13 5 Colligative properties v Dissolving solute in




- Slides: 26

13. 5 Colligative properties v. Dissolving solute in pure liquid will change all physical properties of liquid, Density, Vapor Pressure, Boiling Point, Freezing Point, Osmotic Pressure v Colligative Properties are properties of a liquid that change when a solute is added. v The magnitude of the change depends on the number of solute particles in the solution, not on the identity of the solute particles.

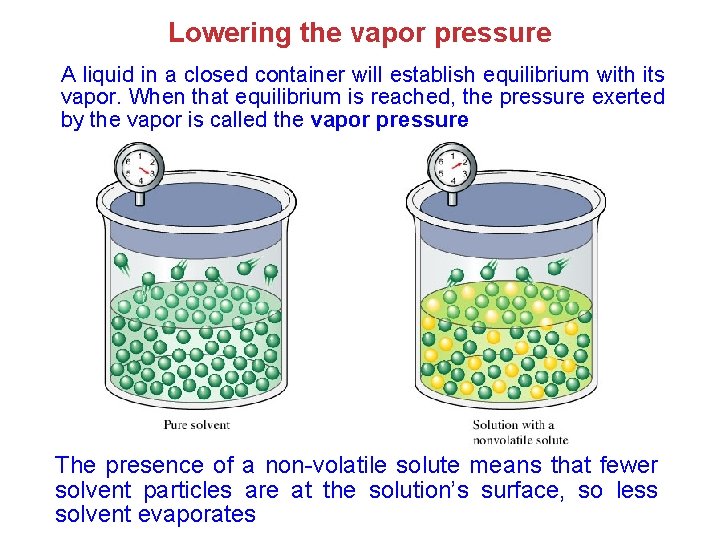
Lowering the vapor pressure A liquid in a closed container will establish equilibrium with its vapor. When that equilibrium is reached, the pressure exerted by the vapor is called the vapor pressure The presence of a non-volatile solute means that fewer solvent particles are at the solution’s surface, so less solvent evaporates

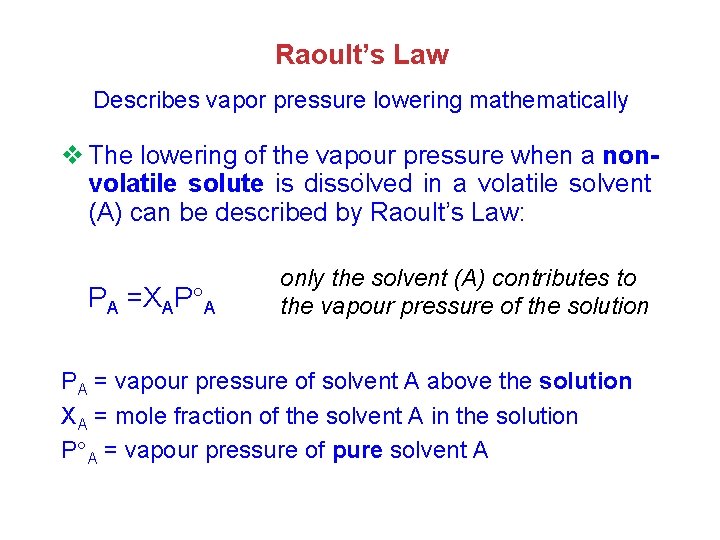
Raoult’s Law Describes vapor pressure lowering mathematically v The lowering of the vapour pressure when a non. volatile solute is dissolved in a volatile solvent (A) can be described by Raoult’s Law: PA =XAP A only the solvent (A) contributes to the vapour pressure of the solution PA = vapour pressure of solvent A above the solution XA = mole fraction of the solvent A in the solution P A = vapour pressure of pure solvent A

Example: What is the vapor pressure of water above a sucrose (MW=342. 3 g/mol) solution prepared by dissolving 158. 0 g of sucrose in 641. 6 g of water at 25 ºC? The vapor pressure of pure water at 25 ºC is 23. 76 mm. Hg. Psoln =XH 2 O P H 2 O ( ( 1 mol C 12 H 22 O 11 g Moles C 12 H 22 O 11 =(158 = 0. 462 342. 3 g C 12 H 22 O 11) mol C 12 H 22 O 11 1 mol H 2 O ( 641. 6 g H O ) Moles H 2 O = 35. 6 2 18 g H 2 O = ( mol ( ( mol H 2 O mol H 2 O + C 12 H 22 O 11 ( XH O = 2 = mol 35. 6 = 0. 987 35. 6+ 0. 462 Psoln =(0. 987)(23. 76 mm. Hg) = 23. 5 mm. Hg

