Transpiration Data 1 Take average for each type





























- Slides: 66

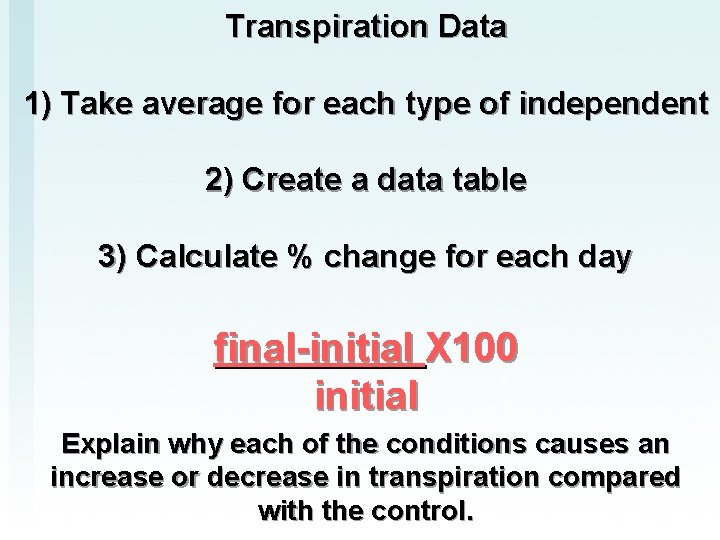
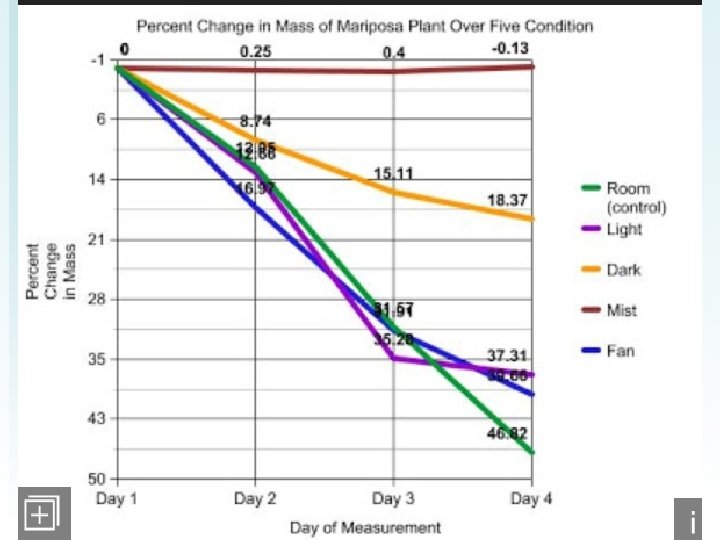
Transpiration Data 1) Take average for each type of independent 2) Create a data table 3) Calculate % change for each day final-initial X 100 initial Explain why each of the conditions causes an increase or decrease in transpiration compared with the control.

Graph • X-axis? ? • Y-axis? ? • Title? ?


1. Why was it important to calculate the PERCENT loss and not just examine water loss per plant? 2. For each condition explain why there was an increase/decrease of water loss when compared to the control.

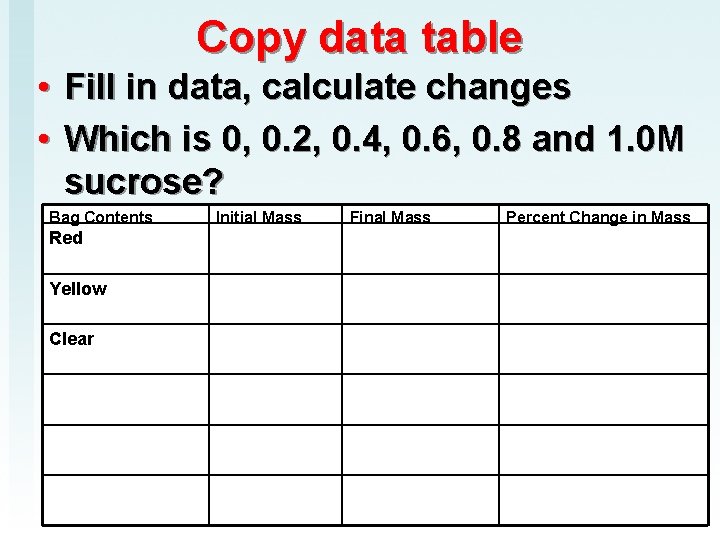
Copy data table • • Fill in data, calculate changes Which is 0, 0. 2, 0. 4, 0. 6, 0. 8 and 1. 0 M sucrose? Bag Contents Red Yellow Clear Initial Mass Final Mass Percent Change in Mass

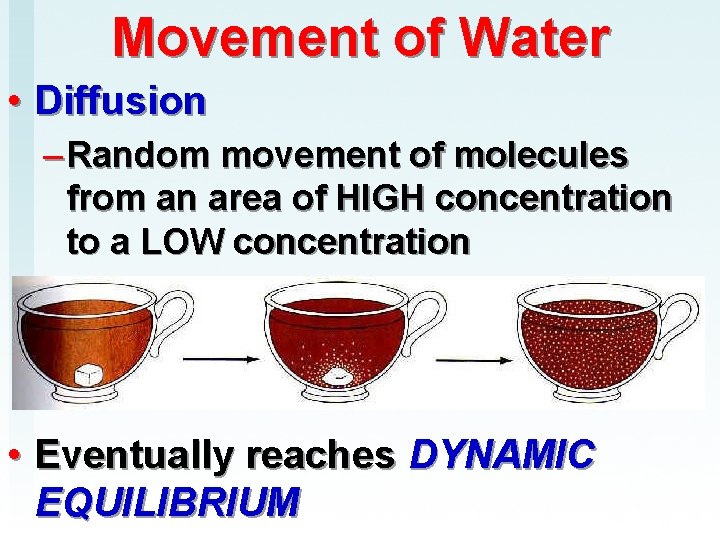
Movement of Water • Diffusion – Random movement of molecules from an area of HIGH concentration to a LOW concentration • Eventually reaches DYNAMIC EQUILIBRIUM

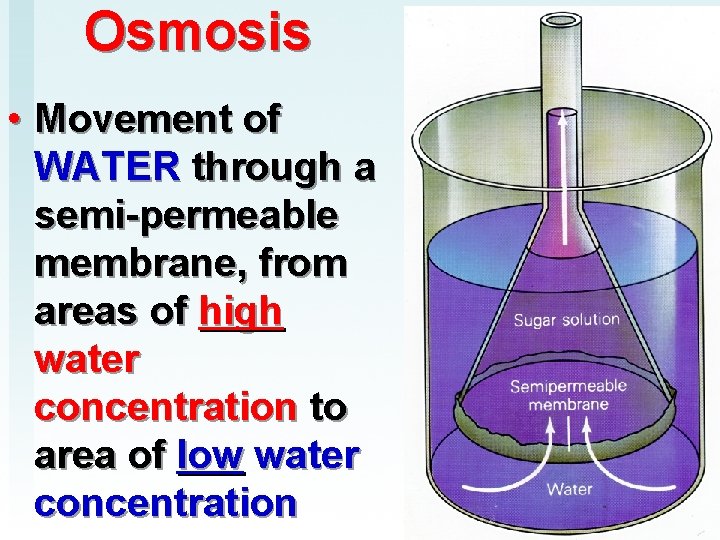
Osmosis • Movement of WATER through a semi-permeable membrane, from areas of high water concentration to area of low water concentration

