Thermochemistry Chapter 6 Thermochemistry Thermodynamics is the science

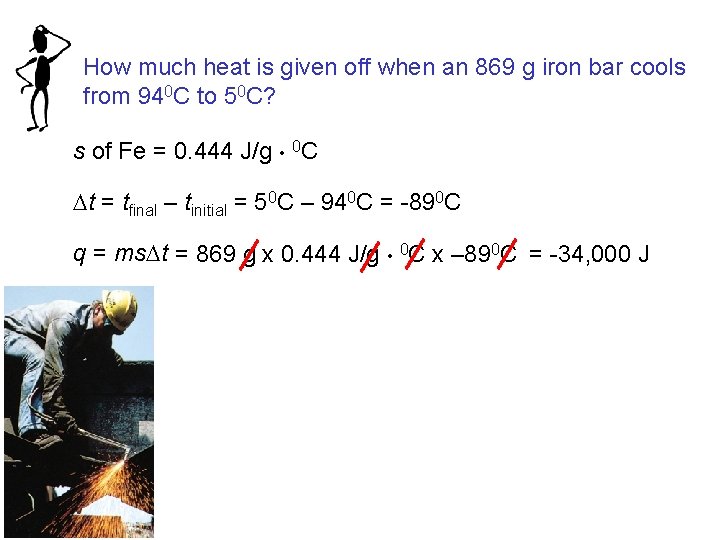











- Slides: 85

Thermochemistry Chapter 6

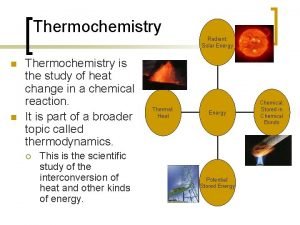
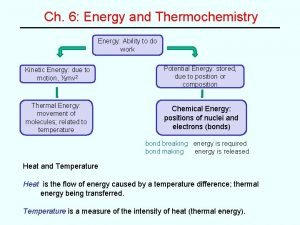
Thermochemistry • Thermodynamics is the science of the relationship between heat and other forms of energy. Thermochemistry is the study of the quantity of heat absorbed or evolved by chemical reactions.

Energy is the capacity to do work • Radiant energy comes from the sun and is earth’s primary energy source • Thermal energy is the energy associated with the random motion of atoms and molecules • Chemical energy is the energy stored within the bonds of chemical substances • Nuclear energy is the energy stored within the collection of neutrons and protons in the atom • Potential energy is the energy available by virtue of an object’s position

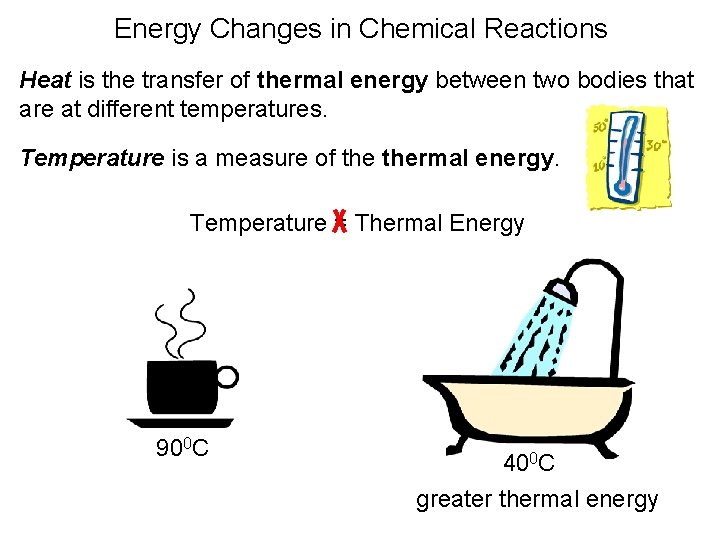
Energy Changes in Chemical Reactions Heat is the transfer of thermal energy between two bodies that are at different temperatures. Temperature is a measure of thermal energy. Temperature = Thermal Energy 900 C 400 C greater thermal energy

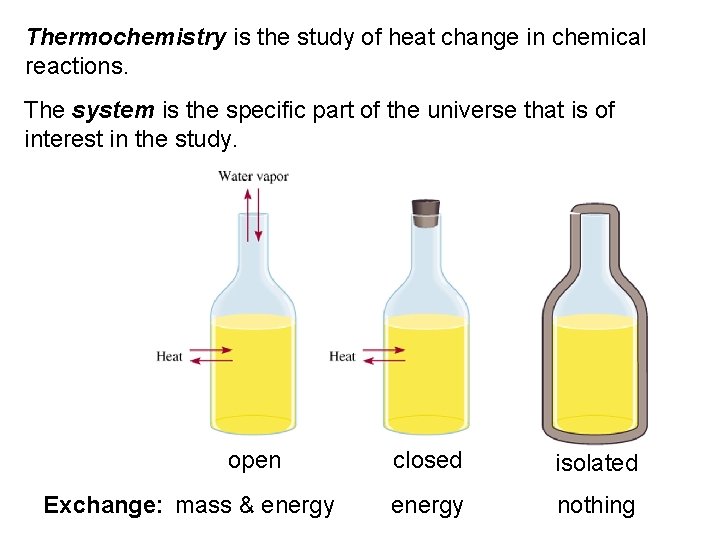
Thermochemistry is the study of heat change in chemical reactions. The system is the specific part of the universe that is of interest in the study. open Exchange: mass & energy closed isolated energy nothing

