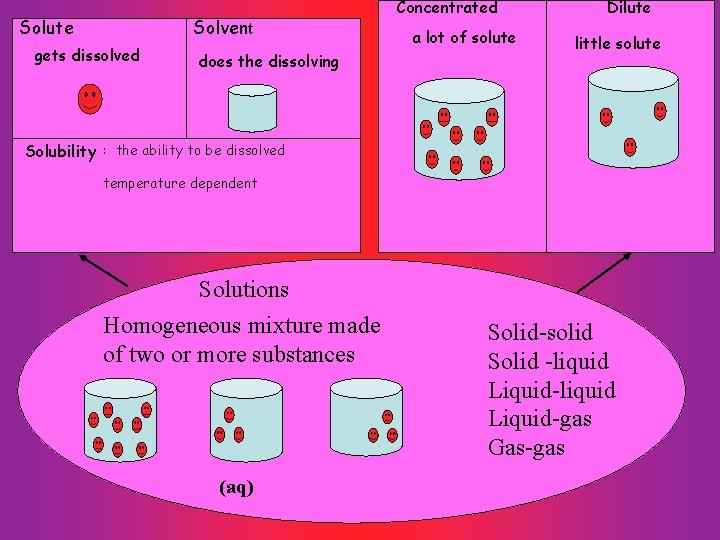
Solutions Solute Solvent gets dissolved does the dissolving




- Slides: 9

Solutions

Solute Solvent gets dissolved does the dissolving Concentrated a lot of solute Dilute little solute Solubility : the ability to be dissolved temperature dependent Solutions Homogeneous mixture made of two or more substances (aq) Solid-solid Solid -liquid Liquid-gas Gas-gas

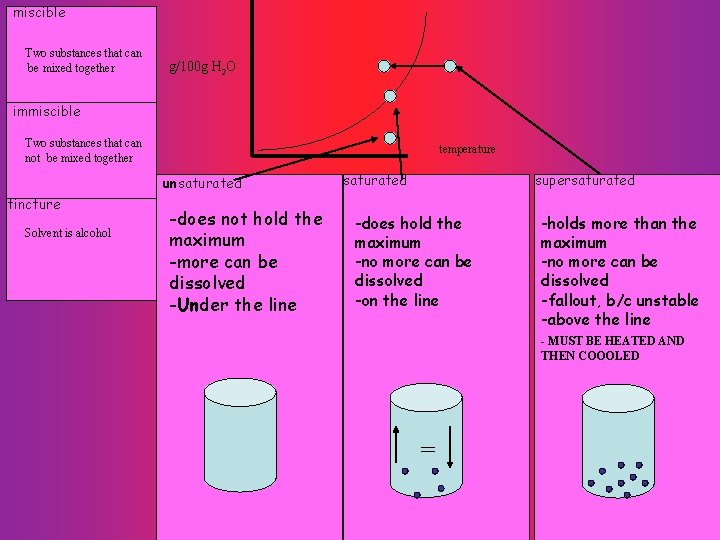
miscible Two substances that can be mixed together g/100 g H 2 O immiscible Two substances that can not be mixed together temperature tincture Solvent is alcohol -does not hold the maximum -more can be dissolved -Under the line saturated supersaturated -does hold the maximum -no more can be dissolved -on the line -holds more than the maximum -no more can be dissolved -fallout, b/c unstable -above the line - MUST BE HEATED AND THEN COOOLED = unsaturated

Table G- Solubility Curve

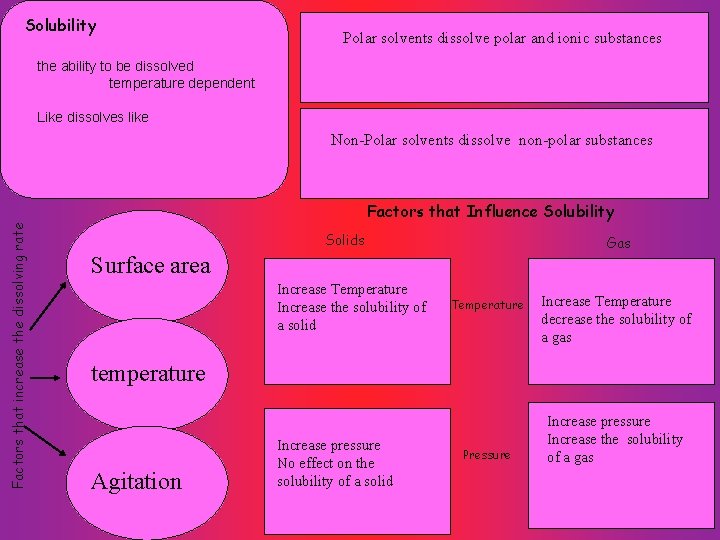
Solubility Polar solvents dissolve polar and ionic substances the ability to be dissolved temperature dependent Like dissolves like Non-Polar solvents dissolve non-polar substances Factors that increase the dissolving rate Factors that Influence Solubility Solids Gas Surface area Increase Temperature Increase the solubility of a solid Temperature Increase Temperature decrease the solubility of a gas temperature Agitation Increase pressure No effect on the solubility of a solid Pressure Increase pressure Increase the solubility of a gas

