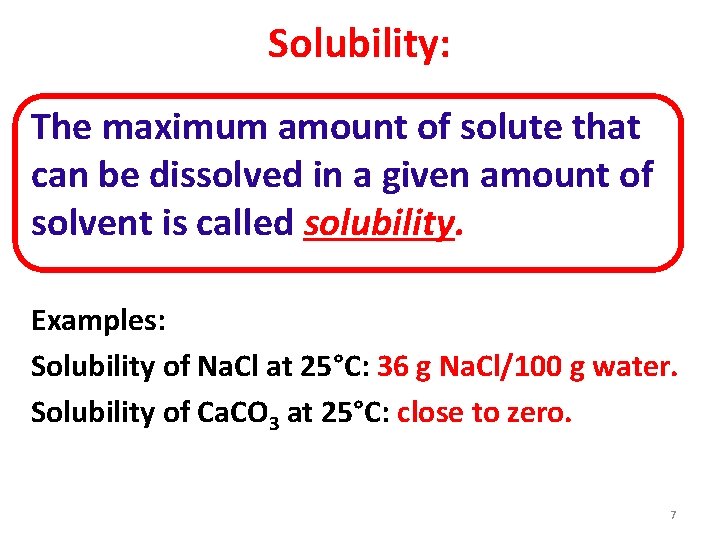
Solubility: The maximum amount of solute that can be dissolved in a given amount of solvent is called solubility. Examples: Solubility of Na. Cl at 25°C: 36 g Na. Cl/100 g water. Solubility of Ca. CO 3 at 25°C: close to zero. 7

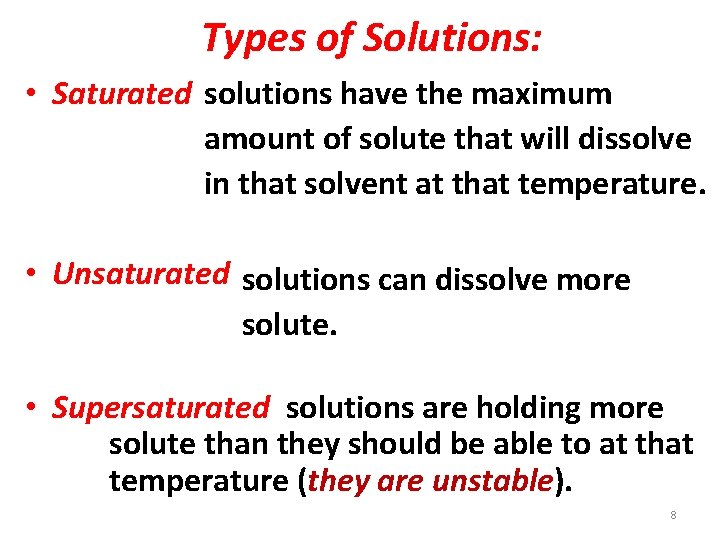
Types of Solutions: • Saturated solutions have the maximum amount of solute that will dissolve in that solvent at that temperature. • Unsaturated solutions can dissolve more solute. • Supersaturated solutions are holding more solute than they should be able to at that temperature (they are unstable). 8

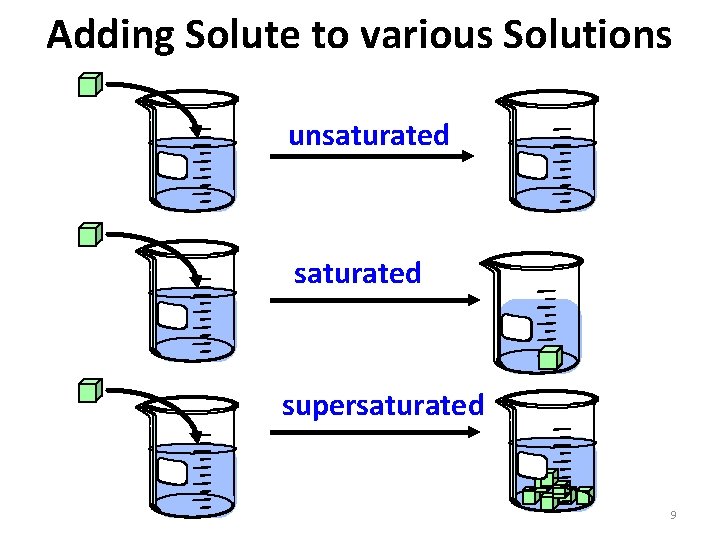
Adding Solute to various Solutions unsaturated supersaturated 9