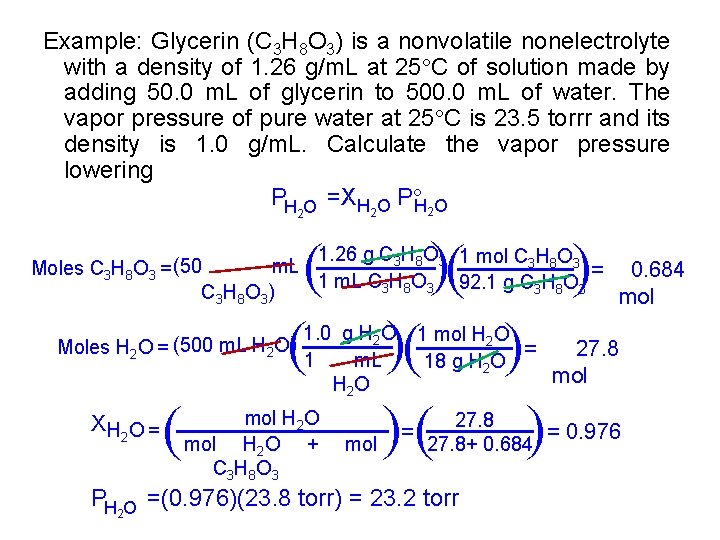
Example: Glycerin (C 3 H 8 O 3) is a nonvolatile nonelectrolyte with a density of 1. 26 g/m. L at 25 C of solution made by adding 50. 0 m. L of glycerin to 500. 0 m. L of water. The vapor pressure of pure water at 25 C is 23. 5 torrr and its density is 1. 0 g/m. L. Calculate the vapor pressure lowering PH O =XH 2 O P H 2 O 2 ( ( 1. 26 g C 3 H 8 O 3 1 mol C 3 H 8 O 3 m. L Moles C 3 H 8 O 3 = (50 = 0. 684 1 m. L C 3 H 8 O 3 92. 1 g C 3 H 8 O 3 C 3 H 8 O 3 ) mol = 1 mol H 2 O = 18 g H 2 O 27. 8 mol 27. 8 = 0. 976 27. 8+ 0. 684 PH O =(0. 976)(23. 8 torr) = 23. 2 torr 2 ( ( mol H 2 O + C 3 H 8 O 3 ( ( ( XH O = 2 1. 0 g H 2 O 1 m. L H 2 O ( Moles H 2 O = (500 m. L H 2 O)

Example: The vapor pressure of pure water at 110 C is 1070 torr. A solution of ethylene glycol and water has a vapor pressure of 1. 00 atm at 110 C. Assuming that Raoult’s law is obeyed, what is the mole fraction of ethylene glycol in the solution? PH O =XH 2 O P H 2 O = 1070 torr 2 P H 2 O = 1 atm = 760 torr P X H O = H 2 O = 760 2 P H O torr 1070 2 = 0. 710 torr C 2 H 6 O X H O + X 2 =1 2 X C H O = 1 0. 71 = 0. 290 2 6 2

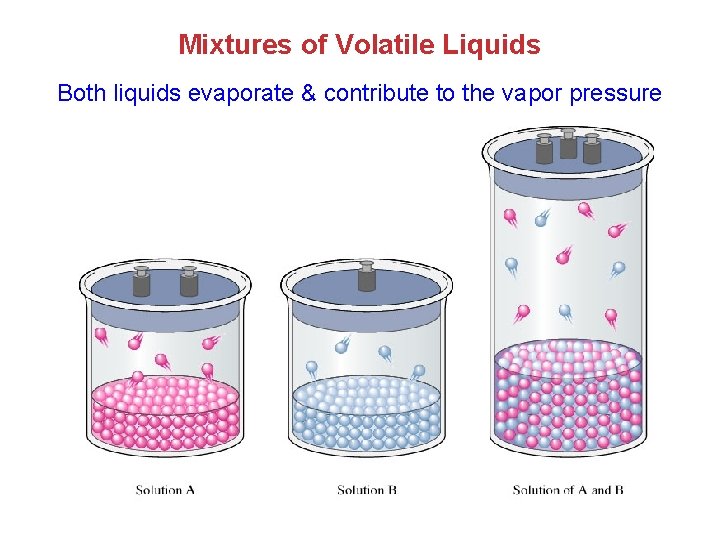
Mixtures of Volatile Liquids Both liquids evaporate & contribute to the vapor pressure

Raoult’s Law: Mixing Two Volatile Liquids Since both liquids are volatile and contribute to the vapour, the total vapor pressure can be represented using Dalton’s Law: PT = P A + P B The vapor pressure from each component follows Raoult’s Law: PT = XAP A + XBP B Also, XA + XB = 1 (since there are 2 components)

Benzene and Toluene A two solvent (volatile) system v. The vapor pressure from each component follows Raoult's Law. v. Benzene - Toluene mixture: Recall that with only two components, XBz + XTol = 1 = 384 torr & Benzene: when XBz = 1, PBz = PBz when XBz = 0 , PBz = 0 Toluene: when XTol = 1, PTol = P Tol = 133 torr & when XTol = 0, PBz = 0

