Chapter 9 Chemical Reactions Chapter 9 Essential Questions

















































- Slides: 78

Chapter 9 Chemical Reactions

Chapter 9 Essential Questions • Key Learning • The number of atoms and mass are conserved in a chemical reaction. • Unit Essential Question • Why do different chemicals react differently when put together?

Chapter 9 Word Study Guide • Word, definition, your definition, picture • Chemical Reaction • Reactant • Product • Coefficient • Synthesis reaction • Combustion reaction • Decomposition reaction • Single-replacement reaction • Doublereplacement reaction • precipitate • Spectator ion • Net ionic equation

Chapter 9, Chemical Reactions Read, Answer, Share, pgs. 282 -285 • Answer the following • What is a chemical reaction? • What are the important indicators of chemical reactions? • Draw and label the different parts of a chemical reaction. • What is the difference between word equations, skeleton equations, and chemical equations.

Word Equation – Write the following skeleton equations for the word equations: Reactants Products • Iron + Oxygen Iron (II) Oxide • Hydrogen Peroxide Water + Oxygen • Methane + Oxygen Carbon Dioxide + Hydrogen USE YOUR PERIODIC TABLE AND POLYATOMIC IONS SHEETS

Add to your Notes • Chemical reactions • Atoms are rearranged to form different substances. • REMEMBER: Composition MUST change for a chemical reaction to have occurred!

Indicators of Chemical Reactions • • • Temperature Change Color change Odor Gas bubbles Precipitate (Solid)

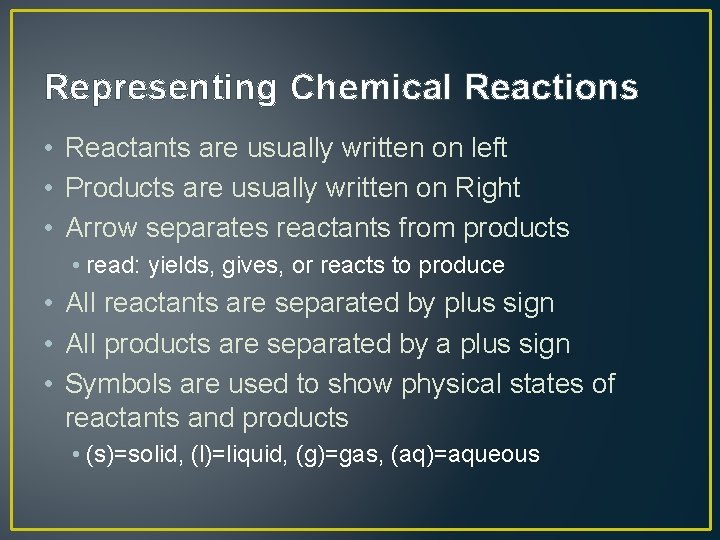
Representing Chemical Reactions • Reactants are usually written on left • Products are usually written on Right • Arrow separates reactants from products • read: yields, gives, or reacts to produce • All reactants are separated by plus sign • All products are separated by a plus sign • Symbols are used to show physical states of reactants and products • (s)=solid, (l)=liquid, (g)=gas, (aq)=aqueous

Skeleton Equation • Fe + O 2 Fe 2 O 3 • Skeleton Equations do not indicate the amounts of reactants and products.

Chemical Equation • Chemical equations must show that matter is conserved during a chemical reaction. • Al(s) + Br 2(l) Al. Br 3 • What is wrong with this equation? ? ?

Classwork • Pg 284 #’s 1 -3

Balancing Equations • Each side of the equation has same number of atoms • Steps to balancing Equations 1. Determine correct formulas 2. Write skeleton equation 3. Determine the number of atoms of each element in reactants and products 4. if polyatomic ion is on both sides of the equation, treat as a single unit 5. Use coefficients to balance 6. Begin balancing elements that appear once on both sides 7. Check to make sure same number of atoms on each side 8. Make sure you have the lowest possible ratio

Examples • H 2 + O 2 H 2 O • Ag. NO 3 + H 2 S Ag 2 S +HNO 3 • Zn(OH)2 + H 3 PO 4 Zn 3(PO 4)2 + H 2 O


Review Check Write the chemical equation (Balance) • Dicarbon dihydride + Oxygen Carbon dioxide + Water • Potassium oxide + Water Potassium hydroxide • Aluminum sulfate + Calcium hydroxide Aluminum hydroxide + Calcium sulfate

Journal • Explain each symbol used in a chemical equation. • Include reactants and products. • Page 980 Section 9. 1 #’s 1 -4

Journal – Write the Chemical Equation 1. zinc + hydrochloric acid ===> zinc chloride + hydrogen 2. copper(II) carbonate + sulfuric acid ===> copper(II) sulfate + water + carbon dioxide 3. magnesium oxide + nitric acid ===> magnesium nitrate + water

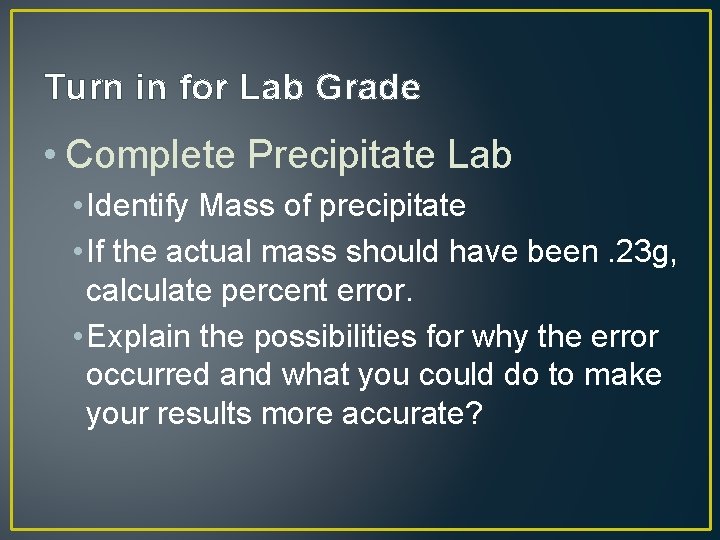
Turn in for Lab Grade • Complete Precipitate Lab • Identify Mass of precipitate • If the actual mass should have been. 23 g, calculate percent error. • Explain the possibilities for why the error occurred and what you could do to make your results more accurate?