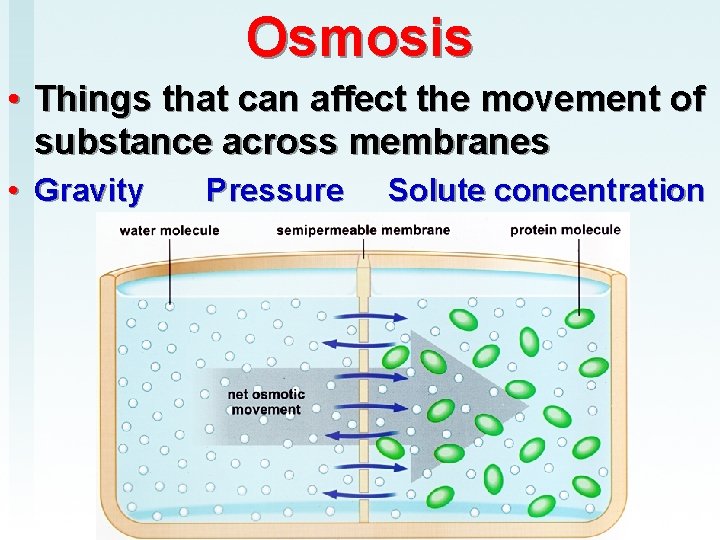
Osmosis • Things that can affect the movement of substance across membranes • Gravity Pressure Solute concentration

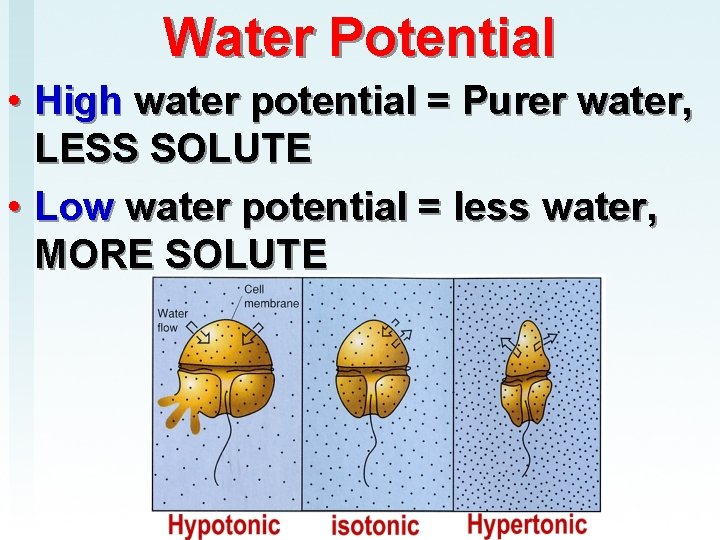
Water Potential • High water potential = Purer water, LESS SOLUTE • Low water potential = less water, MORE SOLUTE

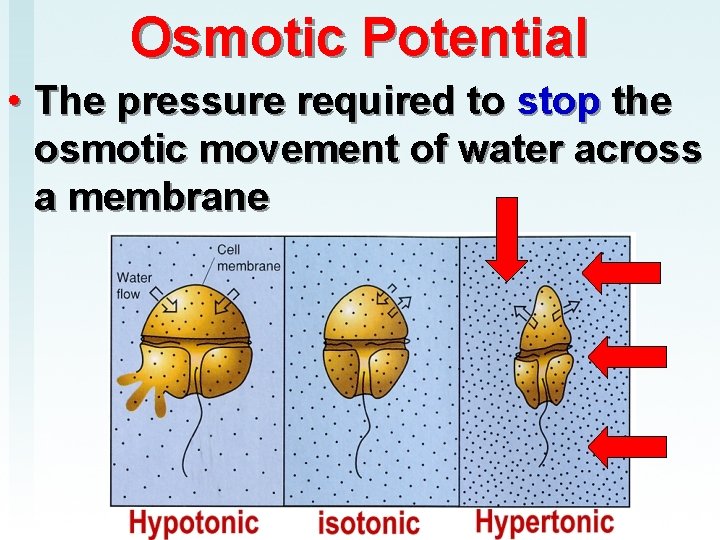
Osmotic Potential • The pressure required to stop the osmotic movement of water across a membrane

Maintaining Structure • Tugor – Water pressure within cells that maintains rigid cell walls • Plasmolysis – Loss of turgor in cells – Water diffuses OUT of cells

Objectives After doing this laboratory, students should be able to • measure the water potential of a solution in a controlled experiment • dermine the osmotic concentration of living tissue or an unknown solution from experimental data • relate osmotic potential to solute concentration and water potential

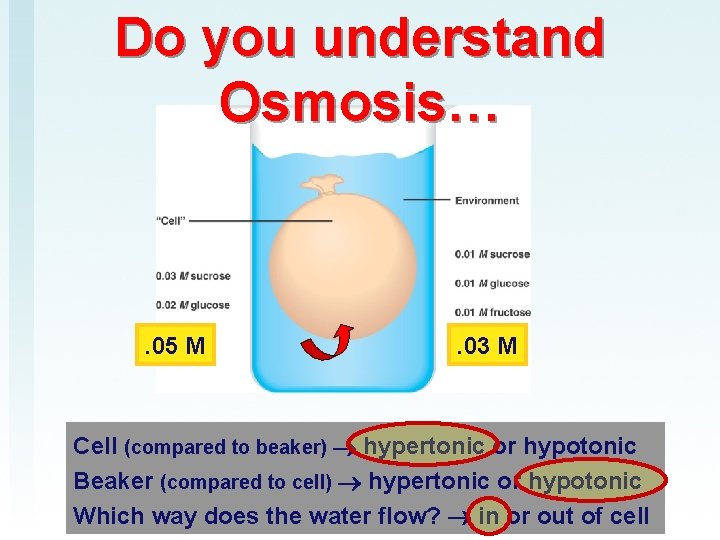
Do you understand Osmosis… . 05 M . 03 M Cell (compared to beaker) hypertonic or hypotonic Beaker (compared to cell) hypertonic or hypotonic Which way does the water flow? in or out of cell

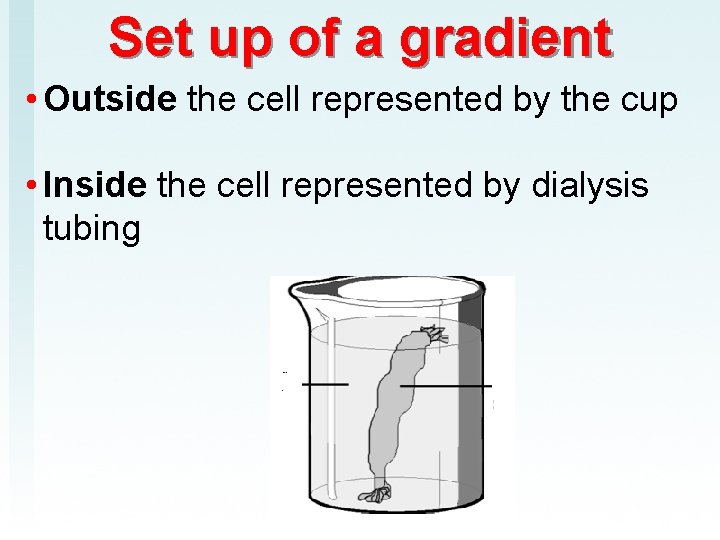
Set up of a gradient • Outside the cell represented by the cup • Inside the cell represented by dialysis tubing

Part A • Make a predication, based on your knowledge of molecular size & movement, which molecule will move in or out of the tubing. • Cups will have water, bags will have 0. 2, 0. 4, 0. 6, 0. 8, 1. 0 M of sucrose

Logistics • Leave space in “cell”. Tie knot at the end of the tubing 1) • 2) Beakers not labeled so record color 3) Label cups