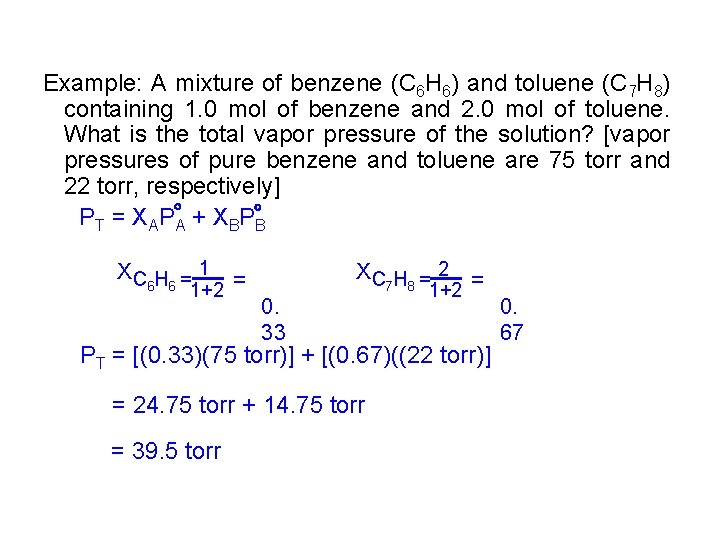
Example: A mixture of benzene (C 6 H 6) and toluene (C 7 H 8) containing 1. 0 mol of benzene and 2. 0 mol of toluene. What is the total vapor pressure of the solution? [vapor pressures of pure benzene and toluene are 75 torr and 22 torr, respectively] PT = XAP A + XBP B XC H = 1 = 6 6 1+2 0. 33 XC H = 2 = 7 8 1+2 PT = [(0. 33)(75 torr)] + [(0. 67)((22 torr)] = 24. 75 torr + 14. 75 torr = 39. 5 torr 0. 67

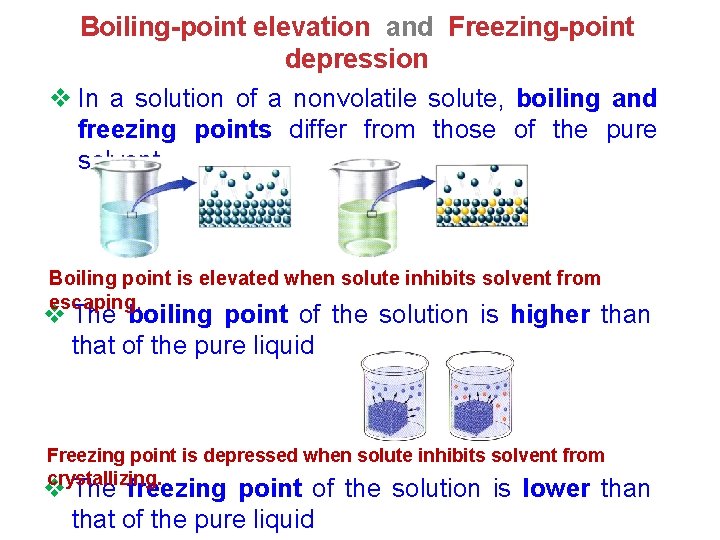
Boiling-point elevation and Freezing-point depression v In a solution of a nonvolatile solute, boiling and freezing points differ from those of the pure solvent Boiling point is elevated when solute inhibits solvent from escaping. v The boiling point of the solution is higher than that of the pure liquid Freezing point is depressed when solute inhibits solvent from crystallizing. v The freezing point of the solution is lower than that of the pure liquid

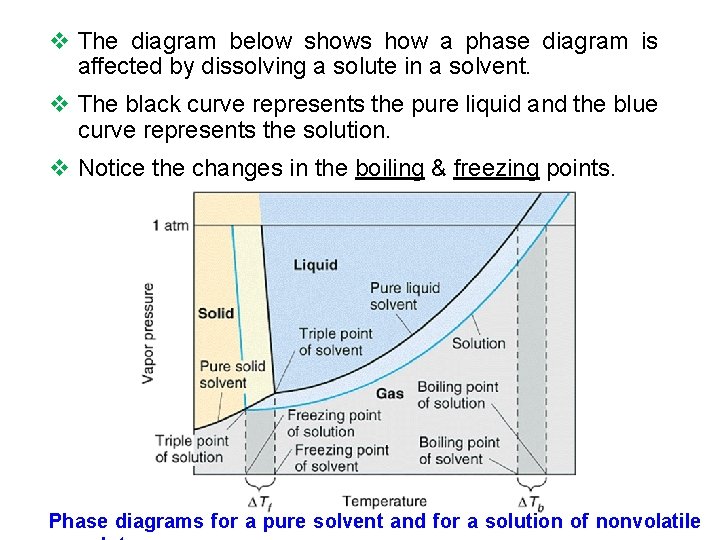
v The diagram below shows how a phase diagram is affected by dissolving a solute in a solvent. v The black curve represents the pure liquid and the blue curve represents the solution. v Notice the changes in the boiling & freezing points. Phase diagrams for a pure solvent and for a solution of nonvolatile

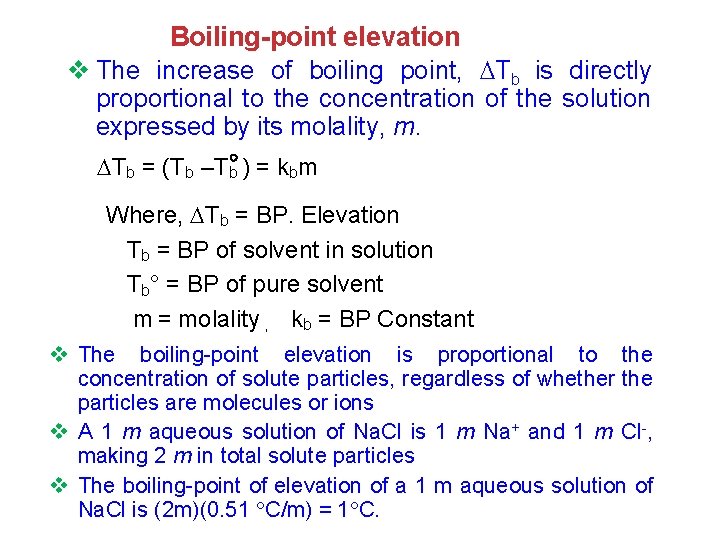
Boiling-point elevation v The increase of boiling point, Tb is directly proportional to the concentration of the solution expressed by its molality, m. Tb = (Tb –Tb ) = kbm Where, Tb = BP. Elevation Tb = BP of solvent in solution Tb° = BP of pure solvent m = molality , kb = BP Constant v The boiling-point elevation is proportional to the concentration of solute particles, regardless of whether the particles are molecules or ions v A 1 m aqueous solution of Na. Cl is 1 m Na+ and 1 m Cl-, making 2 m in total solute particles v The boiling-point of elevation of a 1 m aqueous solution of Na. Cl is (2 m)(0. 51 C/m) = 1 C.