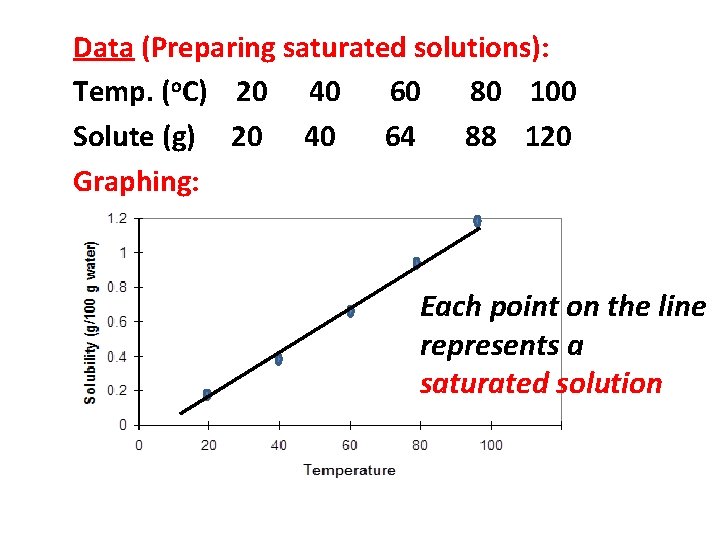
Data (Preparing saturated solutions): Temp. (o. C) 20 40 60 80 100 Solute (g) 20 40 64 88 120 Graphing:

Data (Preparing saturated solutions): Temp. (o. C) 20 40 60 80 100 Solute (g) 20 40 64 88 120 Graphing: Each point on the line represents a saturated solution

Data (Preparing saturated solutions): Temp. (o. C) 20 40 60 80 100 Solute (g) 20 40 64 88 120 Graphing: d e Concentrated t saturated D a r solution u A t sa C r e p B su unsaturated E Diluted solution

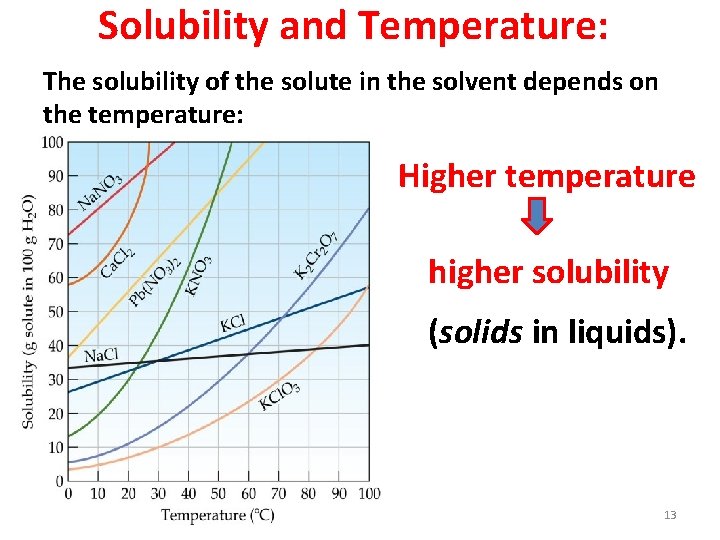
Solubility and Temperature: The solubility of the solute in the solvent depends on the temperature: Higher temperature higher solubility (solids in liquids). 13

Solubility and Temperature: Lower temperature higher solubility (gas in liquid) 14

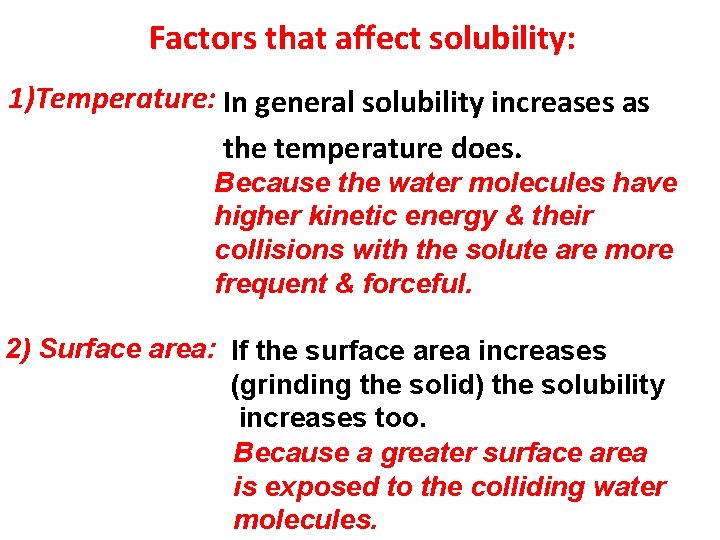
Factors that affect solubility: 1)Temperature: In general solubility increases as the temperature does. Because the water molecules have higher kinetic energy & their collisions with the solute are more frequent & forceful. 2) Surface area: If the surface area increases (grinding the solid) the solubility increases too. Because a greater surface area is exposed to the colliding water molecules.

Factors that affect solubility: 3) Agitation: It increases the rate of solution. (stir, shake) Because it brings fresh solvent (water) into contact with the solute. 4) Pressure: The greater the pressure, the greater the solubility (gases). Smaller volume, collisions between the solute & solvent particles are more frequent.