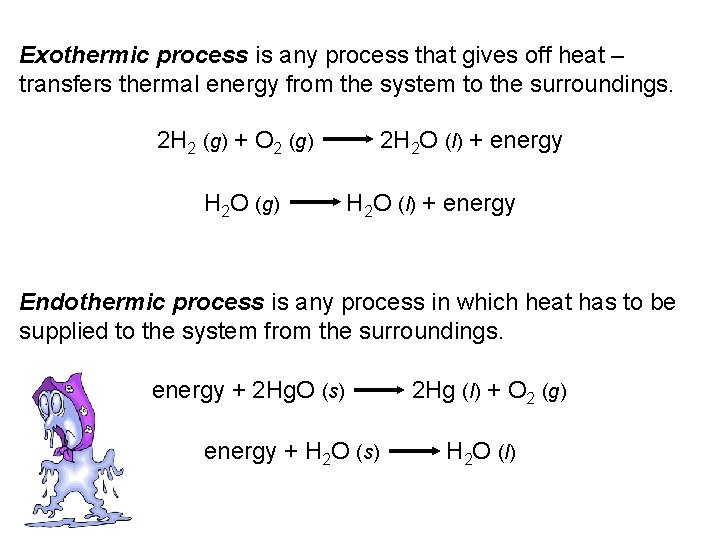
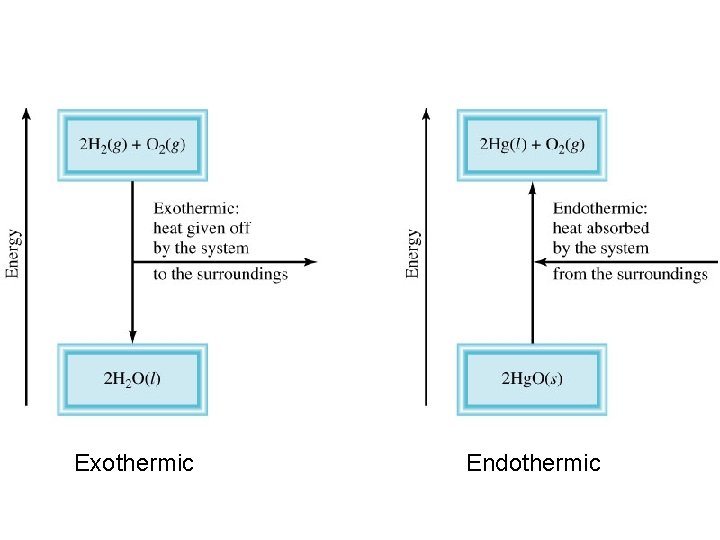
Exothermic process is any process that gives off heat – transfers thermal energy from the system to the surroundings. 2 H 2 (g) + O 2 (g) H 2 O (g) 2 H 2 O (l) + energy Endothermic process is any process in which heat has to be supplied to the system from the surroundings. energy + 2 Hg. O (s) energy + H 2 O (s) 2 Hg (l) + O 2 (g) H 2 O (l)

Exothermic Endothermic

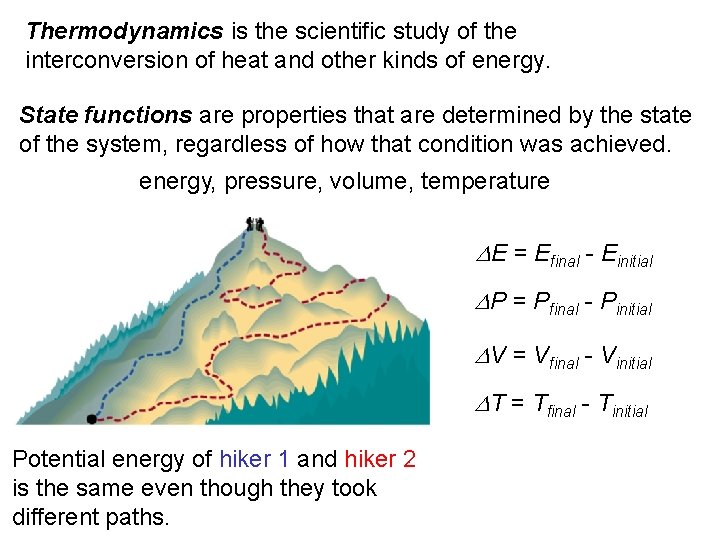
Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy. State functions are properties that are determined by the state of the system, regardless of how that condition was achieved. energy, pressure, volume, temperature DE = Efinal - Einitial DP = Pfinal - Pinitial DV = Vfinal - Vinitial DT = Tfinal - Tinitial Potential energy of hiker 1 and hiker 2 is the same even though they took different paths.

