Energy Chemical Reactions Chemical Reactions and Energy Why







- Slides: 16

Energy & Chemical Reactions

Chemical Reactions and Energy • Why do chemical reactions always involve a change in energy? • What is the difference between an endothermic reaction and an exothermic reaction?

Energy Changes • Chemical bonds contain a form of energy called chemical energy. • Breaking a bond absorbs energy from the surroundings. • The formation of a chemical bond releases energy to the surroundings.

Energy Changes (cont. ) • Some chemical reactions release more energy than they absorb. • Some chemical reactions absorb more energy than they release. • Energy is conserved in all chemical reactions.

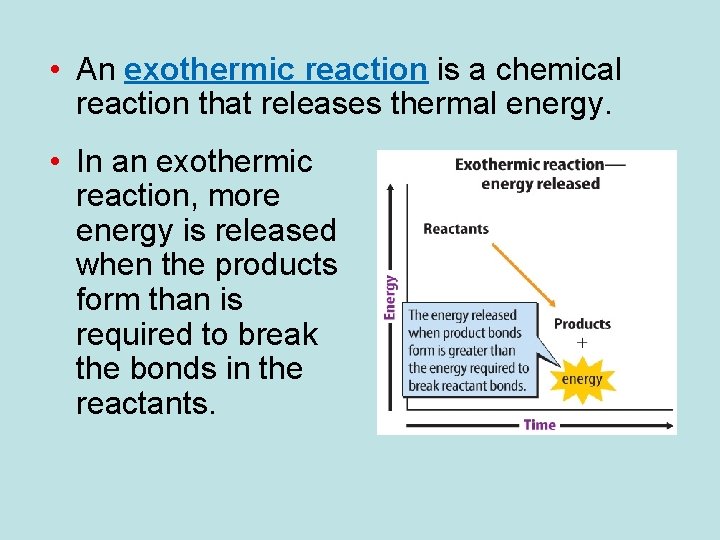
• Chemical reactions that absorb thermal energy are endothermic reactions. • In an endothermic reaction, more energy is required to break the bonds of the reactants than is released when the products form.

• An exothermic reaction is a chemical reaction that releases thermal energy. • In an exothermic reaction, more energy is released when the products form than is required to break the bonds in the reactants.

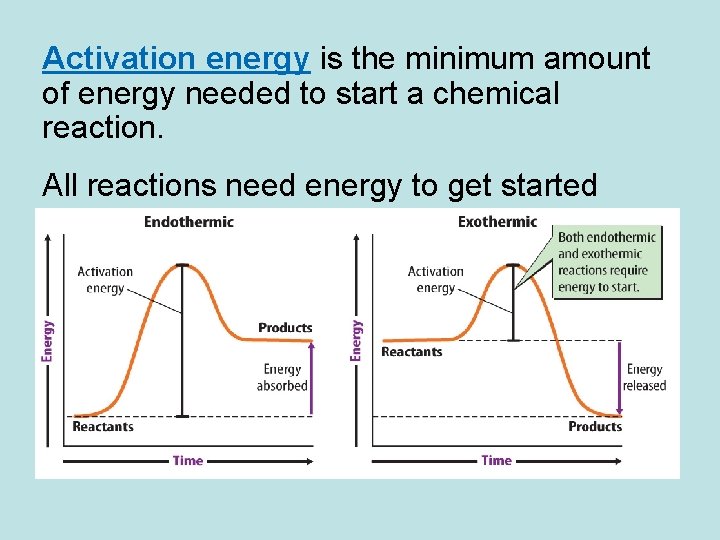
Activation energy is the minimum amount of energy needed to start a chemical reaction. All reactions need energy to get started

Chemical Reactions and Energy Why do chemical reactions involve a change in energy?

Chemical Reactions and Energy • What factors can affect the rate of a chemical reaction?

Reaction Rates • Chemical reactions occur due to particles in substances colliding. • The rate of a reaction is the speed at which it occurs. • Chemical reactions occur faster if particles collide more often or move faster when they collide.