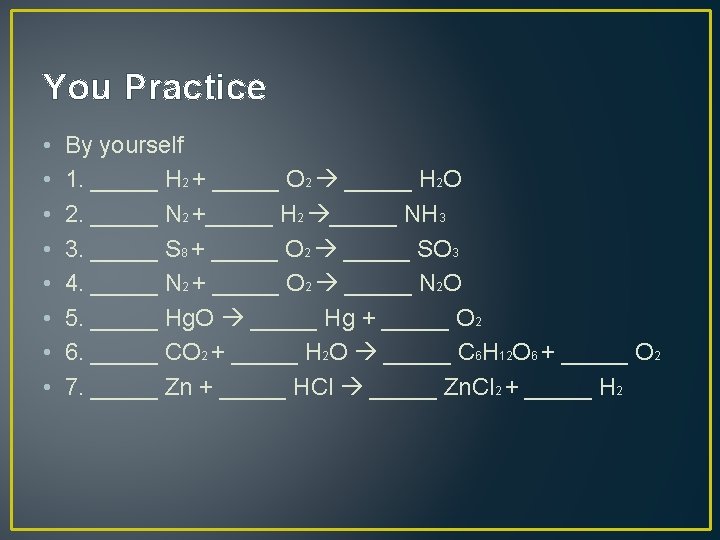
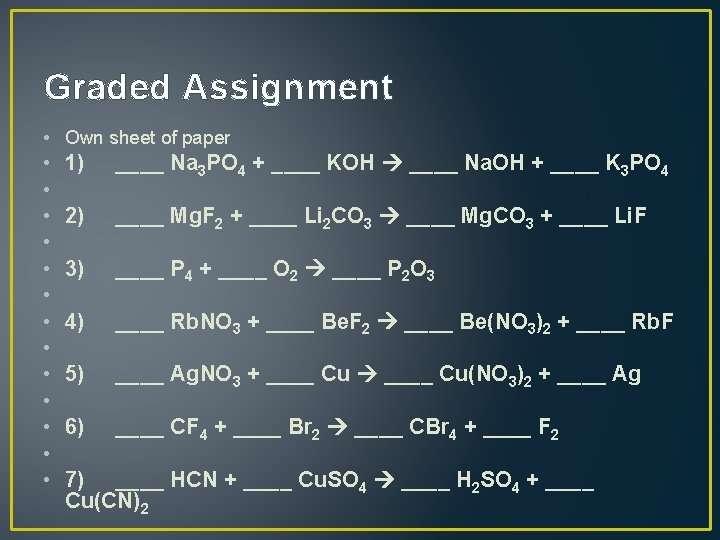
Graded Assignment • Own sheet of paper • • • • 1) ____ Na 3 PO 4 + ____ KOH ____ Na. OH + ____ K 3 PO 4 2) ____ Mg. F 2 + ____ Li 2 CO 3 ____ Mg. CO 3 + ____ Li. F 3) ____ P 4 + ____ O 2 ____ P 2 O 3 4) ____ Rb. NO 3 + ____ Be. F 2 ____ Be(NO 3)2 + ____ Rb. F 5) ____ Ag. NO 3 + ____ Cu(NO 3)2 + ____ Ag 6) ____ CF 4 + ____ Br 2 ____ CBr 4 + ____ F 2 7) ____ HCN + ____ Cu. SO 4 ____ H 2 SO 4 + ____ Cu(CN)2

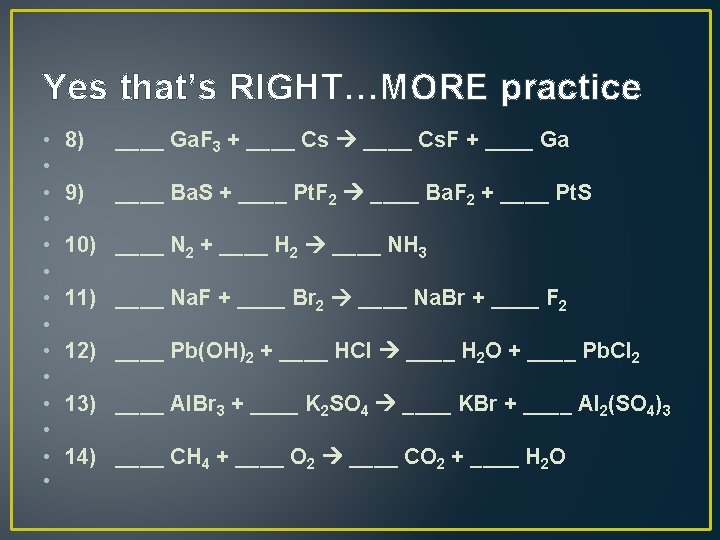
Yes that’s RIGHT…MORE practice • • • • 8) 9) 10) 11) 12) 13) 14) • ____ Ga. F 3 + ____ Cs. F + ____ Ga ____ Ba. S + ____ Pt. F 2 ____ Ba. F 2 + ____ Pt. S ____ N 2 + ____ H 2 ____ NH 3 ____ Na. F + ____ Br 2 ____ Na. Br + ____ F 2 ____ Pb(OH)2 + ____ HCl ____ H 2 O + ____ Pb. Cl 2 ____ Al. Br 3 + ____ K 2 SO 4 ____ KBr + ____ Al 2(SO 4)3 ____ CH 4 + ____ O 2 ____ CO 2 + ____ H 2 O

AND even MORE practice!!!! 15)____ Na 3 PO 4 + ____ Ca. Cl 2 ____ Na. Cl + ____ Ca 3(PO 4)2 • 16)____ K + ____ Cl 2 ____ KCl • 17)____ Al + ____ HCl ____ H 2 + ____ Al. Cl 3 • 18)____ N 2 + ____ F 2 ____ NF 3 • 19)____ SO 2 + ____ Li 2 Se ____ SSe 2 + ____ Li 2 O • 20)____ NH 3 + ____ H 2 SO 4 ____ (NH 4)2 SO 4

More Practice – Composition Book • Pg 287 #’s 4 -6 • Pg 288 #’s 7 -13

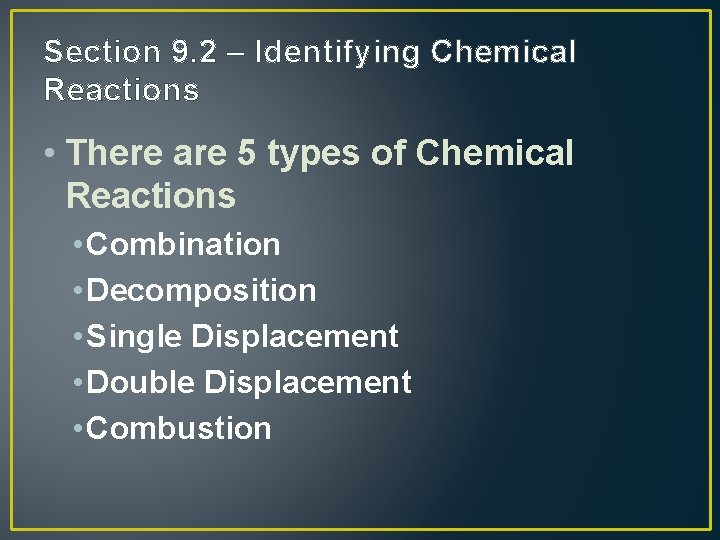
Section 9. 2 – Identifying Chemical Reactions • There are 5 types of Chemical Reactions • Combination • Decomposition • Single Displacement • Double Displacement • Combustion