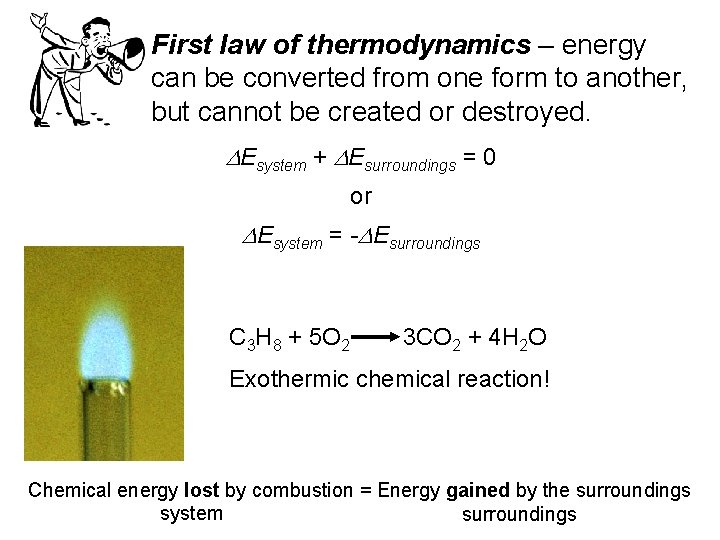
First law of thermodynamics – energy can be converted from one form to another, but cannot be created or destroyed. DEsystem + DEsurroundings = 0 or DEsystem = -DEsurroundings C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O Exothermic chemical reaction! Chemical energy lost by combustion = Energy gained by the surroundings system surroundings

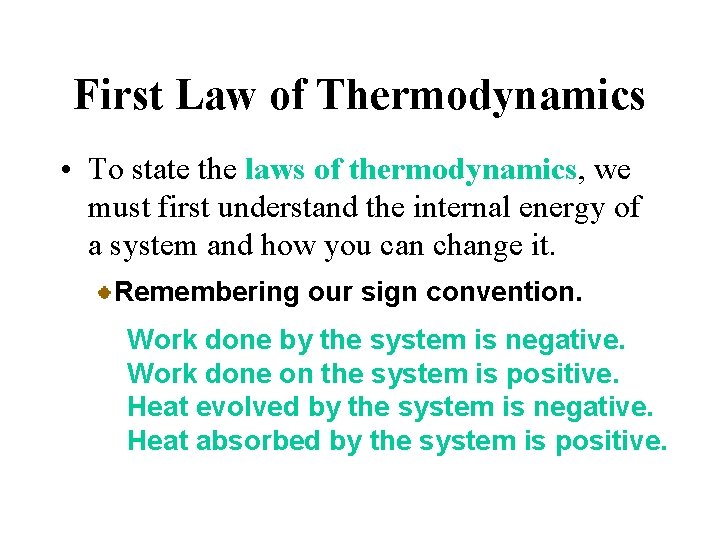
First Law of Thermodynamics • To state the laws of thermodynamics, we must first understand the internal energy of a system and how you can change it. Heat is energy that moves into or out of a system because of a temperature difference between system and surroundings. Work, on the other hand, is the energy exchange that results when a force F moves an object through a distance d; work (w) = F d

First Law of Thermodynamics • To state the laws of thermodynamics, we must first understand the internal energy of a system and how you can change it. Remembering our sign convention. Work done by the system is negative. Work done on the system is positive. Heat evolved by the system is negative. Heat absorbed by the system is positive.

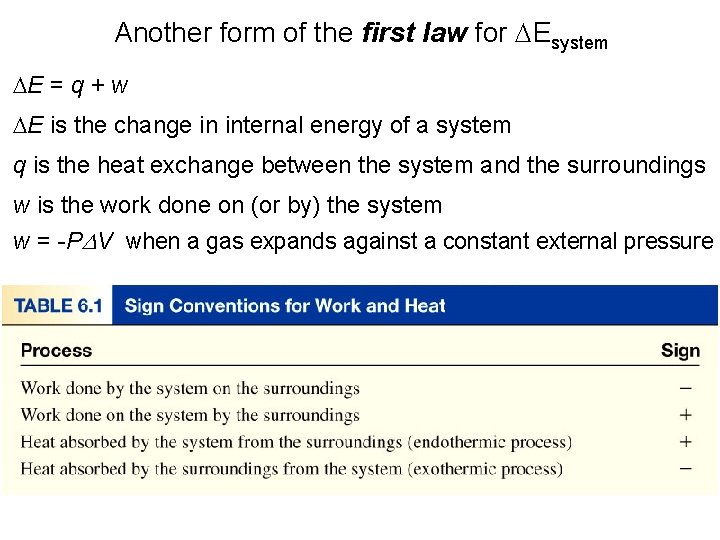
Another form of the first law for DEsystem DE = q + w DE is the change in internal energy of a system q is the heat exchange between the system and the surroundings w is the work done on (or by) the system w = -PDV when a gas expands against a constant external pressure