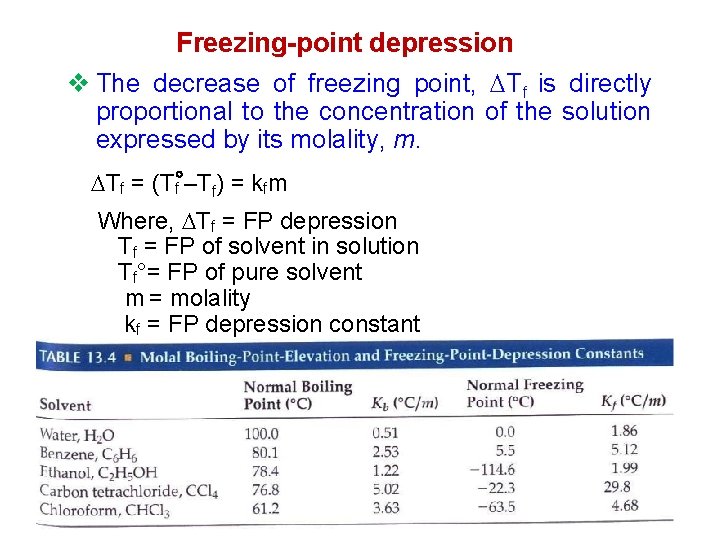
Freezing-point depression v The decrease of freezing point, Tf is directly proportional to the concentration of the solution expressed by its molality, m. Tf = (Tf –Tf) = kfm Where, Tf = FP depression Tf = FP of solvent in solution Tf°= FP of pure solvent m = molality kf = FP depression constant

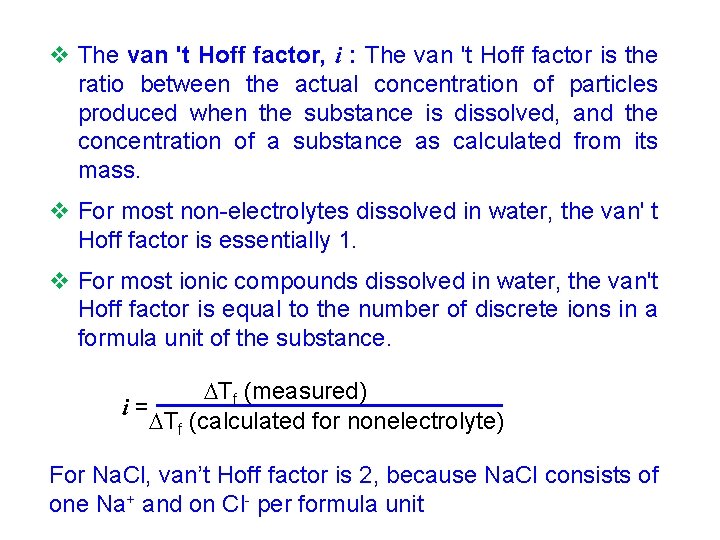
v The van 't Hoff factor, i : The van 't Hoff factor is the ratio between the actual concentration of particles produced when the substance is dissolved, and the concentration of a substance as calculated from its mass. v For most non-electrolytes dissolved in water, the van' t Hoff factor is essentially 1. v For most ionic compounds dissolved in water, the van't Hoff factor is equal to the number of discrete ions in a formula unit of the substance. Tf (measured) i= Tf (calculated for nonelectrolyte) For Na. Cl, van’t Hoff factor is 2, because Na. Cl consists of one Na+ and on Cl- per formula unit