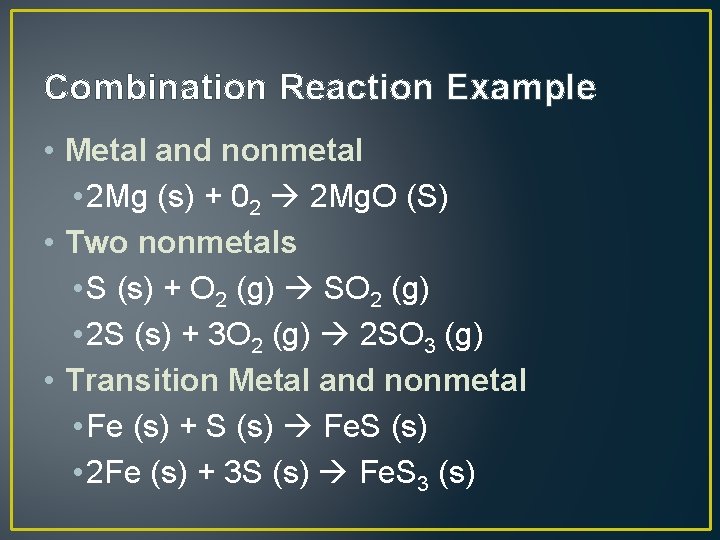
Combination Reaction • Also called synthesis • Two or more substances react to form a single new substance. • Can include • Metal and Nonmetal (only one possible product) • Two nonmetals (more than one product is possible) • Transition metal and nonmetal (more than one product is possible)

Combination Reaction Example • Metal and nonmetal • 2 Mg (s) + 02 2 Mg. O (S) • Two nonmetals • S (s) + O 2 (g) SO 2 (g) • 2 S (s) + 3 O 2 (g) 2 SO 3 (g) • Transition Metal and nonmetal • Fe (s) + S (s) Fe. S (s) • 2 Fe (s) + 3 S (s) Fe. S 3 (s)

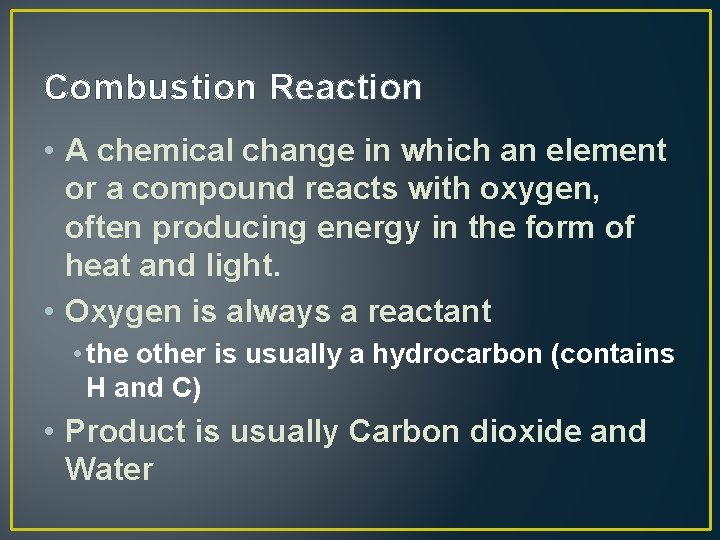
Combustion Reaction • A chemical change in which an element or a compound reacts with oxygen, often producing energy in the form of heat and light. • Oxygen is always a reactant • the other is usually a hydrocarbon (contains H and C) • Product is usually Carbon dioxide and Water

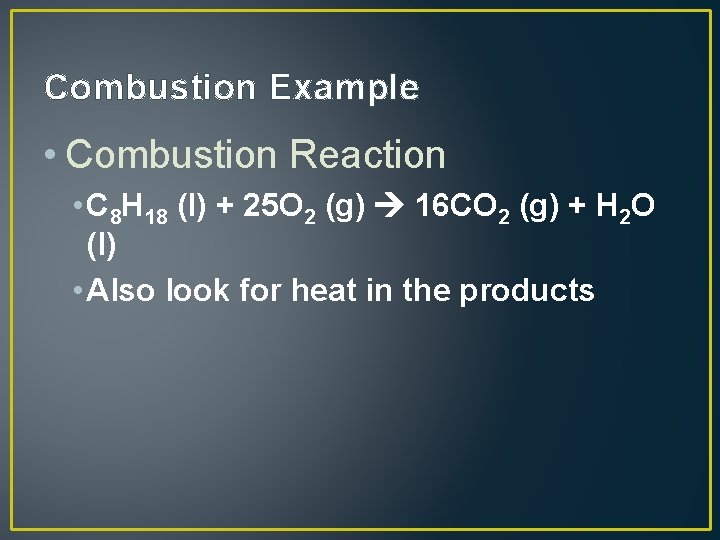
Combustion Example • Combustion Reaction • C 8 H 18 (l) + 25 O 2 (g) 16 CO 2 (g) + H 2 O (l) • Also look for heat in the products

Practice Problems –Review with shoulder partner • Page 291 • 1’s teach 2’s 14 and 15 • 2’s teach ‘s 16 and 17

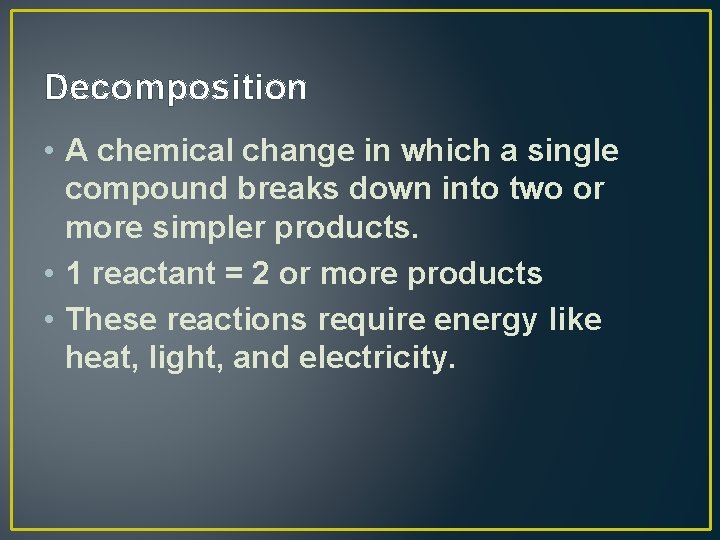
Decomposition • A chemical change in which a single compound breaks down into two or more simpler products. • 1 reactant = 2 or more products • These reactions require energy like heat, light, and electricity.

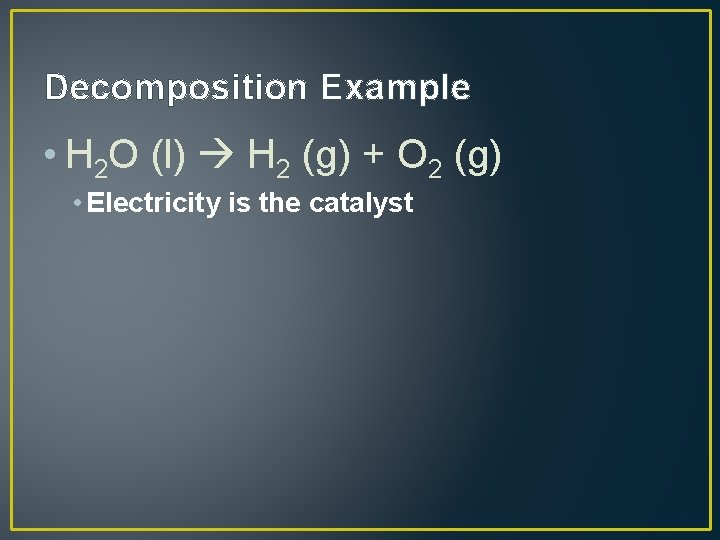
Decomposition Example • H 2 O (l) H 2 (g) + O 2 (g) • Electricity is the catalyst