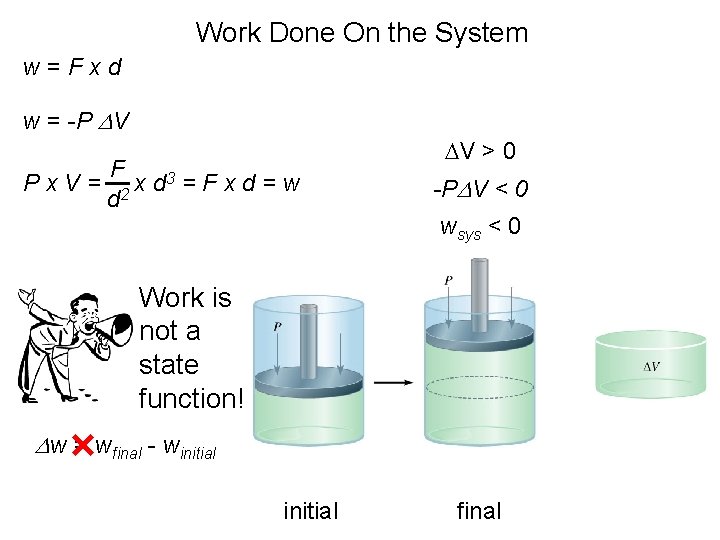
Work Done On the System w=Fxd w = -P DV Px. V= F 3 = F x d = w x d d 2 DV > 0 -PDV < 0 wsys < 0 Work is not a state function! Dw = wfinal - winitial final

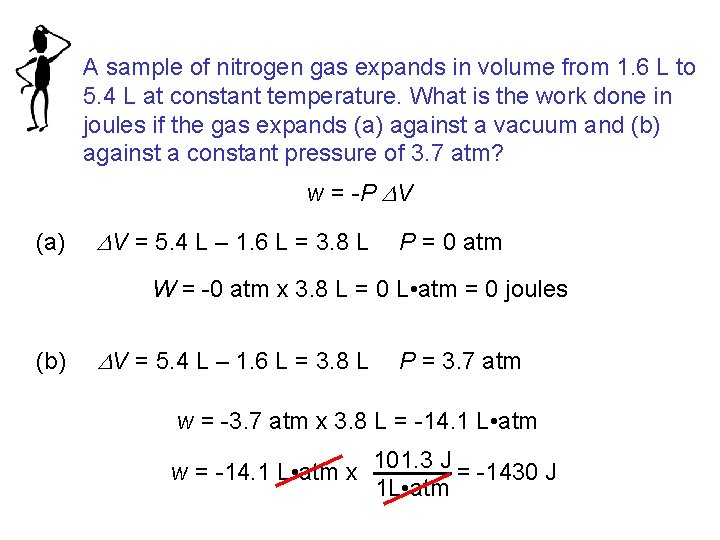
A sample of nitrogen gas expands in volume from 1. 6 L to 5. 4 L at constant temperature. What is the work done in joules if the gas expands (a) against a vacuum and (b) against a constant pressure of 3. 7 atm? w = -P DV (a) DV = 5. 4 L – 1. 6 L = 3. 8 L P = 0 atm W = -0 atm x 3. 8 L = 0 L • atm = 0 joules (b) DV = 5. 4 L – 1. 6 L = 3. 8 L P = 3. 7 atm w = -3. 7 atm x 3. 8 L = -14. 1 L • atm 101. 3 J = -1430 J w = -14. 1 L • atm x 1 L • atm

Chemistry in Action: Making Snow DE = q + w q=0 w < 0, DE < 0 D E = CD T DT < 0, SNOW!

Enthalpy and the First Law of Thermodynamics DE = q + w At constant pressure: q = DH and w = -PDV DE = DH - PDV DH = DE + PDV

Heat of Reaction • Heat is defined as the energy that flows into or out of a system because of a difference in temperature between the system and its surroundings. Heat flows from a region of higher temperature to one of lower temperature; once the temperatures become equal, heat flow stops.

Enthalpy and Enthalpy Change • Enthalpy, denoted H, is an extensive property of a substance that can be used to obtain the heat absorbed or evolved in a chemical reaction. An extensive property is one that depends on the quantity of substance. Enthalpy is a state function, a property of a system that depends only on its present state and is independent of any previous history of the system.