Next time • Set up potatoes • Mass bags from part A—look at math • Water potential math • Plasmolysis with Elodea • HM: Background of lab

Dispatch Take out lab packet • What is the math equation for part A? • What is the math equation for part B? Fix to Ys not Ypie • Annotate fun with water potential side 1 • Pick up poster headings • Come up if you hear your name—this is regarding your last test • Poster Symposium moved to Thur/Fri

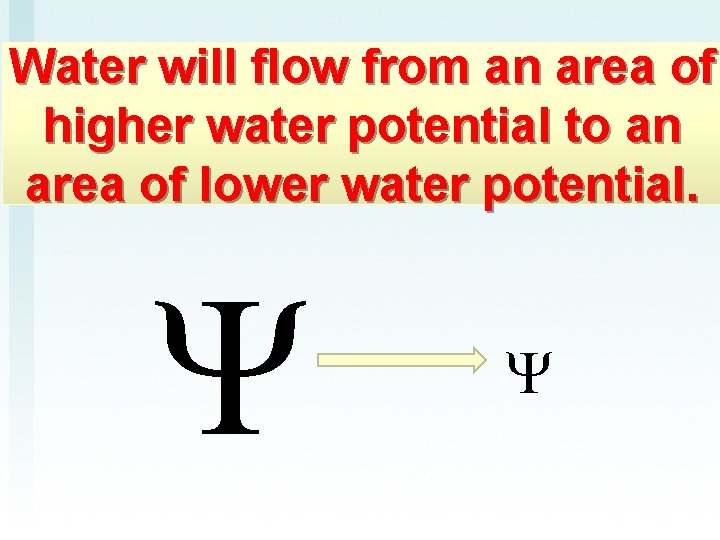
Water will flow from an area of higher water potential to an area of lower water potential.

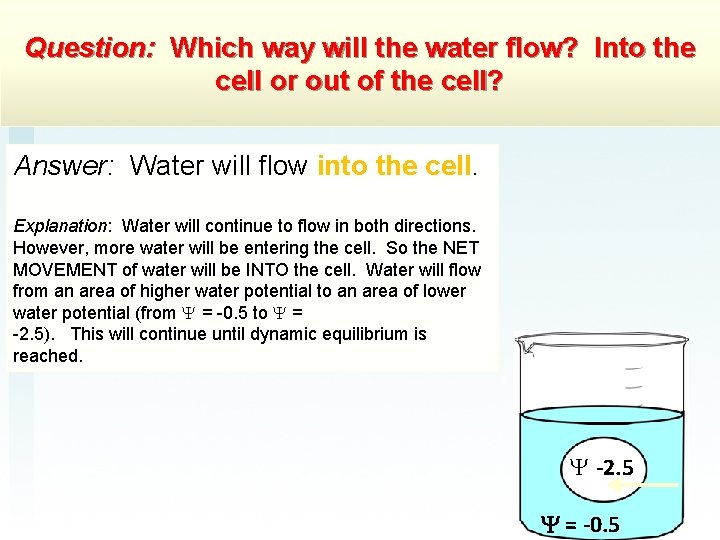
Question: Which way will the water flow? Into the cell or out of the cell? Answer: Water will flow into the cell. Explanation: Water will continue to flow in both directions. However, more water will be entering the cell. So the NET MOVEMENT of water will be INTO the cell. Water will flow from an area of higher water potential to an area of lower water potential (from = -0. 5 to = -2. 5). This will continue until dynamic equilibrium is reached. -2. 5 = -0. 5

Water Potential Video

• Solve problems on the back of the 1) The solute potential of pure water is _________. 2) The solute potential of the plant cell is (greater/less than)pure water. Therefore the greater water potential is (in the cell/in the solution). Circle your choice. 3) If solute potential in the plant cell above is – 6. 25 bars and pressure potential is 0, what is water potential of the plant cell? _____________ What does this indicate in terms of water movement? 4) If solute potential in the plant cell above is – 6. 25 bars and pressure potential is 6. 25 bars, what is water potential of the plant cell? ____________ What does this indicate in terms of water movement? 5) A plant cell has a solute potential of – 4. 0 and a pressure potential of 1. 0. It is then placed in a solution with a water potential of – 5. 0. What will happen to this plant cell? 6) A plant cell has a solute potential of – 2. 0 and a pressure potential of 0. 0. It is placed in a solution with a water potential of – 1. 0. What will happen to this plant cell?

Mingle at table • Lab Report/Poster —Background info

Your tasks 1) Set up potatoes—part B. Reuse cups. Excess potatoes go to worms 2) Mass your bags—dump glucose water outside for plant. Keep or throw away bags 3) Calculate % change in mass for A in notebook. Everyone needs to do math. (Final mass-Initial mass / Initial mass )X 100= 4) Figure out which color solution is 0. 0 M glucose, 0. 2, 0. 4, 0. 6, 0. 8, 1. 0 and confirm with Morris. O’Hearn. Justify with data 5) Share with group WHYs for part A (hypertonic, hypotonic, water potential, diffusion/osmosis, sucrose, solute, membrane, water etc) 6)Graph zucchini data

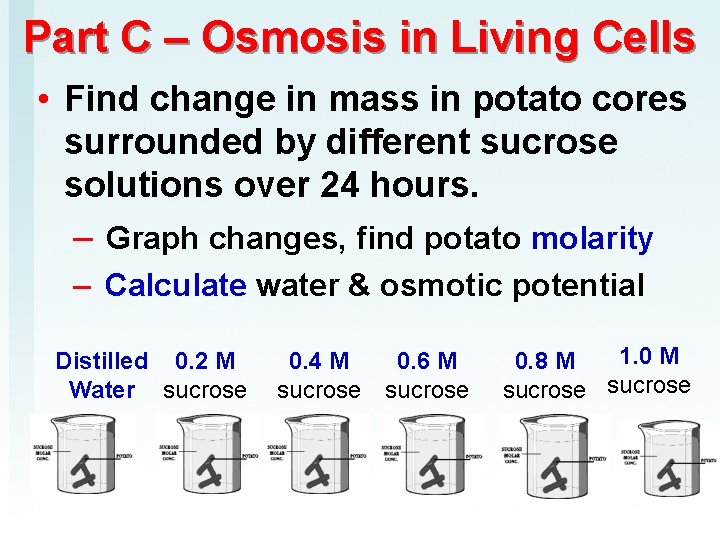
Part C – Osmosis in Living Cells • Find change in mass in potato cores surrounded by different sucrose solutions over 24 hours. – Graph changes, find potato molarity – Calculate water & osmotic potential Distilled 0. 2 M Water sucrose 0. 4 M 0. 6 M sucrose 1. 0 M 0. 8 M sucrose

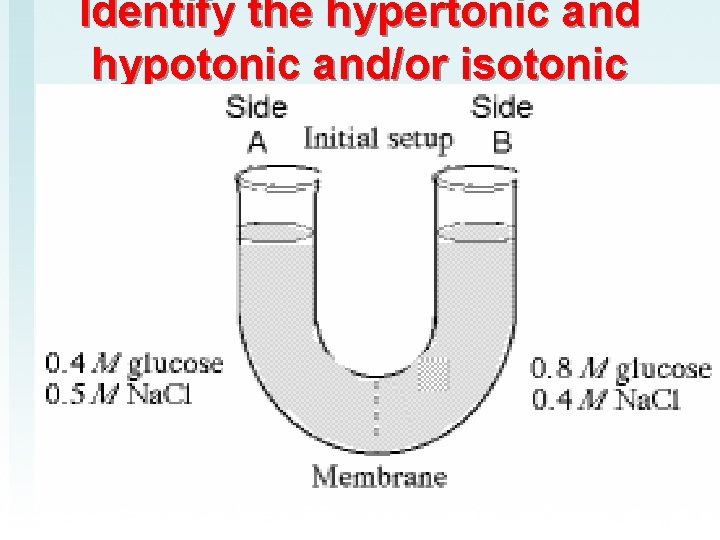
Dispatch 1) Label each side as hypertonic, hypotonic or isotonic 2) What is plasmolysis? 3) What needs to be in this lab report? 4) What would you ask a former Lawndale student who is now in college?