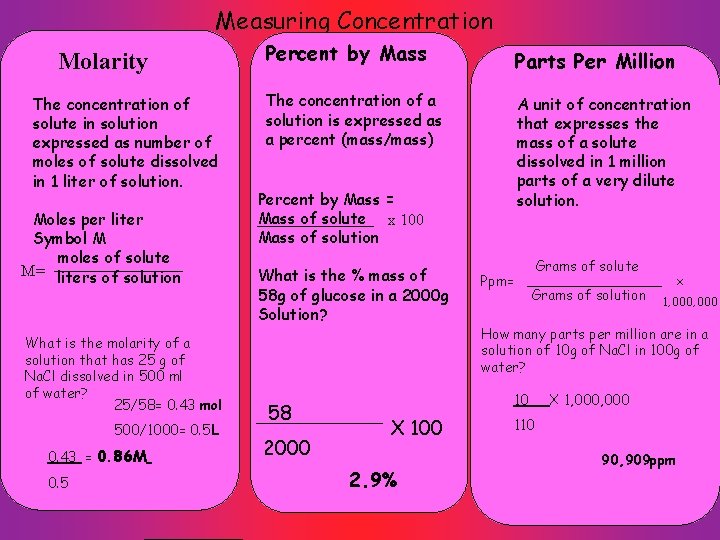
Measuring Concentration Molarity The concentration of solute in solution expressed as number of moles of solute dissolved in 1 liter of solution. Moles per liter Symbol M moles of solute M= liters of solution What is the molarity of a solution that has 25 g of Na. Cl dissolved in 500 ml of water? 25/58= 0. 43 mol 500/1000= 0. 5 L 0. 43 = 0. 86 M 0. 5 Percent by Mass Parts Per Million The concentration of a solution is expressed as a percent (mass/mass) A unit of concentration that expresses the mass of a solute dissolved in 1 million parts of a very dilute solution. Percent by Mass = Mass of solute x 100 Mass of solution What is the % mass of 58 g of glucose in a 2000 g Solution? 58 2000 Ppm= Grams of solute Grams of solution 2. 9% 1, 000 How many parts per million are in a solution of 10 g of Na. Cl in 100 g of water? 10 X 100 x X 1, 000 110 90, 909 ppm

Effect of Ionic Substances on the Boiling and freezing point : The more ions you have the higher the boiling point and the lower the freezing point!!!

Colligative Properties-- factors that determine boiling and freezing point of a liquid by the changing the concentration of solute (number of particles) Boiling Point Elevation When a nonvolatile solute is added to a solvent , the boiling point will increase When a nonvolatile solute is added to a solvent , the freezing point will decrease Freezing Point Depression Effect of Electrolytes Need to look at how many particles are produced when solute dissolved A solution that can conduct electricity due to the presence of ions Ionic-- metal/nonmetal --- splits Na. Cl --- Na+ + Cl- --- 2 particles Covalent --nonmetals -- do not split C 6 H 12 O 6 ---- 1 particle Other examples: Ba(NO 3)2 ------Ba 2+ + 2 NO 3 - -----3 particles. CH 3 OH----- does not split ---CH 3 OH The more particles you have, the greater the change one particle

Molarity by Dilution- Concentrated solutions can be mixed with solvent to make weaker or dilute solutions. In dilutions the amount of solvent is increased, but the amount of solute is kept constant. The result is a decreased concentration, but a greater volume. number moles original = number of moles final Use the following formula: M 1 V 1=M 2 V 2 M 1= original concentration V 1= original volume M 2= final concentration V 2= final volume Ex. You have 53 ml of a 1. 5 M solution of Na. Cl, but 0. 8 M solution is needed. How many ml of 0. 8 M can you make? M 1 V 1=M 2 V 2 (1. 5 M) (53 m. L) = (0. 8 M)x 79. 5 =0. 8 x 99. 4 m. L