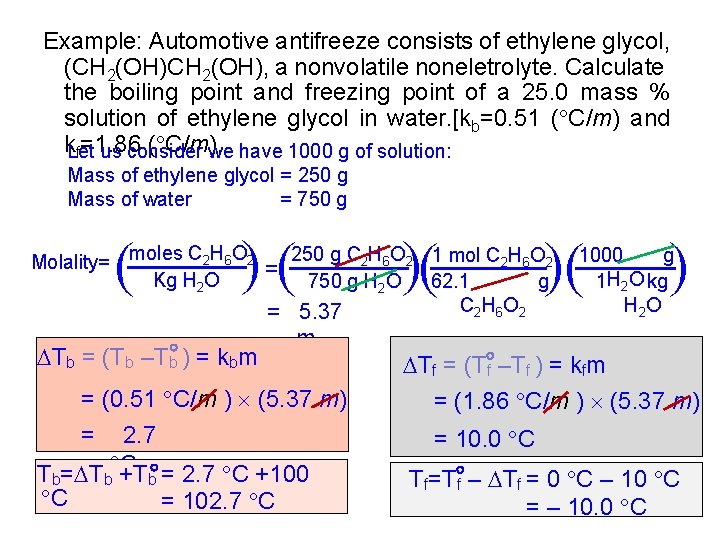
Example: Automotive antifreeze consists of ethylene glycol, (CH 2(OH), a nonvolatile noneletrolyte. Calculate the boiling point and freezing point of a 25. 0 mass % solution of ethylene glycol in water. [kb=0. 51 ( C/m) and k. Let ( C/m). us consider we have 1000 g of solution: f=1. 86 Mass of ethylene glycol = 250 g Mass of water = 750 g ( ( ( m ( = (0. 51 C/m ) (5. 37 m) = 2. 7 Tb= Tb C +Tb = 2. 7 C +100 C = 102. 7 C ( Tb = (Tb –Tb ) = kbm ( ( ( Molality= moles C 2 H 6 O 2 = 250 g C 2 H 6 O 2 1 mol C 2 H 6 O 2 Kg H 2 O 750 g H 2 O 62. 1 g C 2 H 6 O 2 = 5. 37 1000 g 1 H 2 O kg H 2 O Tf = (T f –Tf ) = kfm = (1. 86 C/m ) (5. 37 m) = 10. 0 C Tf=T f – Tf = 0 C – 10 C = – 10. 0 C

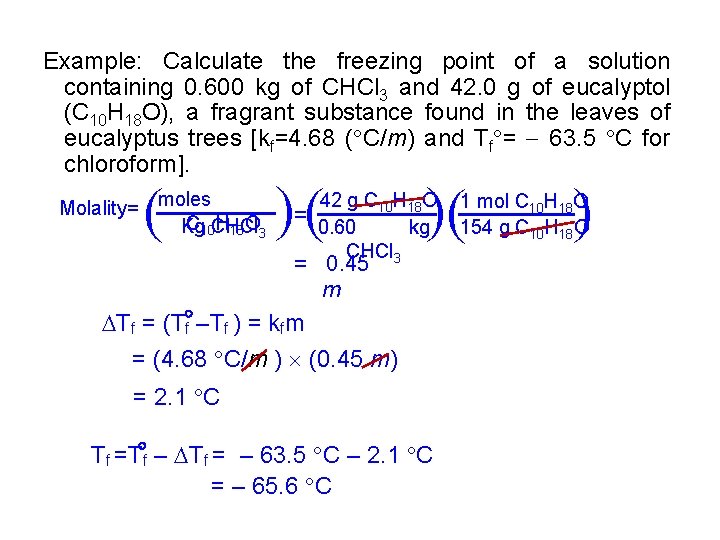
Example: Calculate the freezing point of a solution containing 0. 600 kg of CHCl 3 and 42. 0 g of eucalyptol (C 10 H 18 O), a fragrant substance found in the leaves of eucalyptus trees [kf=4. 68 ( C/m) and Tf = 63. 5 C for chloroform]. ( ( ( 42 g C 10 H 18 O = 0. 60 kg CHCl 3 = 0. 45 m Tf = (T f –Tf ) = kfm = (4. 68 C/m ) (0. 45 m) = 2. 1 C Tf =T f – Tf = – 63. 5 C – 2. 1 C = – 65. 6 C ( ( ( Molality= moles C 10 CHCl H 18 O 3 Kg 1 mol C 10 H 18 O 154 g C 10 H 18 O

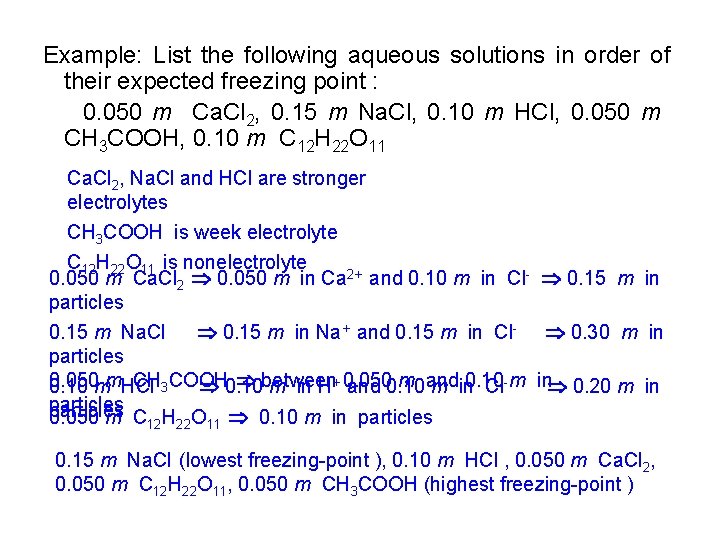
Example: List the following aqueous solutions in order of their expected freezing point : 0. 050 m Ca. Cl 2, 0. 15 m Na. Cl, 0. 10 m HCl, 0. 050 m CH 3 COOH, 0. 10 m C 12 H 22 O 11 Ca. Cl 2, Na. Cl and HCl are stronger electrolytes CH 3 COOH is week electrolyte C 12 H 22 O 11 is nonelectrolyte 0. 050 m Ca. Cl 2 0. 050 m in Ca 2+ and 0. 10 m in Cl- 0. 15 m in particles 0. 15 m Na. Cl 0. 15 m in Na+ and 0. 15 m in Cl- 0. 30 m in particles 0. 050 CH 3 COOH between m and 0. 10 mm. HCl 0. 10 m in H+ 0. 050 and 0. 10 m in 0. 10 Cl- m in 0. 20 m in particles 0. 050 m C H O 0. 10 m in particles 12 22 11 0. 15 m Na. Cl (lowest freezing-point ), 0. 10 m HCl , 0. 050 m Ca. Cl 2, 0. 050 m C 12 H 22 O 11, 0. 050 m CH 3 COOH (highest freezing-point )