Practice Problems • Page 292 • 2’s teach 1’s • 18 and 19 • 1’s teach 2’s • 20

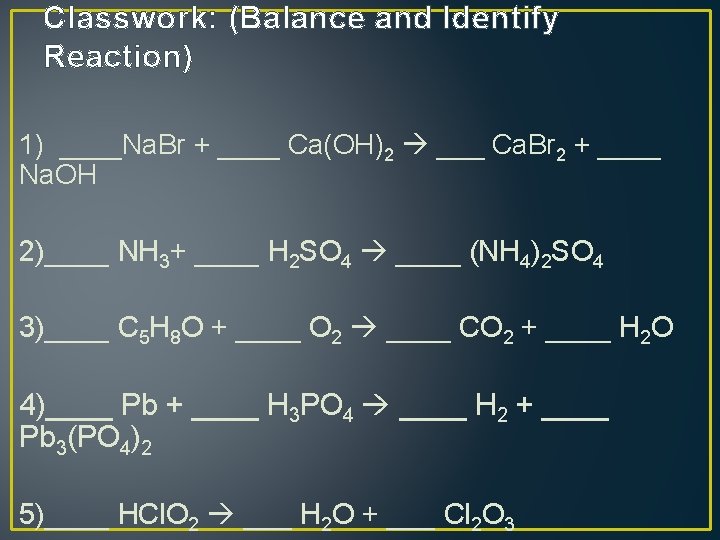
Classwork: (Balance and Identify Reaction) 1) ____Na. Br + ____ Ca(OH)2 ___ Ca. Br 2 + ____ Na. OH 2)____ NH 3+ ____ H 2 SO 4 ____ (NH 4)2 SO 4 3)____ C 5 H 8 O + ____ O 2 ____ CO 2 + ____ H 2 O 4)____ Pb + ____ H 3 PO 4 ____ H 2 + ____ Pb 3(PO 4)2 5)____ HCl. O 2 ___ H 2 O + ___ Cl 2 O 3

Single Replacement Reactions • Metal replaces H or another metal • Nonmetal can replace nonmetal • Can identify by noting that both the reactants and the products consist of an element and a compound. • Understand activity series • Page 293, figure 9. 13

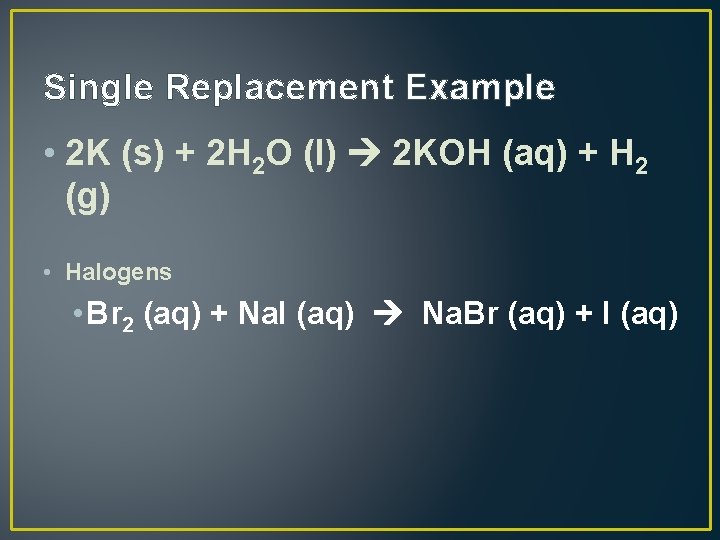
Single Replacement Example • 2 K (s) + 2 H 2 O (l) 2 KOH (aq) + H 2 (g) • Halogens • Br 2 (aq) + Na. I (aq) Na. Br (aq) + I (aq)

Review Practice Problems • Page 295 • 1’s teach 2’s • 21 and 22 • 2’s teach 1’s • 23 and 24

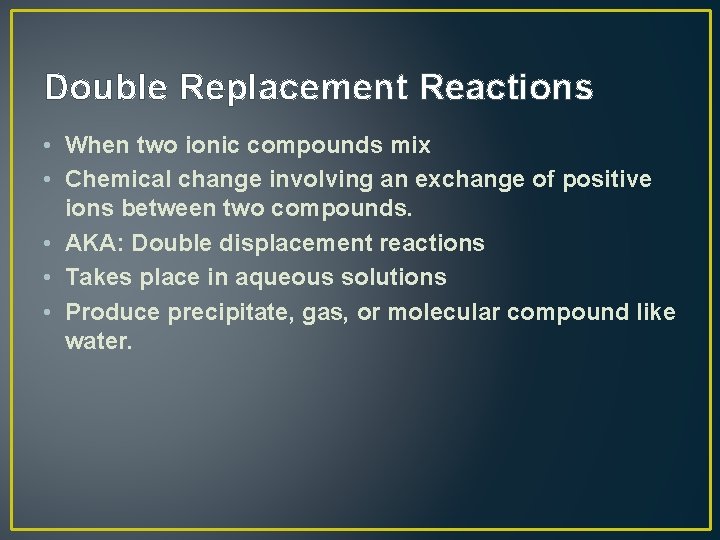
Double Replacement Reactions • When two ionic compounds mix • Chemical change involving an exchange of positive ions between two compounds. • AKA: Double displacement reactions • Takes place in aqueous solutions • Produce precipitate, gas, or molecular compound like water.

Double Replacement Example • Na 2 S (aq) + Cd(NO 3)2 (aq) Cd. S (s) + 2 Na. NO 3 (aq)

Practice Problems • Page 297 • 2’s teach 1’s 25 and 26 • 1’s teach 2’s 27 and 28

Practice Problems • Write the chemical equation and identify the type of reaction. • 1’s teach 2’s • Magnesium chloride (aq) reacts with potassium carbonate (aq) • Br 2(l) + Li. I(aq)

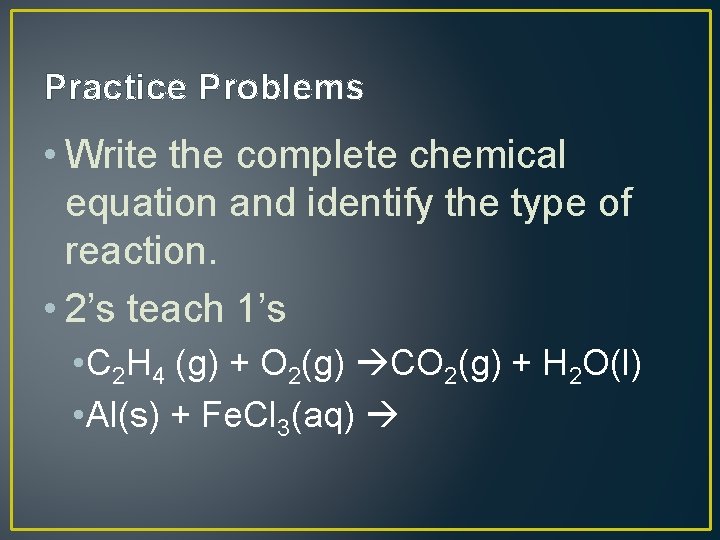
Practice Problems • Write the complete chemical equation and identify the type of reaction. • 2’s teach 1’s • C 2 H 4 (g) + O 2(g) CO 2(g) + H 2 O(l) • Al(s) + Fe. Cl 3(aq)