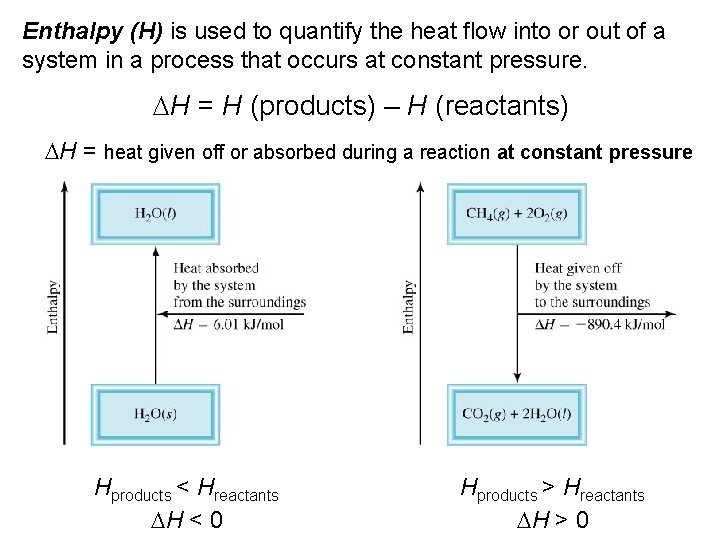
Enthalpy (H) is used to quantify the heat flow into or out of a system in a process that occurs at constant pressure. DH = H (products) – H (reactants) DH = heat given off or absorbed during a reaction at constant pressure Hproducts < Hreactants DH < 0 Hproducts > Hreactants DH > 0

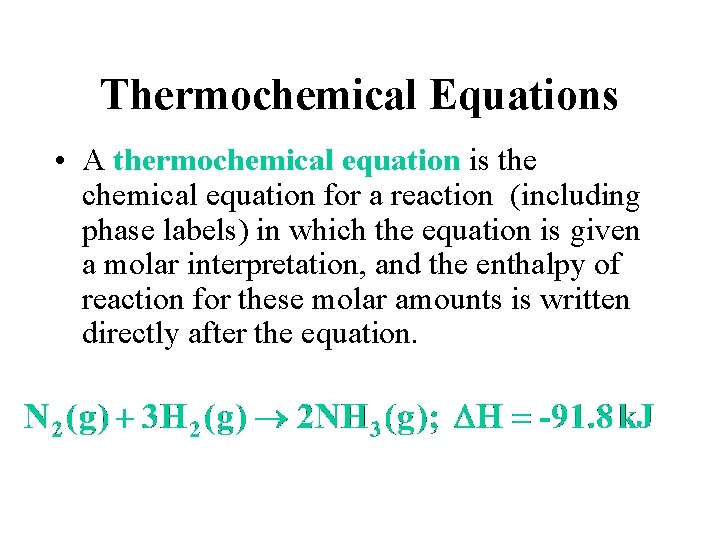
Thermochemical Equations • A thermochemical equation is the chemical equation for a reaction (including phase labels) in which the equation is given a molar interpretation, and the enthalpy of reaction for these molar amounts is written directly after the equation.

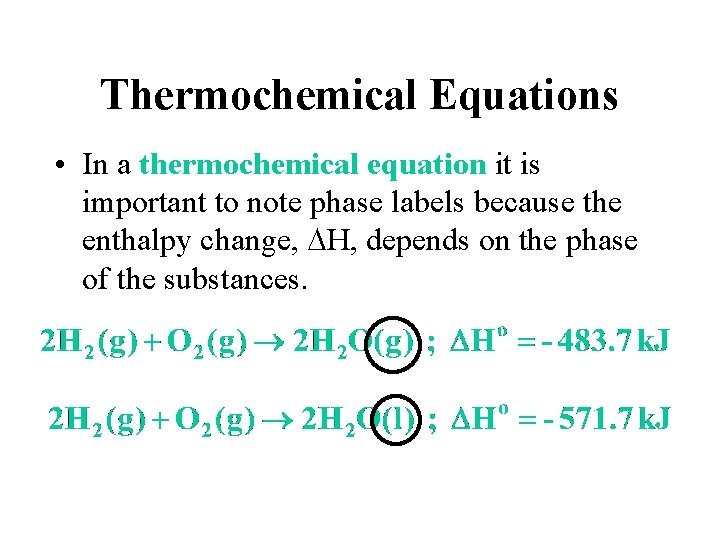
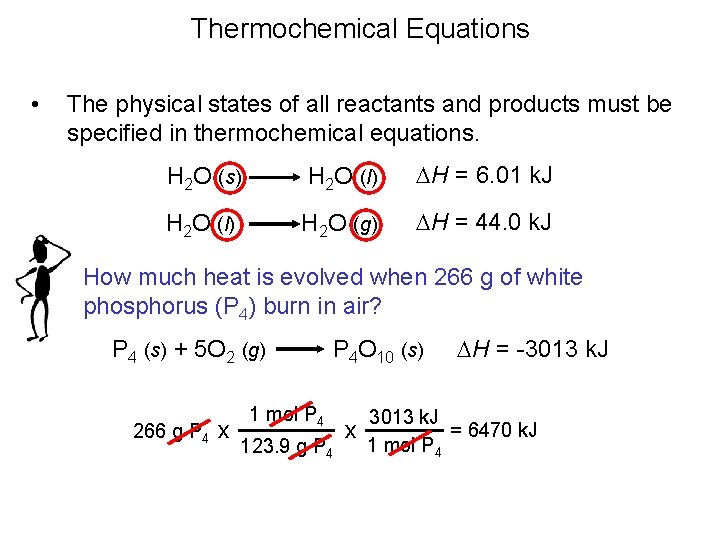
Thermochemical Equations • In a thermochemical equation it is important to note phase labels because the enthalpy change, DH, depends on the phase of the substances.