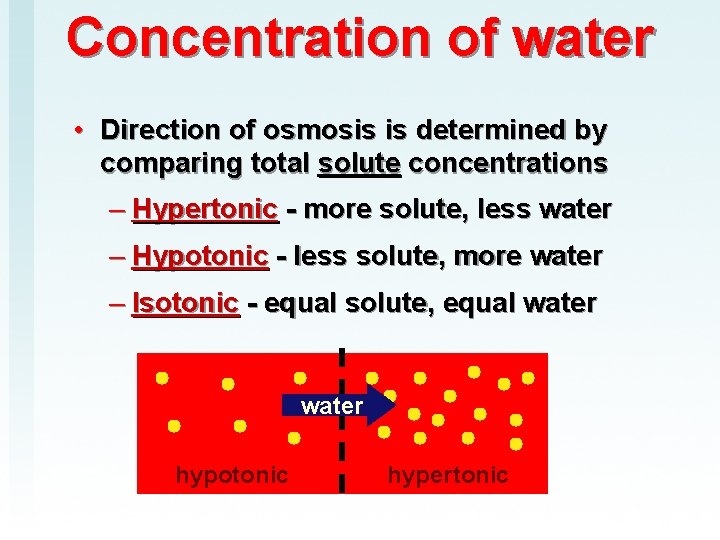
Concentration of water • Direction of osmosis is determined by comparing total solute concentrations – Hypertonic - more solute, less water – Hypotonic - less solute, more water – Isotonic - equal solute, equal water hypotonic hypertonic

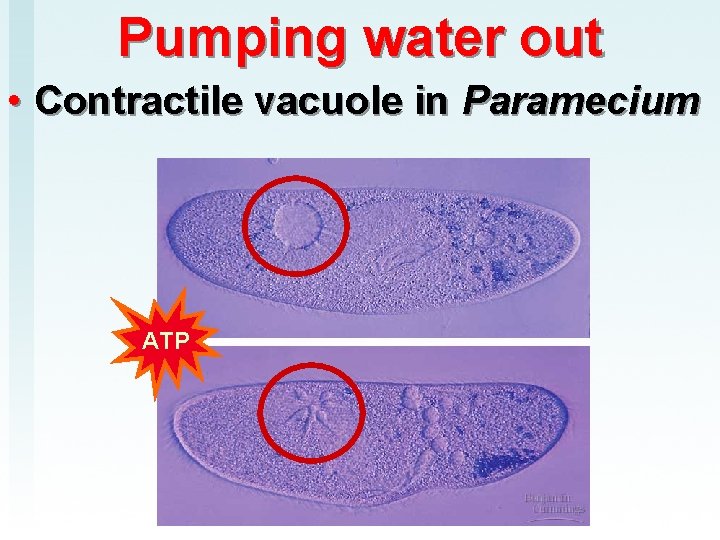
Pumping water out • Contractile vacuole in Paramecium ATP

Dispatch 1) Draw and explain exocytosis. Use the book pg 138 2) What would Darwin say about genetic engineering? Use book pg 397 and your knowledge of Darwin 3) What would Darwin say about Hardy-Weinberg? 3) The allele frequency of dominant is 0. 7, what percent of population is heterozygous? Take out FRQ paper


• • • PROBLEM ONE: The initial molar concentration of the cytoplasm inside a cell is 2 M and the cell is placed in a solution with a concentration of 2. 5 M. Initially, free energy is greater inside the cell than outside It is possible that this cell is already in equilibrium with its surroundings. Initially, solute concentration is greater outside the cell than inside. Water will enter the cell because solute potential is lower inside the cell than outside. The cell will become flaccid because the pressure potential is greater outside the cell than inside. The cell is already in equilibrium with its surroundings because of the combination of pressure potential and solute potential inside and outside the cell. Initially, the cytoplasm is hypertonic to the surrounding solution. Initially, the numerical value of the solute potential is more negative inside the cell than outside. Net diffusion of water will be from inside the cell to outside the cell. At equilibrium, the molarity of the cytoplasm will have increased. At equilibrium, the pressure potential inside the cell will have increased.

• • • • PROBLEM ONE: A B The cell must lose water to reach equilibrium. There is no way pressure can build up to bring the cell to equilibrium. A B Water will leave the cell because solute potential is higher inside the cell than outside. B The cell will become more flaccid because the solute potential is greater inside the cell than outside. B The cell is not in equilibrium because there is no pressure potential inside the cell and none will build up when water leaves the cell. B . . . cell is hypotonic to the surrounding solution. B . . . more negative outside the cell than inside. A A B . . . pressure potential inside the cell will remain zero.

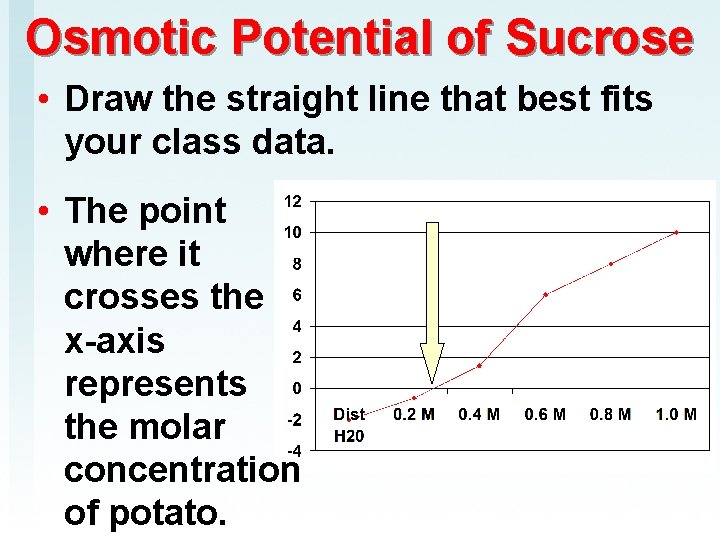
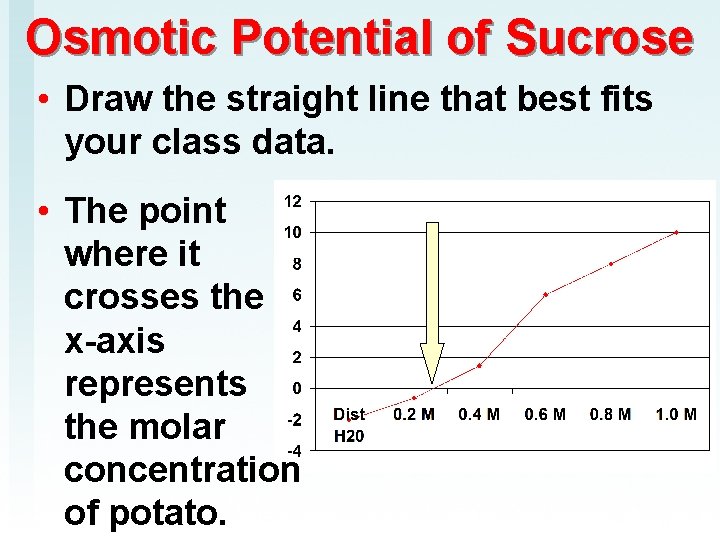
Osmotic Potential of Sucrose • Draw the straight line that best fits your class data. • The point where it crosses the x-axis represents the molar concentration of potato.