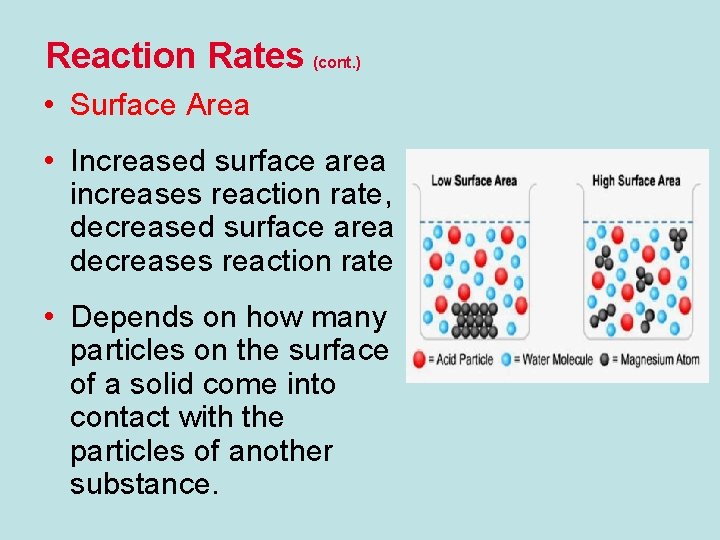
Reaction Rates (cont. ) • Surface Area • Increased surface area increases reaction rate, decreased surface area decreases reaction rate • Depends on how many particles on the surface of a solid come into contact with the particles of another substance.

Reaction Rates (cont. ) • Temperature • Higher temperatures speed up reactions, lower temperatures slow down reactions • Depends on the average speed of reactant particles able to collide with other reactant particles

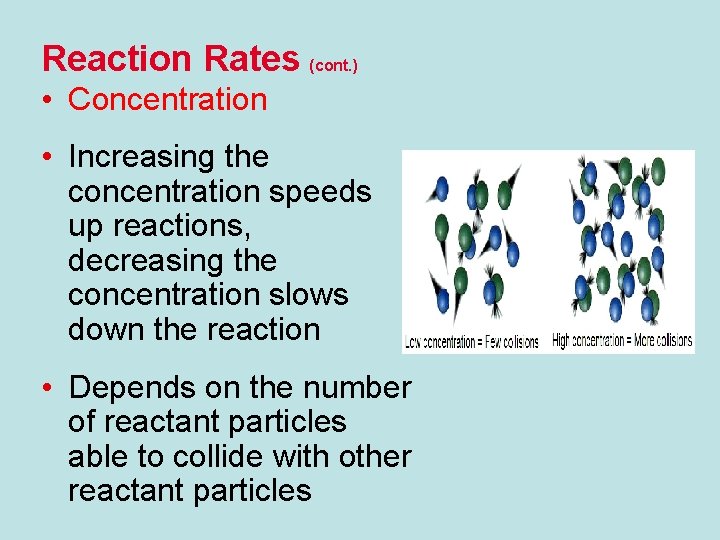
Reaction Rates (cont. ) • Concentration • Increasing the concentration speeds up reactions, decreasing the concentration slows down the reaction • Depends on the number of reactant particles able to collide with other reactant particles

Reaction Rates (cont. ) • Catalysts • A catalyst is a substance that increases reaction rate by lowering the activation energy of a reaction. • Special catalysts called enzymes work in the human body Ex) making cheese, making paper products

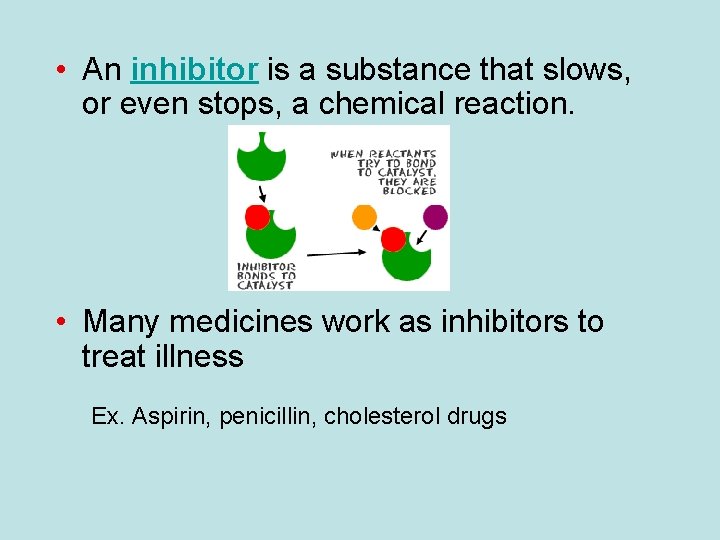
• An inhibitor is a substance that slows, or even stops, a chemical reaction. • Many medicines work as inhibitors to treat illness Ex. Aspirin, penicillin, cholesterol drugs

Reaction Rates (cont. ) What factors can affect the rate of a chemical reaction?
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Hey hey bye bye
Hey hey bye bye Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Enzym
Enzym How to write half reactions
How to write half reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Don't ask why why why
Don't ask why why why Chemical reactions reactants and products
Chemical reactions reactants and products Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Balancing chemical equations definition
Balancing chemical equations definition Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry