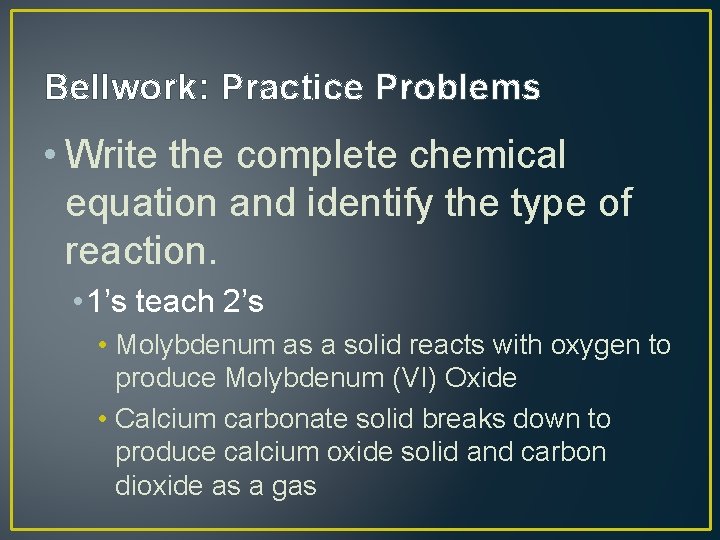
Bellwork: Practice Problems • Write the complete chemical equation and identify the type of reaction. • 1’s teach 2’s • Molybdenum as a solid reacts with oxygen to produce Molybdenum (VI) Oxide • Calcium carbonate solid breaks down to produce calcium oxide solid and carbon dioxide as a gas

Practice • Write the complete chemical equation and identify the type of reaction. • 2’s teach 1’s • Bismuth (III) nitrate(aq) reacts with Sodium sulfide(aq) • Copper solid reacts with magnesium sulfate aqueous

Complete by Yourself: Write Chemical Equation and identify the type of reaction 1. Calcium chlorate Calcium chloride + Oxygen 2. Lithium oxide + Water Lithium hydroxide 3. Aluminum + Hydrogen chloride 4. Tricarbon octahydride + Oxygen 5. Aluminum sulfate + Calcium hydroxide

Group Rotation • Instructions • Go to your numbered table • You will have 5 min to answer the questions. • When the timer goes off, you will rotate to the next table (RIGHT) • After you have been at each table • Go to your new group • Come up with a consensus for each answer • 1 paper turned in for the group

Quiz • Write complete chemical equations for the following reactions and identify the type of reaction 1. Tetracarbon hexahydride + Oxygen 2. Sodium phosphate + calcium chloride 3. Aluminum Hydroxide + Calcium 4. Water

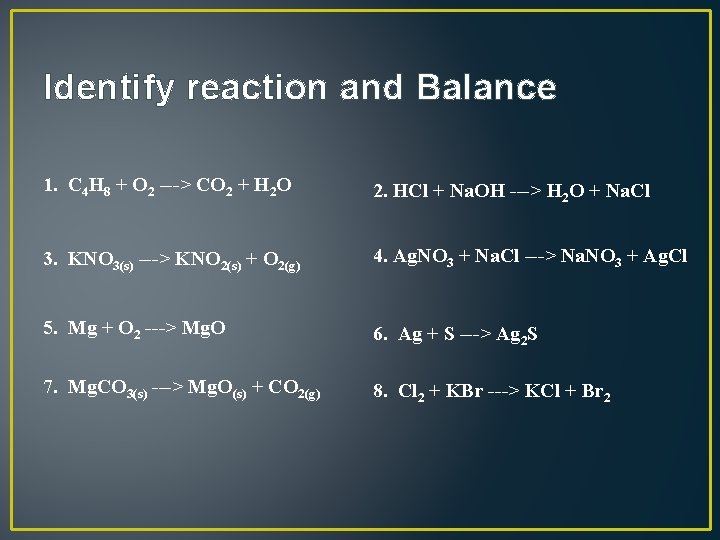
Identify reaction and Balance 1. C 4 H 8 + O 2 ---> CO 2 + H 2 O 2. HCl + Na. OH ---> H 2 O + Na. Cl 3. KNO 3(s) ---> KNO 2(s) + O 2(g) 4. Ag. NO 3 + Na. Cl ---> Na. NO 3 + Ag. Cl 5. Mg + O 2 ---> Mg. O 6. Ag + S ---> Ag 2 S 7. Mg. CO 3(s) ---> Mg. O(s) + CO 2(g) 8. Cl 2 + KBr ---> KCl + Br 2

Journal • Complete the following Reactions • Potassium Iodide and silver nitrate are mixed to produce

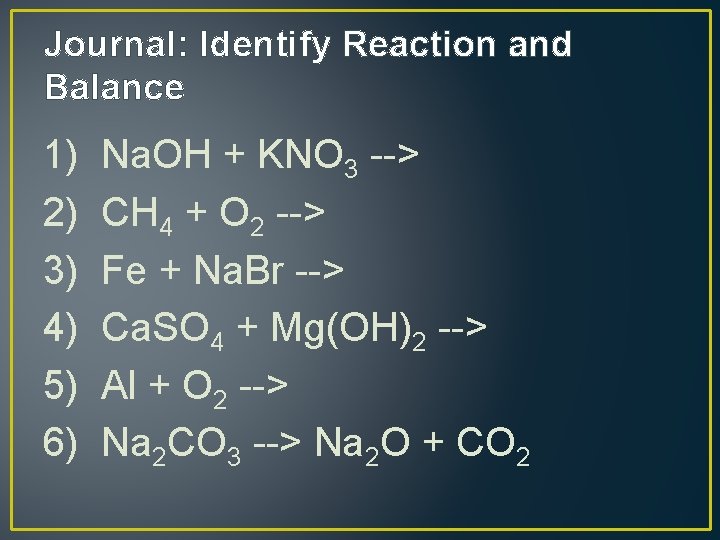
Journal: Identify Reaction and Balance 1) Na. OH + KNO 3 --> 2) CH 4 + O 2 --> 3) Fe + Na. Br --> 4) Ca. SO 4 + Mg(OH)2 --> 5) Al + O 2 --> 6) Na 2 CO 3 --> Na 2 O + CO 2

Bellwork • Take out a sheet of paper and something to write with. • put everything else away.

Reactions in Aqueous Solution Section 9. 3

Net Ionic Equations • Many important chemical reactions take place in water: aka aqueous solution • Ag. NO 3 (aq) + Na. Cl (aq) Ag. Cl (s) + Na. NO 3 (aq) • This does not show that the reactants and at least one product dissociate into cations and anions when dissolved in water. • WHAT DOES A NET IONIC EQUATION LOOK LIKE?

Net Ionic Equation • Can only break apart aqueous compounds. • All unchanged elements are eliminated. • Not involved in reaction = Spectator ions • LEAVING OUT SPECTATOR IONS RESULTS IN THE NET IONIC EQUATION. • Charge must be balanced on both sides of the equation • If not, use coefficients!