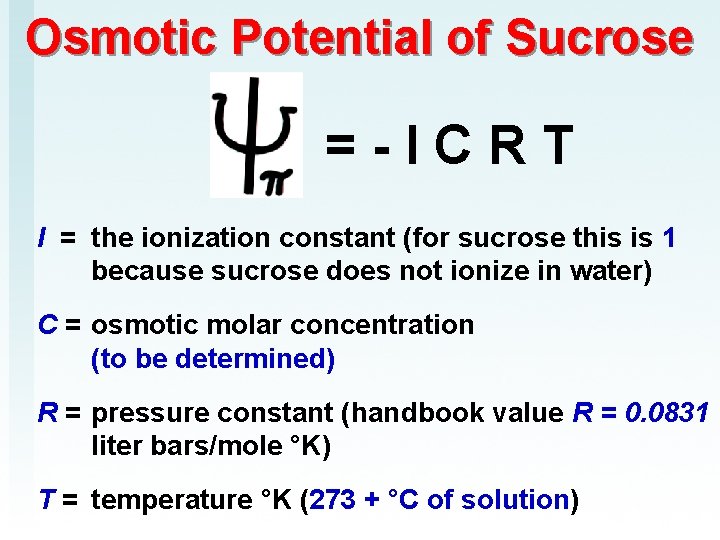
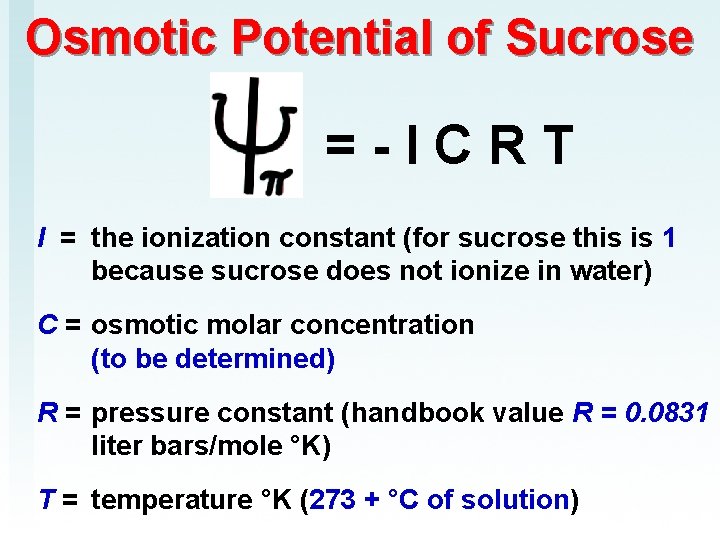
Osmotic Potential of Sucrose = - I C R T I = the ionization constant (for sucrose this is 1 because sucrose does not ionize in water) C = osmotic molar concentration (to be determined) R = pressure constant (handbook value R = 0. 0831 liter bars/mole °K) T = temperature °K (273 + °C of solution)

Designed by Anne F. Maben These images are for viewing only and may not be published in any form

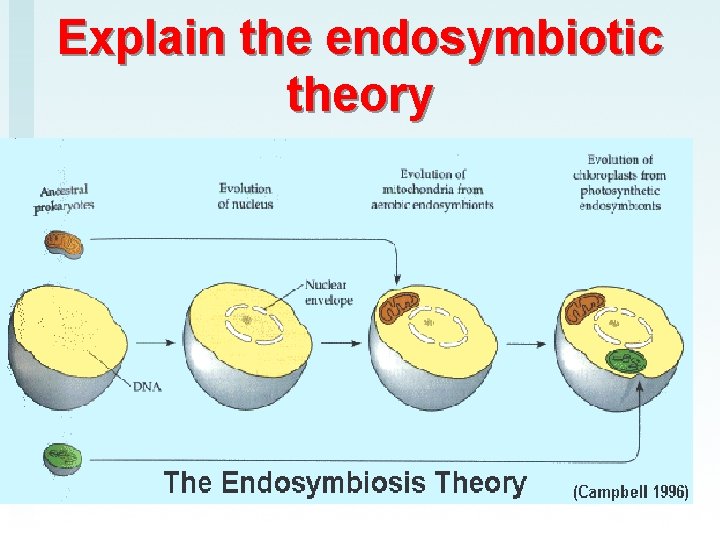
Explain the endosymbiotic theory

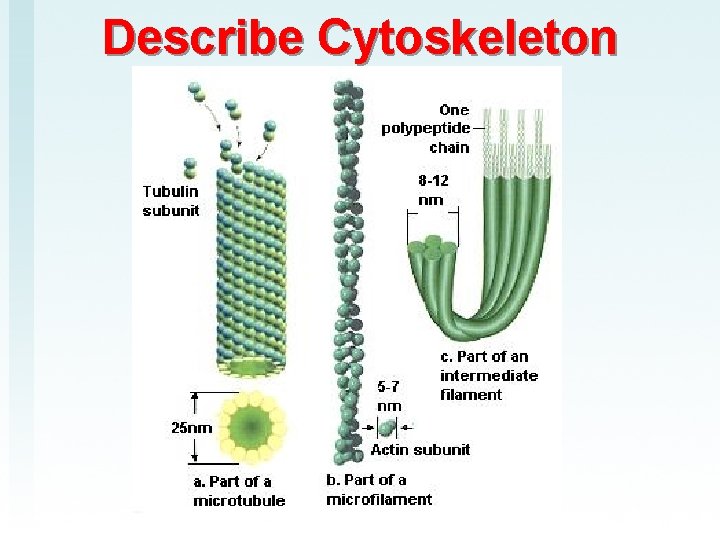
Describe Cytoskeleton

Identify the hypertonic and hypotonic and/or isotonic

Results • • • Yellow— 0. 0 M Dark green— 0. 2 M Black— 0. 4 M Blue— 0. 6 M Clear— 0. 8 M Olive green— 1. 0 M Why + 5 WORDS FROM WORD BANK

Water potential • http: //www. phschool. com/science/ biology_place/labbench/lab 1/watcal c. html

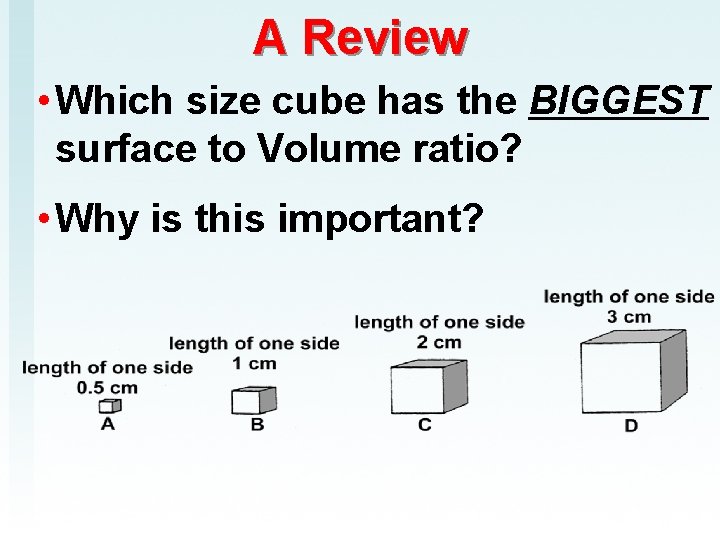
A Review • Which size cube has the BIGGEST surface to Volume ratio? • Why is this important?