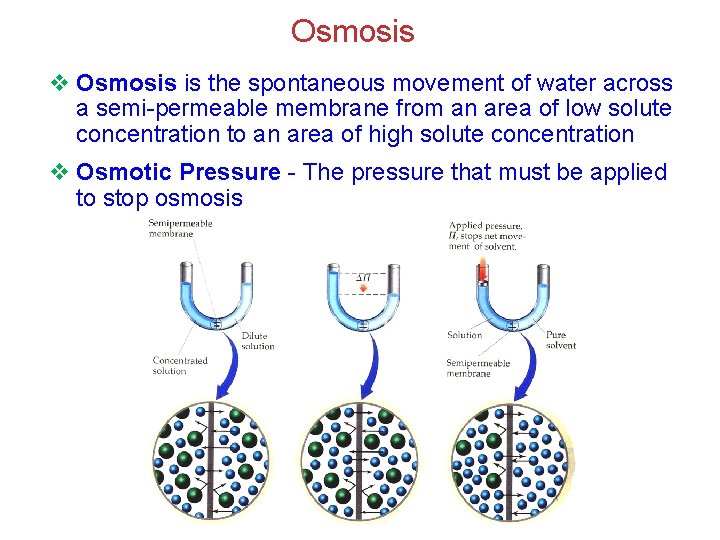
Osmosis v Osmosis is the spontaneous movement of water across a semi-permeable membrane from an area of low solute concentration to an area of high solute concentration v Osmotic Pressure - The pressure that must be applied to stop osmosis

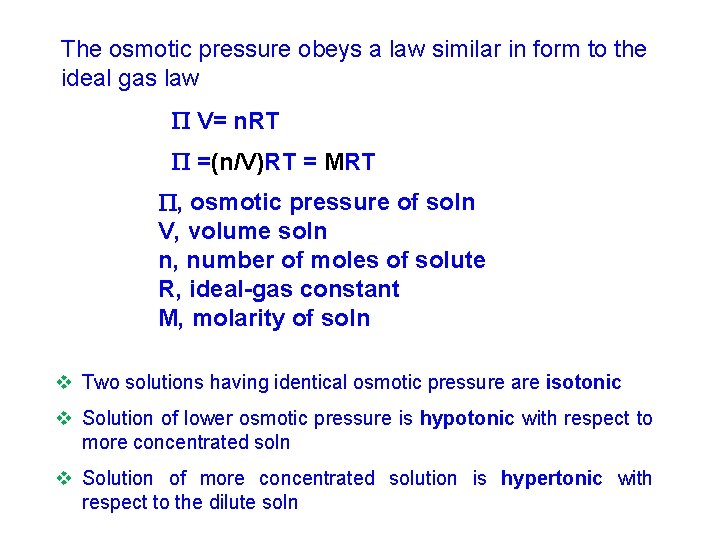
The osmotic pressure obeys a law similar in form to the ideal gas law V= n. RT =(n/V)RT = MRT , osmotic pressure of soln V, volume soln n, number of moles of solute R, ideal-gas constant M, molarity of soln v Two solutions having identical osmotic pressure are isotonic v Solution of lower osmotic pressure is hypotonic with respect to more concentrated soln v Solution of more concentrated solution is hypertonic with respect to the dilute soln

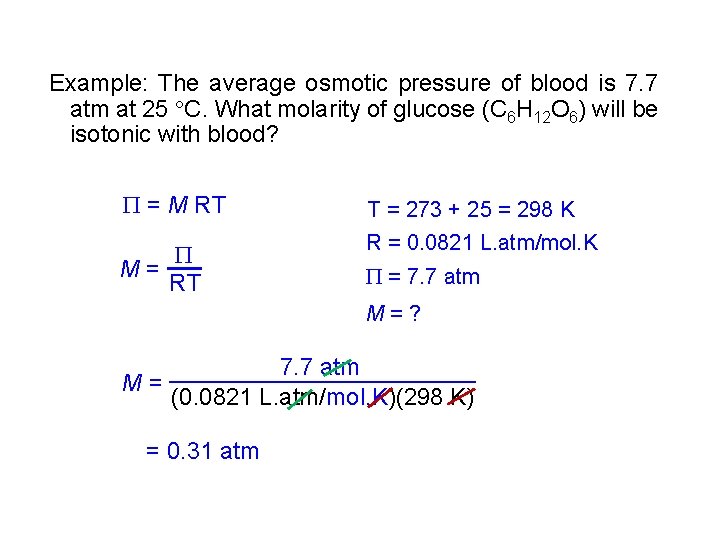
Example: The average osmotic pressure of blood is 7. 7 atm at 25 C. What molarity of glucose (C 6 H 12 O 6) will be isotonic with blood? = M RT M= RT T = 273 + 25 = 298 K R = 0. 0821 L. atm/mol. K = 7. 7 atm M=? 7. 7 atm M= (0. 0821 L. atm/mol. K)(298 K) = 0. 31 atm