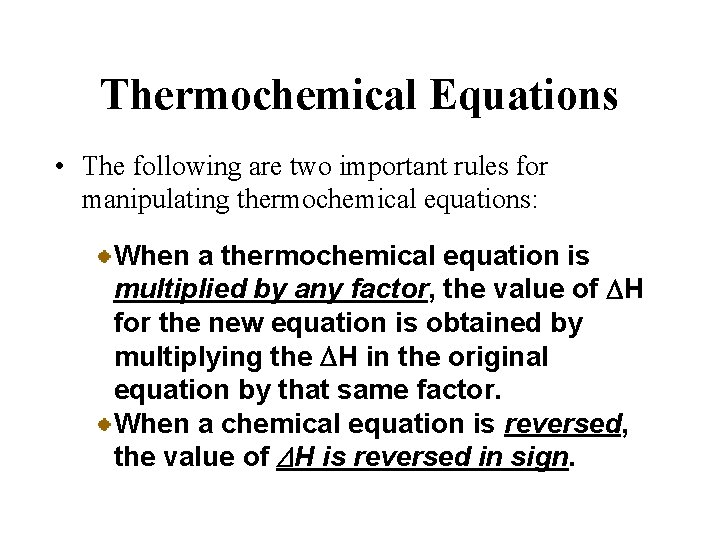
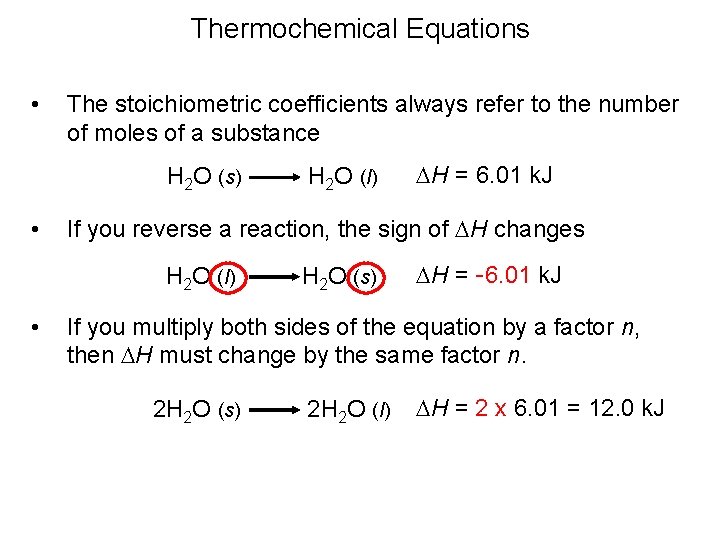
Thermochemical Equations • The following are two important rules for manipulating thermochemical equations: When a thermochemical equation is multiplied by any factor, the value of DH for the new equation is obtained by multiplying the DH in the original equation by that same factor. When a chemical equation is reversed, the value of DH is reversed in sign.

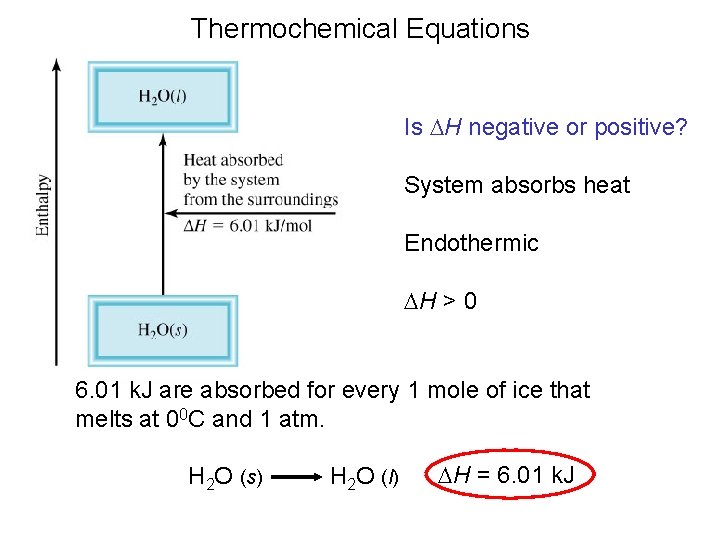
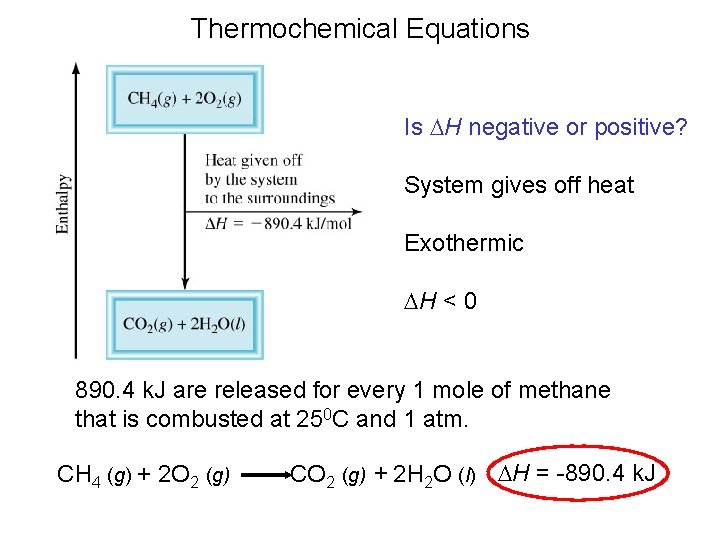
Thermochemical Equations Is DH negative or positive? System absorbs heat Endothermic DH > 0 6. 01 k. J are absorbed for every 1 mole of ice that melts at 00 C and 1 atm. H 2 O (s) H 2 O (l) DH = 6. 01 k. J