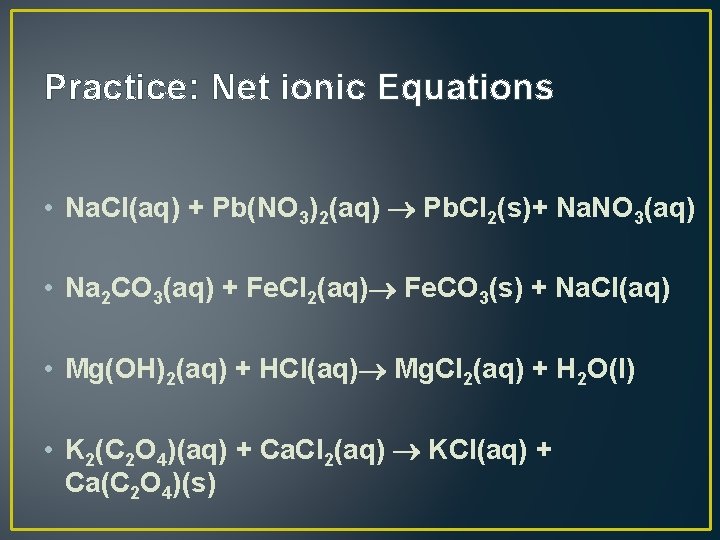
Practice: Net ionic Equations • Na. Cl(aq) + Pb(NO 3)2(aq) Pb. Cl 2(s)+ Na. NO 3(aq) • Na 2 CO 3(aq) + Fe. Cl 2(aq) Fe. CO 3(s) + Na. Cl(aq) • Mg(OH)2(aq) + HCl(aq) Mg. Cl 2(aq) + H 2 O(l) • K 2(C 2 O 4)(aq) + Ca. Cl 2(aq) KCl(aq) + Ca(C 2 O 4)(s)

Bellwork • Write the net ionic equation for the following chemical equation. • Na 2 SO 4 (aq) + Pb(NO 3)2 (aq) • SO 4 compound will be your solid

Predicting the formation of a precipitate • Whether or not a precipitate forms depends upon the solubility of new compounds that form. • There are 5 Rules for predicting solubility.

Rule 1 • Salts of Alkali metals and ammonia are soluble. • Ammonia (NH 3)or Ammonium (NH 4+) • Group 1 A metals • Li, Na, K, Rb, Cs, Fr

Rule 2 • Nitrate Salts and Chlorate Salts are soluble • Nitrate = NO 3 • Chlorate = Cl. O 3 -

Rule 3 • Sulfate salts are soluble • SO 4 -2 • Exceptions • Pb+2 • Ag+ • Hg 2+2 • Ba+2 • Sr+2 • Ca+2

Rule 4 • Chloride and Iodide Salts are soluble • Cl • I- • Exceptions • Ag+ • Pb+2 • Hg 2+2

Rule 5 • Carbonates, Phosphates, Chromates, sulfides, and hydroxides are INSOLUBLE • CO 3 -2 • PO 4 -3 • Cr. O 4 -2 • S-2 • OH-

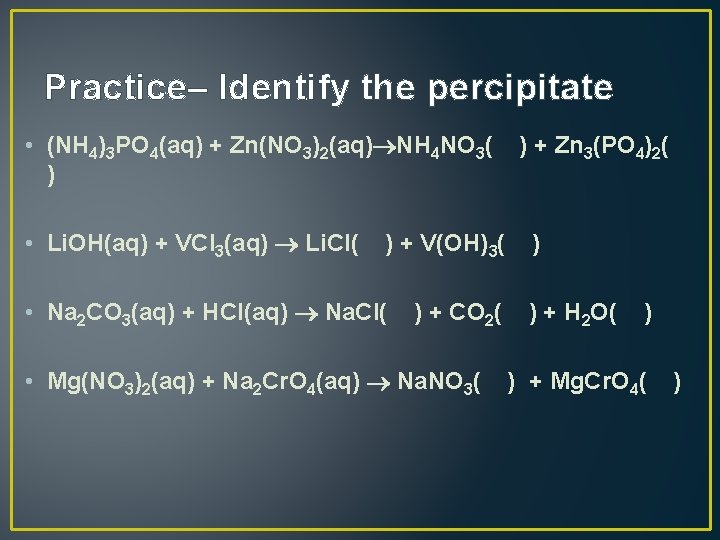
Practice– Identify the percipitate • (NH 4)3 PO 4(aq) + Zn(NO 3)2(aq) NH 4 NO 3( ) + Zn 3(PO 4)2( ) • Li. OH(aq) + VCl 3(aq) Li. Cl( ) + V(OH)3( ) • Na 2 CO 3(aq) + HCl(aq) Na. Cl( ) + CO 2( ) + H 2 O( ) • Mg(NO 3)2(aq) + Na 2 Cr. O 4(aq) Na. NO 3( ) + Mg. Cr. O 4( )

With your shoulder partner • Pg 302 • #’s 35 -39

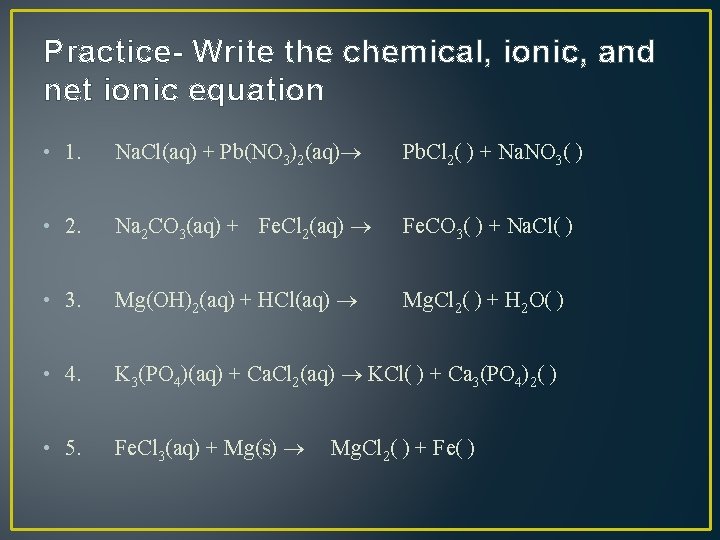
Practice- Write the chemical, ionic, and net ionic equation • 1. Na. Cl(aq) + Pb(NO 3)2(aq) Pb. Cl 2( ) + Na. NO 3( ) • 2. Na 2 CO 3(aq) + Fe. Cl 2(aq) Fe. CO 3( ) + Na. Cl( ) • 3. Mg(OH)2(aq) + HCl(aq) Mg. Cl 2( ) + H 2 O( ) • 4. K 3(PO 4)(aq) + Ca. Cl 2(aq) KCl( ) + Ca 3(PO 4)2( ) • 5. Fe. Cl 3(aq) + Mg(s) Mg. Cl 2( ) + Fe( )