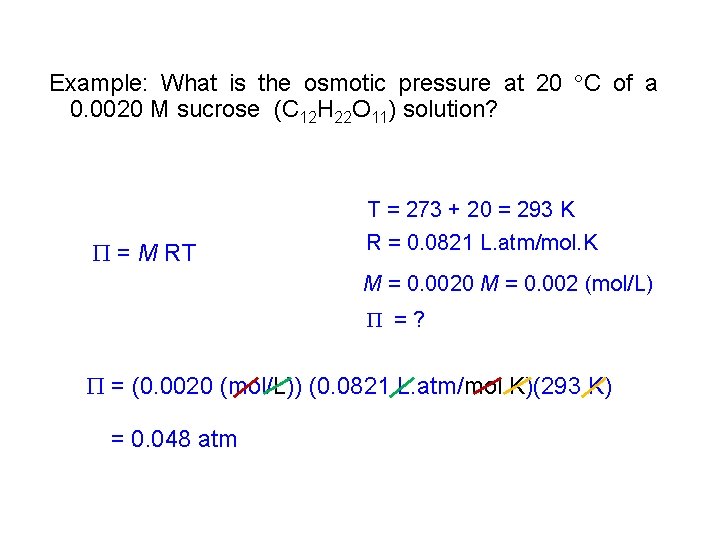
Example: What is the osmotic pressure at 20 C of a 0. 0020 M sucrose (C 12 H 22 O 11) solution? = M RT T = 273 + 20 = 293 K R = 0. 0821 L. atm/mol. K M = 0. 0020 M = 0. 002 (mol/L) =? = (0. 0020 (mol/L)) (0. 0821 L. atm/mol. K)(293 K) = 0. 048 atm

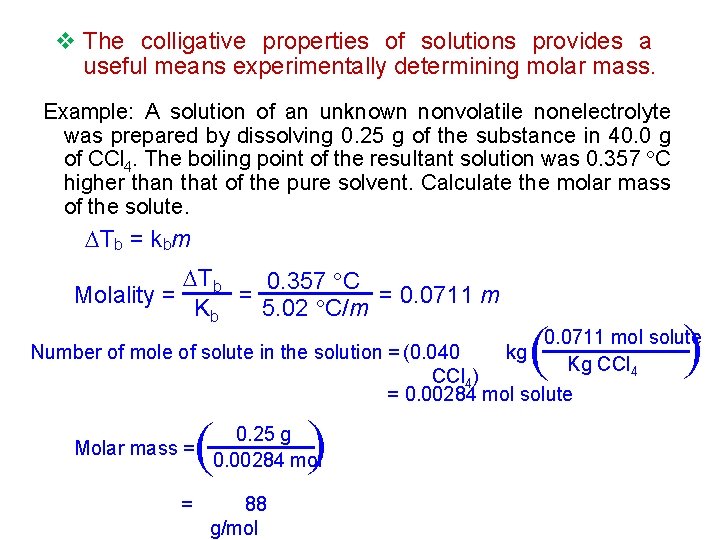
v The colligative properties of solutions provides a useful means experimentally determining molar mass. Example: A solution of an unknown nonvolatile nonelectrolyte was prepared by dissolving 0. 25 g of the substance in 40. 0 g of CCl 4. The boiling point of the resultant solution was 0. 357 C higher than that of the pure solvent. Calculate the molar mass of the solute. Tb = kbm ( ( Tb 0. 357 C = = 0. 0711 m Molality = 5. 02 C/m Kb 0. 0711 mol solute Number of mole of solute in the solution = (0. 040 kg Kg CCl 4) = 0. 00284 mol solute = ( ( Molar mass = 0. 25 g 0. 00284 mol 88 g/mol

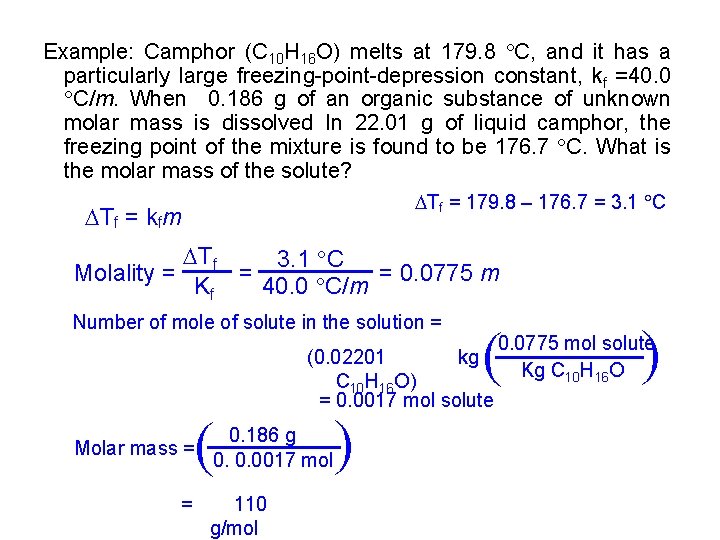
Example: Camphor (C 10 H 16 O) melts at 179. 8 C, and it has a particularly large freezing-point-depression constant, kf =40. 0 C/m. When 0. 186 g of an organic substance of unknown molar mass is dissolved In 22. 01 g of liquid camphor, the freezing point of the mixture is found to be 176. 7 C. What is the molar mass of the solute? Tf = 179. 8 – 176. 7 = 3. 1 C Tf = kfm Tf 3. 1 C = = 0. 0775 m Molality = Kf 40. 0 C/m = 0. 186 g 0. 0. 0017 mol 110 g/mol 0. 0775 mol solute kg Kg C 10 H 16 O (0. 02201 C 10 H 16 O) = 0. 0017 mol solute ( ( Molar mass = ( ( Number of mole of solute in the solution =