Osmotic Potential of Sucrose • Draw the straight line that best fits your class data. • The point where it crosses the x-axis represents the molar concentration of potato.

Osmotic Potential of Sucrose = - I C R T I = the ionization constant (for sucrose this is 1 because sucrose does not ionize in water) C = osmotic molar concentration (to be determined) R = pressure constant (handbook value R = 0. 0831 liter bars/mole °K) T = temperature °K (273 + °C of solution)

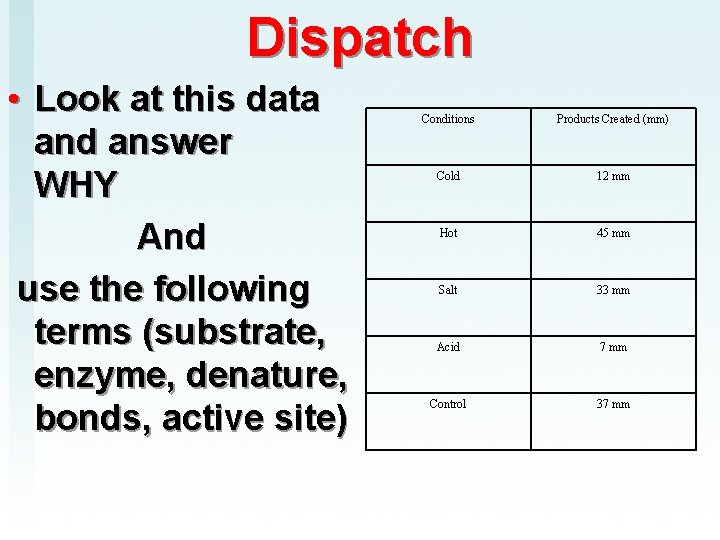
Dispatch • Look at this data and answer WHY And use the following terms (substrate, enzyme, denature, bonds, active site) Conditions Products Created (mm) Cold 12 mm Hot 45 mm Salt 33 mm Acid 7 mm Control 37 mm

Dispatch 1) A plant cell has a solute potential of – 3. 0 and a pressure potential of 0. 0. It is placed in a solution with a water potential of – 1. 0. What will happen to this plant cell? Draw and write the answer 2) Explain what would happen to a red blood cell (RBC) placed in distilled water. Be sure to include which solution has the higher water potential in your explanation and why? 3) Take out lab packet. Summarize part C—microscopes of lab

http: //icell. hudsonalpha. org/

Announcements • Retakes must be started and finished in the same session so study BEFORE hand. Ask questions • 2 B assessment moved to Dec • Poster Sympossium—Thurs/Fri • Register for ACT/SAT and get pts in this class!

Office Hours • Week of November 18 M--Nutrition T--After School W--Lunch TH--None F--Nutrition

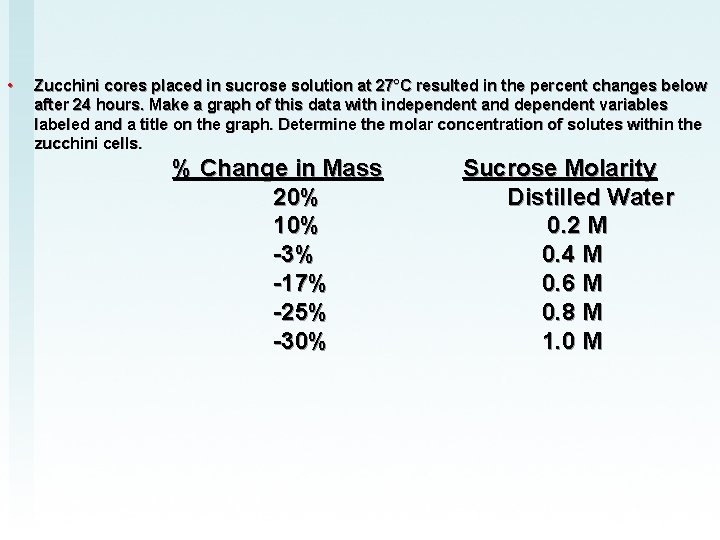
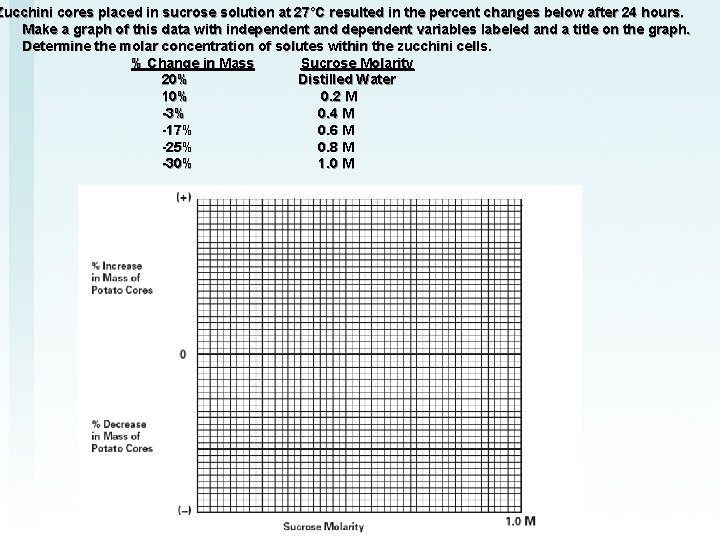
• Zucchini cores placed in sucrose solution at 27°C resulted in the percent changes below after 24 hours. Make a graph of this data with independent and dependent variables labeled and a title on the graph. Determine the molar concentration of solutes within the zucchini cells. % Change in Mass Sucrose Molarity 20% Distilled Water 10% 0. 2 M -3% 0. 4 M -17% 0. 6 M -25% 0. 8 M -30% 1. 0 M

Zucchini cores placed in sucrose solution at 27°C resulted in the percent changes below after 24 hours. Make a graph of this data with independent and dependent variables labeled and a title on the graph. Determine the molar concentration of solutes within the zucchini cells. % Change in Mass Sucrose Molarity 20% Distilled Water 10% 0. 2 M -3% 0. 4 M -17% 0. 6 M -25% 0. 8 M -30% 1. 0 M

• Potatoes and Onion are living cells • Dialysis tubing was a model cell

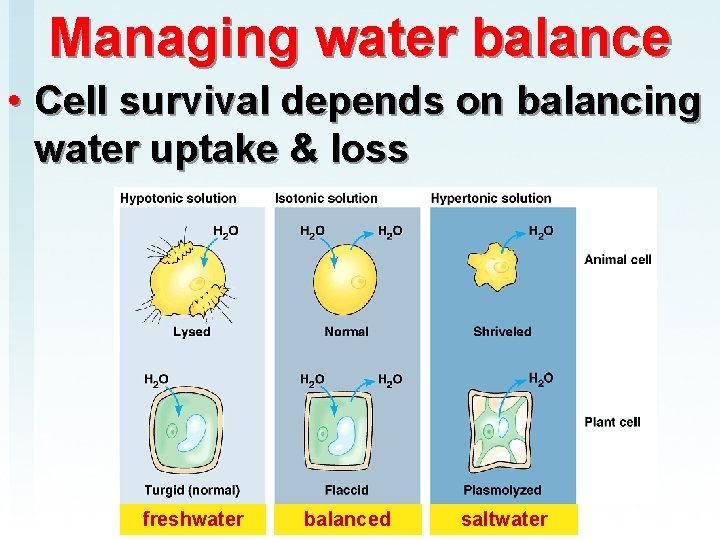
Managing water balance • Cell survival depends on balancing water uptake & loss freshwater balanced saltwater