Thermochemical Equations Is DH negative or positive? System gives off heat Exothermic DH < 0 890. 4 k. J are released for every 1 mole of methane that is combusted at 250 C and 1 atm. CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (l) DH = -890. 4 k. J

Thermochemical Equations • The stoichiometric coefficients always refer to the number of moles of a substance H 2 O (s) • DH = 6. 01 k. J If you reverse a reaction, the sign of DH changes H 2 O (l) • H 2 O (l) H 2 O (s) DH = -6. 01 k. J If you multiply both sides of the equation by a factor n, then DH must change by the same factor n. 2 H 2 O (s) 2 H 2 O (l) DH = 2 x 6. 01 = 12. 0 k. J

Thermochemical Equations • The physical states of all reactants and products must be specified in thermochemical equations. H 2 O (s) H 2 O (l) DH = 6. 01 k. J H 2 O (l) H 2 O (g) DH = 44. 0 k. J How much heat is evolved when 266 g of white phosphorus (P 4) burn in air? P 4 (s) + 5 O 2 (g) 266 g P 4 x P 4 O 10 (s) 1 mol P 4 123. 9 g P 4 x DH = -3013 k. J = 6470 k. J 1 mol P 4

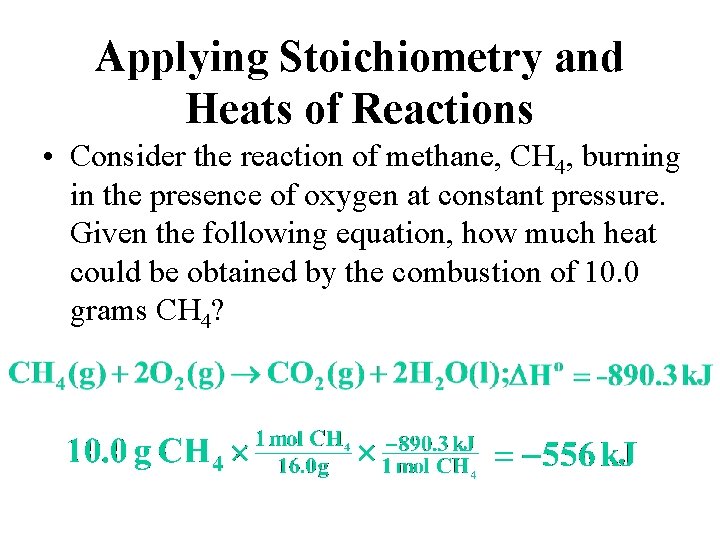
Applying Stoichiometry and Heats of Reactions • Consider the reaction of methane, CH 4, burning in the presence of oxygen at constant pressure. Given the following equation, how much heat could be obtained by the combustion of 10. 0 grams CH 4?

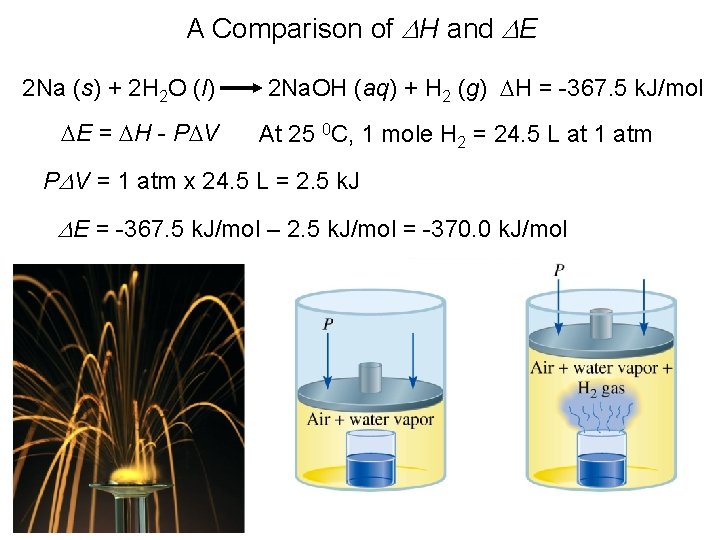
A Comparison of DH and DE 2 Na (s) + 2 H 2 O (l) DE = DH - PDV 2 Na. OH (aq) + H 2 (g) DH = -367. 5 k. J/mol At 25 0 C, 1 mole H 2 = 24. 5 L at 1 atm PDV = 1 atm x 24. 5 L = 2. 5 k. J DE = -367. 5 k. J/mol – 2. 5 k. J/mol = -370. 0 k. J/mol