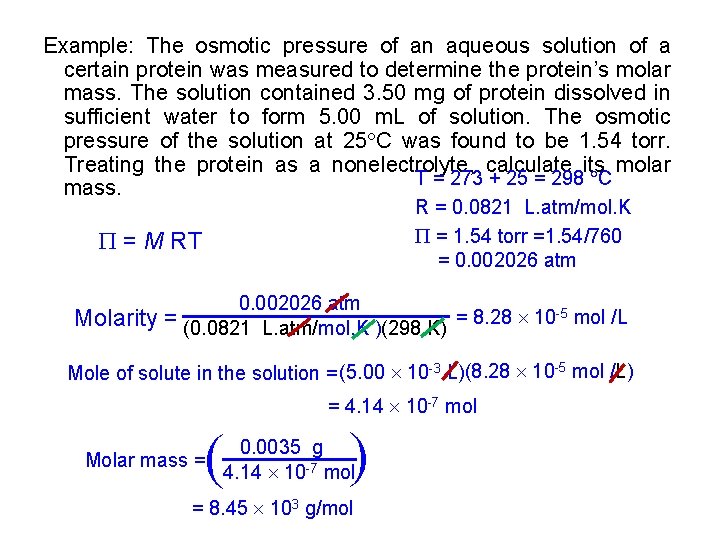
Example: The osmotic pressure of an aqueous solution of a certain protein was measured to determine the protein’s molar mass. The solution contained 3. 50 mg of protein dissolved in sufficient water to form 5. 00 m. L of solution. The osmotic pressure of the solution at 25 C was found to be 1. 54 torr. Treating the protein as a nonelectrolyte, calculate its molar T = 273 + 25 = 298 C mass. R = 0. 0821 L. atm/mol. K = 1. 54 torr =1. 54/760 = 0. 002026 atm = M RT Molarity 0. 002026 atm = (0. 0821 L. atm/mol. K )(298 K) = 8. 28 10 -5 mol /L Mole of solute in the solution = (5. 00 10 -3 L)(8. 28 10 -5 mol /L) = 4. 14 10 -7 mol ( ( Molar mass = 0. 0035 g 4. 14 10 -7 mol = 8. 45 103 g/mol

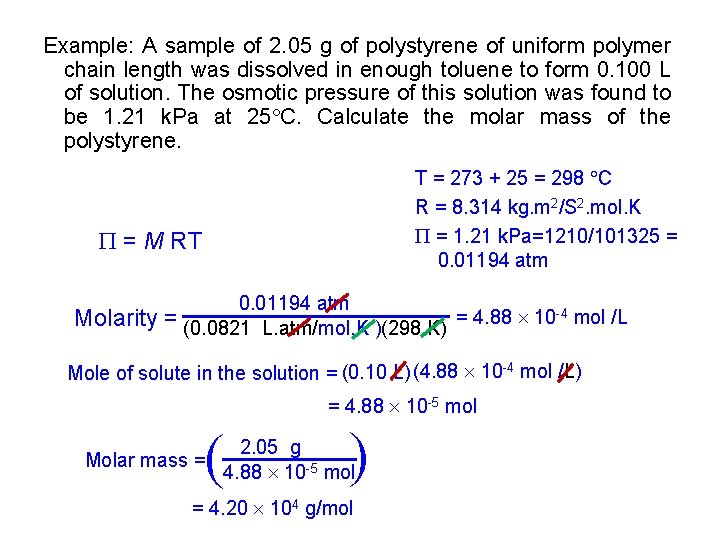
Example: A sample of 2. 05 g of polystyrene of uniform polymer chain length was dissolved in enough toluene to form 0. 100 L of solution. The osmotic pressure of this solution was found to be 1. 21 k. Pa at 25 C. Calculate the molar mass of the polystyrene. T = 273 + 25 = 298 C R = 8. 314 kg. m 2/S 2. mol. K = 1. 21 k. Pa=1210/101325 = 0. 01194 atm = M RT Molarity 0. 01194 atm = (0. 0821 L. atm/mol. K )(298 K) = 4. 88 10 -4 mol /L Mole of solute in the solution = (0. 10 L) (4. 88 10 -4 mol /L) = 4. 88 10 -5 mol ( ( Molar mass = 2. 05 g 4. 88 10 -5 mol = 4. 20 104 g/mol