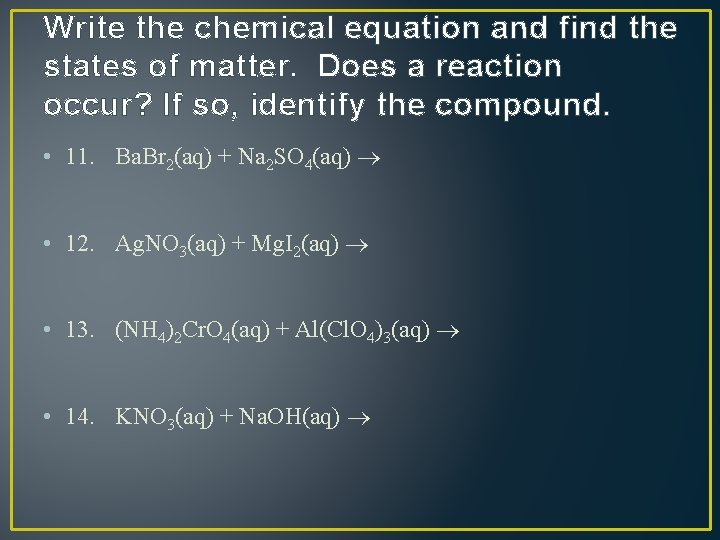
Write the chemical equation and find the states of matter. Does a reaction occur? If so, identify the compound. • 11. Ba. Br 2(aq) + Na 2 SO 4(aq) • 12. Ag. NO 3(aq) + Mg. I 2(aq) • 13. (NH 4)2 Cr. O 4(aq) + Al(Cl. O 4)3(aq) • 14. KNO 3(aq) + Na. OH(aq)

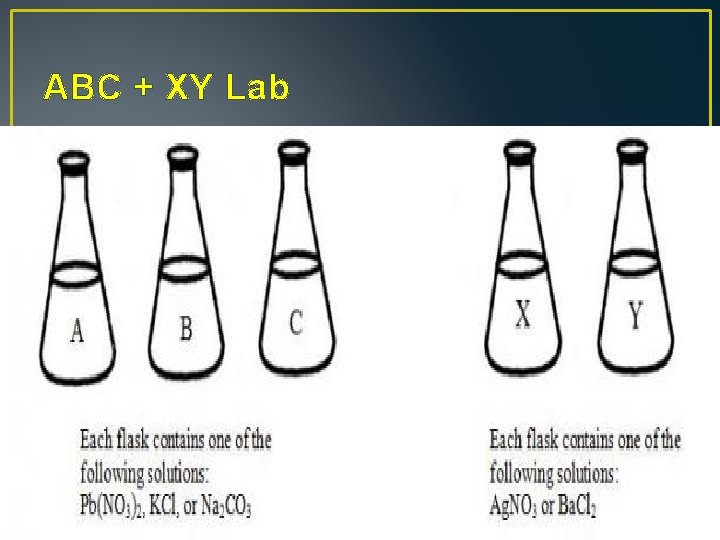
ABC + XY Lab © 2013, Robert Ayton. All rights reserved. www. mrayton. com

Bellwork-Get lab set up • Complete the following instructtions • Get apron, gloves, and goggles • Set up your reaction chart • Label all pipets • Discuss your next move with your partner and wait for further instructions • DO NOT TOUCH ANY CHEMICALS UNTIL YOU ARE TOLD TO DO SO!

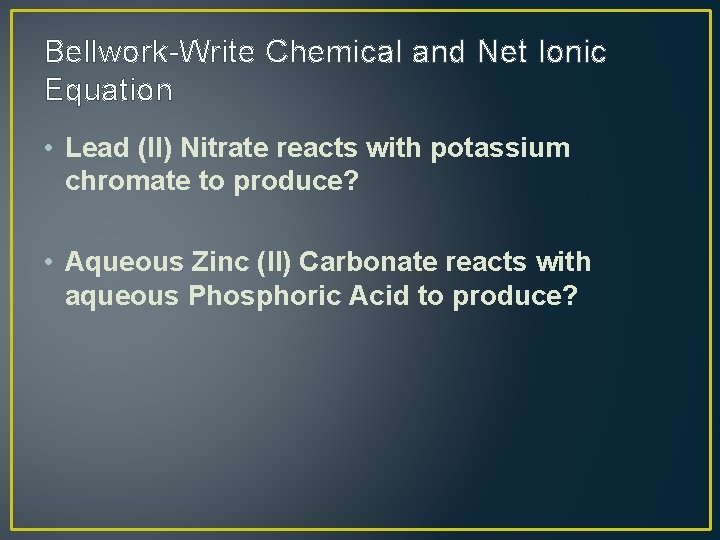
Bellwork-Write Chemical and Net Ionic Equation • Lead (II) Nitrate reacts with potassium chromate to produce? • Aqueous Zinc (II) Carbonate reacts with aqueous Phosphoric Acid to produce?

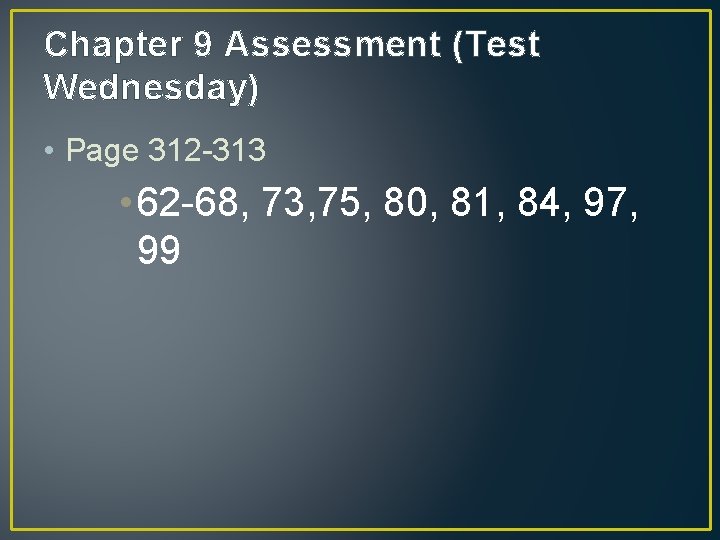
Chapter 9 Assessment (Test Wednesday) • Page 312 -313 • 62 -68, 73, 75, 80, 81, 84, 97, 99

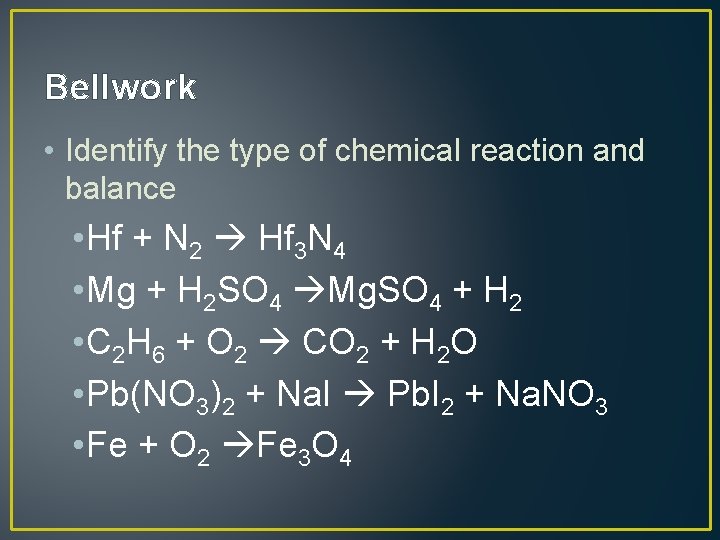
Bellwork • Identify the type of chemical reaction and balance • Hf + N 2 Hf 3 N 4 • Mg + H 2 SO 4 Mg. SO 4 + H 2 • C 2 H 6 + O 2 CO 2 + H 2 O • Pb(NO 3)2 + Na. I Pb. I 2 + Na. NO 3 • Fe + O 2 Fe 3 O 4