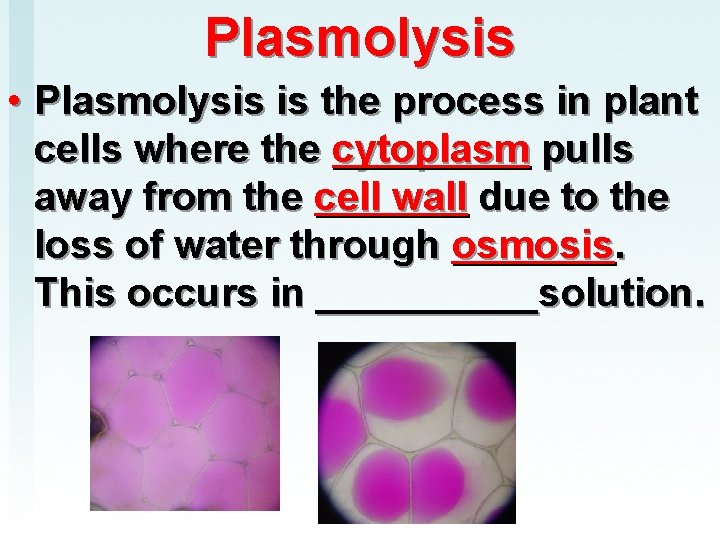
Plasmolysis • Plasmolysis is the process in plant cells where the cytoplasm pulls away from the cell wall due to the loss of water through osmosis. This occurs in _____solution.

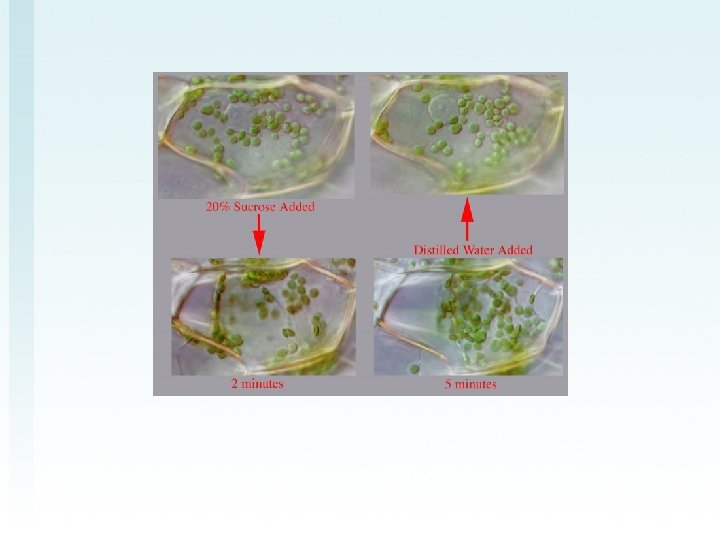
Part C—Use Elodea cells to view plasmolysis. This must be verified • PLASMOLYSIS


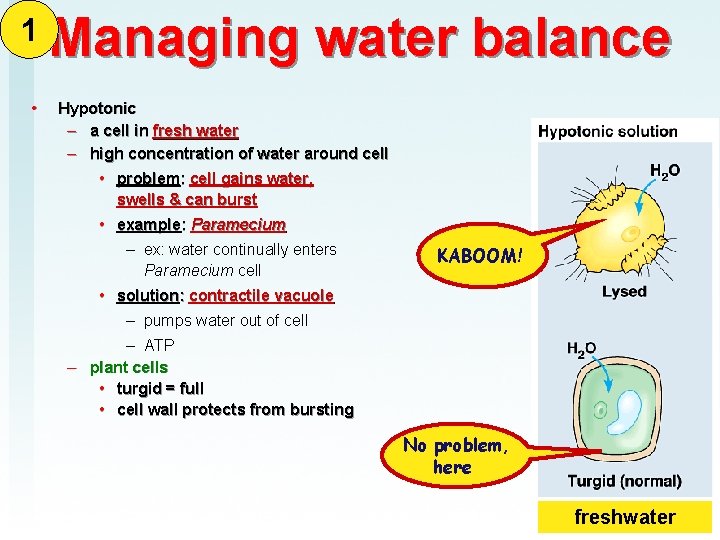
1 • Managing water balance Hypotonic – a cell in fresh water – high concentration of water around cell • problem: cell gains water, swells & can burst • example: Paramecium – ex: water continually enters Paramecium cell KABOOM! • solution: contractile vacuole – pumps water out of cell – ATP – plant cells • turgid = full • cell wall protects from bursting No problem, here freshwater

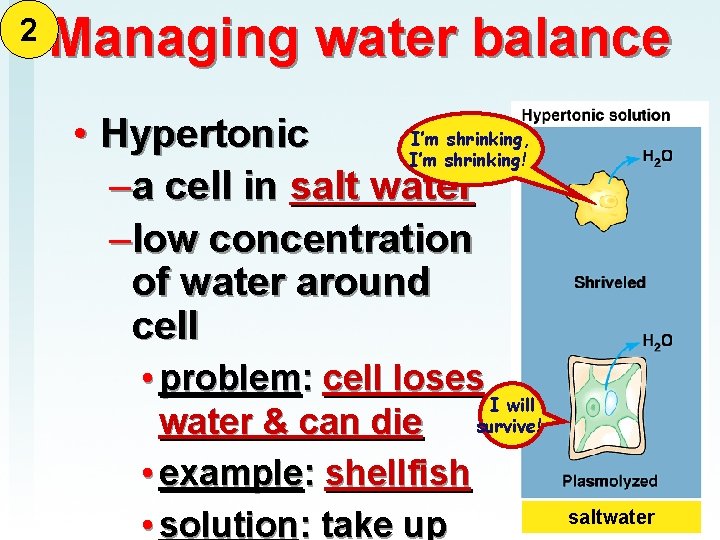
2 Managing water balance I’m shrinking, • Hypertonic I’m shrinking! –a cell in salt water –low concentration of water around cell • problem: cell loses I will water & can die survive! • example: shellfish • solution: take up saltwater

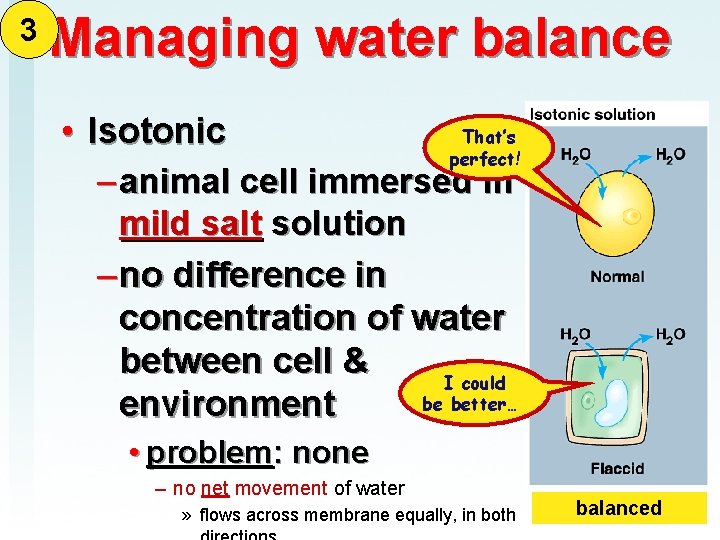
3 Managing water balance That’s • Isotonic perfect! – animal cell immersed in mild salt solution – no difference in concentration of water between cell & I could be better… environment • problem: none – no net movement of water » flows across membrane equally, in both balanced