Chapter 9 Study Guide 1. Define the Following Terms • • • Product Reactant Chemical Equation Balanced Equation Skeleton Equation Activity Series of metals Single-replacement Combustion Decomposition Double-replacement Combination/Synthesis 2. Where do chemical reactions take place? 3. Know the following symbols 2. 3. ∆ (g), (l), (aq), (s) 4. What is a catalyst? 5. Balancing Chemical Equations 6. Identify the 5 types of chemical reactions 7. Know how to write a skeleton equation given a word equation 8. Writing net ionic equations (spectator ions) 1. Predicting precipitates

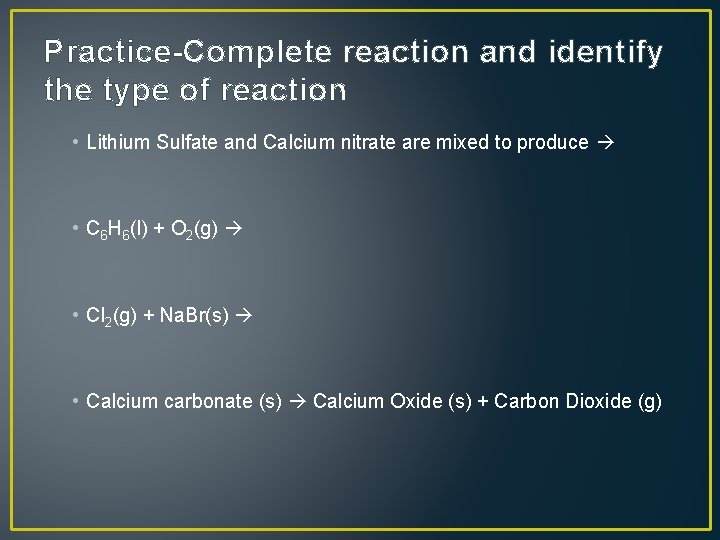
Practice-Complete reaction and identify the type of reaction • Lithium Sulfate and Calcium nitrate are mixed to produce • C 6 H 6(l) + O 2(g) • Cl 2(g) + Na. Br(s) • Calcium carbonate (s) Calcium Oxide (s) + Carbon Dioxide (g)

Practice- Write the chemical, ionic, net ionic equation, and finding states of matter 6. (NH 4)3 PO 4(aq) + Zn(NO 3)2(aq) 7. Li. OH(aq) + VCl 3(aq) 8. Na 2 CO 3(aq) + HCl(aq) Na. Cl( ) + CO 2( ) + H 2 O( 9. Mg(NO 3)2(aq) + Na 2 Cr. O 4(aq) 10. Zr(OH)4(s)+ HNO 3(aq) Zr(NO 3)4( ) + H 2 O( ) )

Prepare for Test • ALL BACKPACKS UP FRONT • ALL CELLPHONES OFF AND IN BACKPACK • YOU NEED: • A PIECE OF PAPER • PENCIL

Review • Review last night’s homework with your shoulder partner

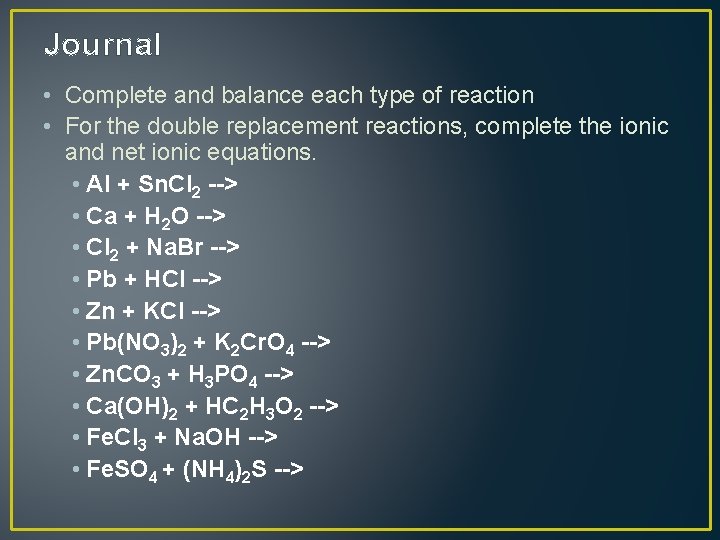
Journal • Complete and balance each type of reaction • For the double replacement reactions, complete the ionic and net ionic equations. • Al + Sn. Cl 2 --> • Ca + H 2 O --> • Cl 2 + Na. Br --> • Pb + HCl --> • Zn + KCl --> • Pb(NO 3)2 + K 2 Cr. O 4 --> • Zn. CO 3 + H 3 PO 4 --> • Ca(OH)2 + HC 2 H 3 O 2 --> • Fe. Cl 3 + Na. OH --> • Fe. SO 4 + (NH 4)2 S -->

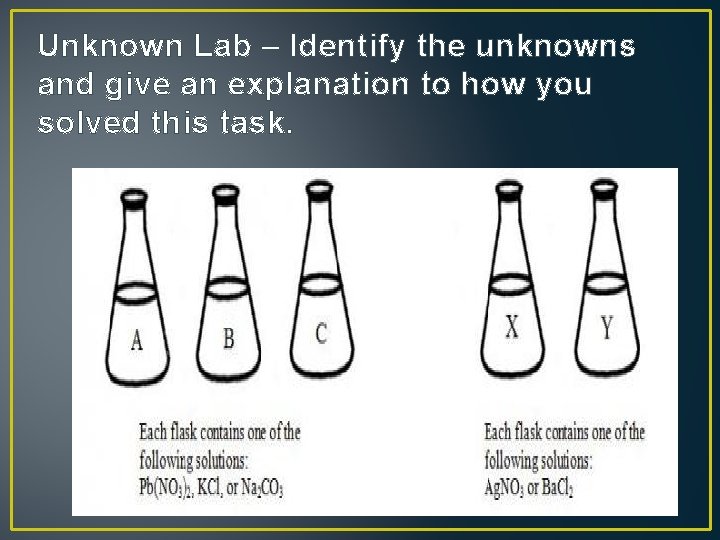
Unknown Lab – Identify the unknowns and give an explanation to how you solved this task.

Chemistry 1 • Complete Lab • Class Discussion on Lab Write up • State the Problem (purpose) • Gather information • What is a chemical reaction? • What type of reaction are we dealing with? How do you know? • What is a precipitate? What is the precipitate in this lab? • Form a hypothesis • Define: • Experiment • Write down materials and procedures (list) • Collect and Analyze • In a table organize the following • • Average mass of filter paper Mass of precipitate and filter paper Mass of precipitate Percent error • In this section also include qualitative information

Lab Continue • Draw Conclusion • Summarize what you did • Restate purpose of the experiment • What do you think led to your error? • Give at least two reasons.