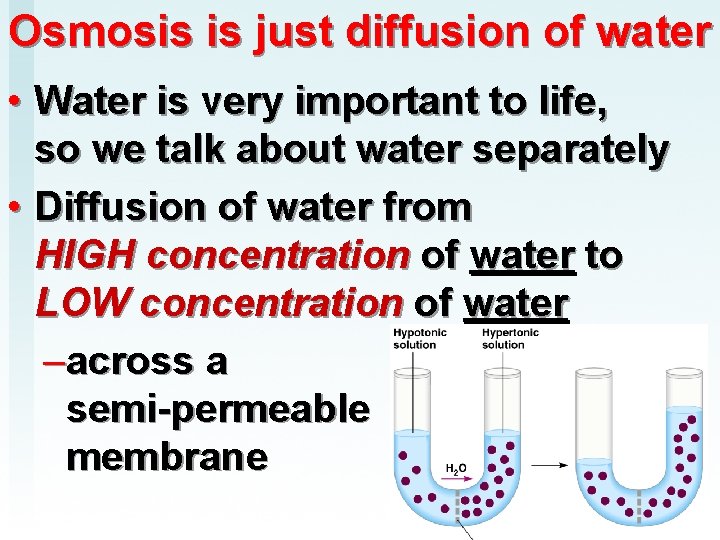
Osmosis is just diffusion of water • Water is very important to life, so we talk about water separately • Diffusion of water from HIGH concentration of water to LOW concentration of water –across a semi-permeable membrane

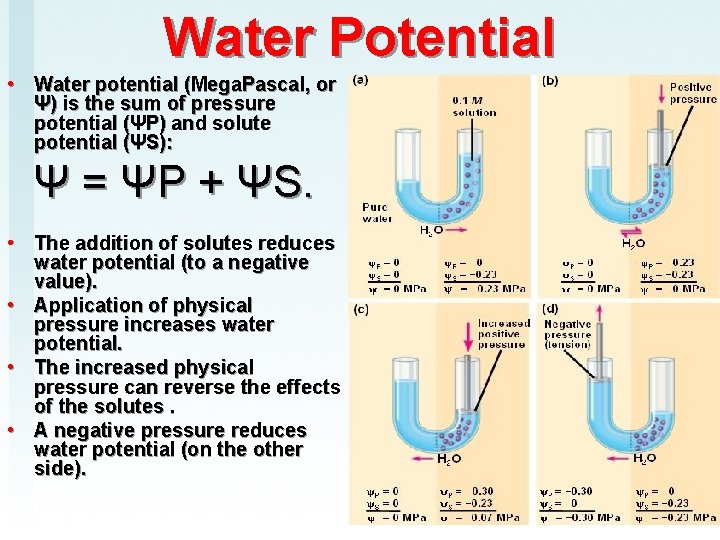
Water Potential • Water potential (Mega. Pascal, or Ψ) is the sum of pressure potential (ΨP) and solute potential (ΨS): Ψ = ΨP + ΨS. • The addition of solutes reduces water potential (to a negative value). • Application of physical pressure increases water potential. • The increased physical pressure can reverse the effects of the solutes. • A negative pressure reduces water potential (on the other side).

Tasks 1) 2) 3) 4) 5) 6) Mass potatoes for final mass Part C Microscopes and Plasmolysis in a Onion cell— 25 min Group check in with me for ticket out the door, data, calculations, questions, guess the color to M Start analysis questions Zucchini graph (this is how you will graph potatoes—look for where your line crosses 0) Test corrections + Grade Reports

Solutions • Clear • Yellow • Light green • Dark green • Turquioise • Blue

Evolution Test Answers • Pick up a key • For all of your wrong answers (QAE) Question Answer Explanation

Analysis/Conclusions: • • • Compare and contrast osmosis and diffusion. In part A, did osmosis occur? Did diffusion occur? Explain your reasoning. Did the dialysis tubing serve as a selectively permeable membrane? Explain your reasoning. In part B, what caused the mass of the dialysis bags to change? Was there more or less water in the bag at the conclusion of the experiment? Explain. Was the distilled water in the beakers hypertonic or hypotonic in relation to the sucrose solution found in the dialysis bags? Suppose the dialysis bags were placed in beakers containing a 0. 6 M sucrose solution as opposed to distilled water. How do you think your results would change? Sketch a graph below to show the mass of each of the bags would be affected. Study the graph you have plotted for part C of the experiment. What is important about the point where the best fit line crosses the x-axis? What is the concentration of sucrose in your potato? The water potential of a solution is equal to the osmotic potential plus the pressure potential. Since there is no differential pressure action on the solution the pressure potential is equal to zero, making the water potential equal to the osmotic potential. If the equilibrium point between the solutions and the potato cylinders indicates the point where the two water potentials are equal, what is the water potential of the potato cells? Would the water potential of the potato cells change if the cylinders were allowed to dry out? In what way? What are the effects on cells when they are placed in a hypotonic solution, a hypertonic solution, and an isotonic solution?

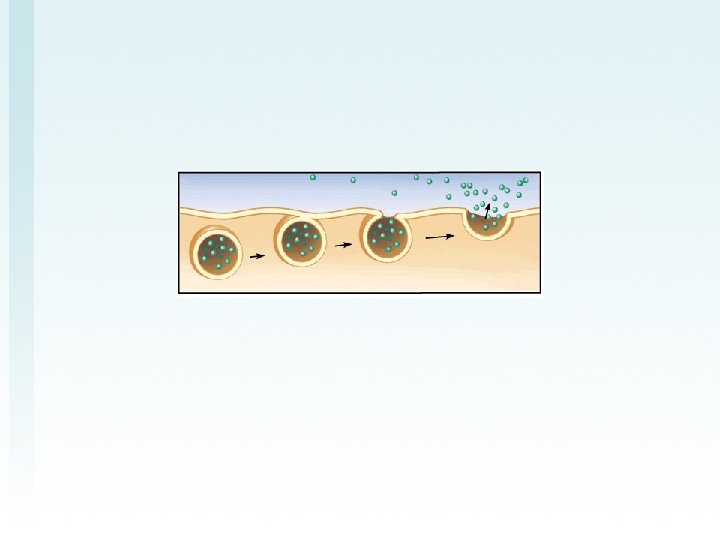
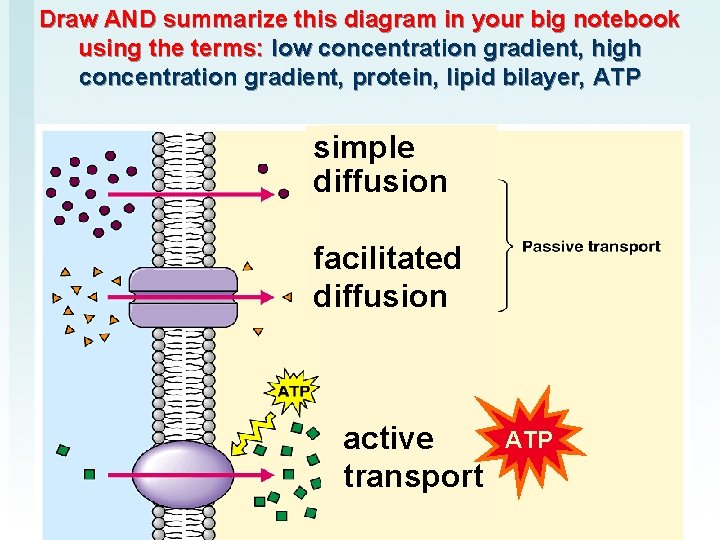
Draw AND summarize this diagram in your big notebook using the terms: low concentration gradient, high concentration gradient, protein, lipid bilayer, ATP simple diffusion facilitated diffusion active transport ATP

Copy terms Hypertonic—HIGH solute in relation to other side Hypotonic—LOW solute in relation to other side Isotonic—EQUAL solute on both side