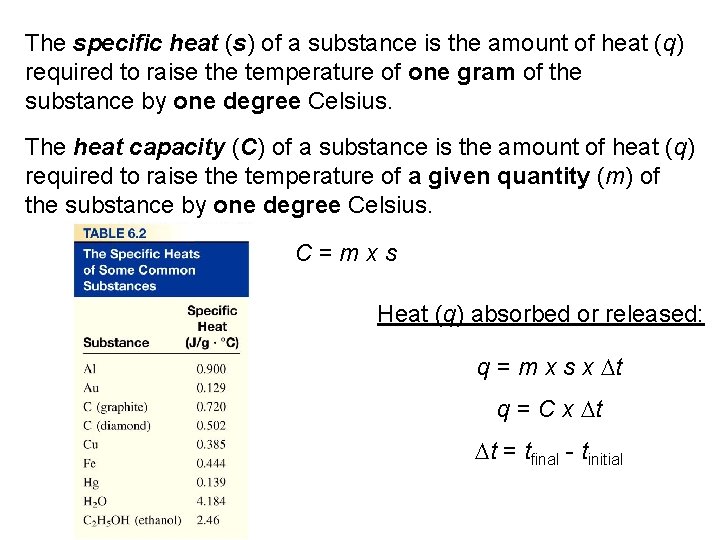
The specific heat (s) of a substance is the amount of heat (q) required to raise the temperature of one gram of the substance by one degree Celsius. The heat capacity (C) of a substance is the amount of heat (q) required to raise the temperature of a given quantity (m) of the substance by one degree Celsius. C=mxs Heat (q) absorbed or released: q = m x s x Dt q = C x Dt Dt = tfinal - tinitial

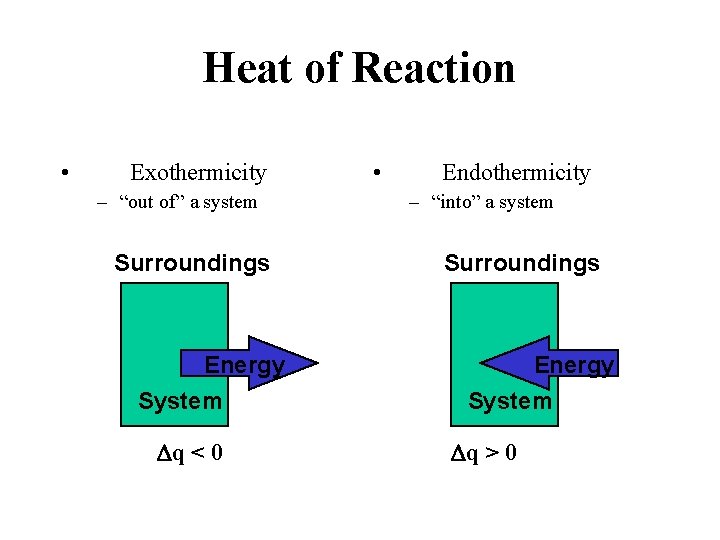
Heat of Reaction • Exothermicity – “out of” a system Surroundings Energy System Dq < 0 • Endothermicity – “into” a system Surroundings Energy System Dq > 0
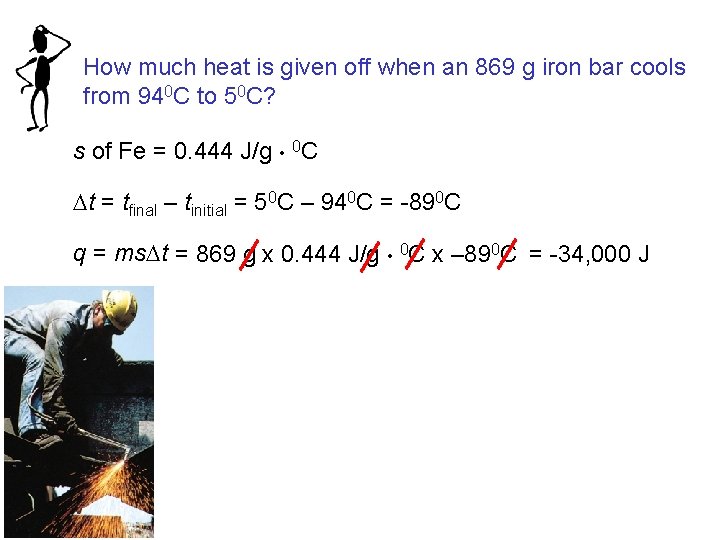
How much heat is given off when an 869 g iron bar cools from 940 C to 50 C? s of Fe = 0. 444 J/g • 0 C Dt = tfinal – tinitial = 50 C – 940 C = -890 C q = ms. Dt = 869 g x 0. 444 J/g • 0 C x – 890 C = -34, 000 J

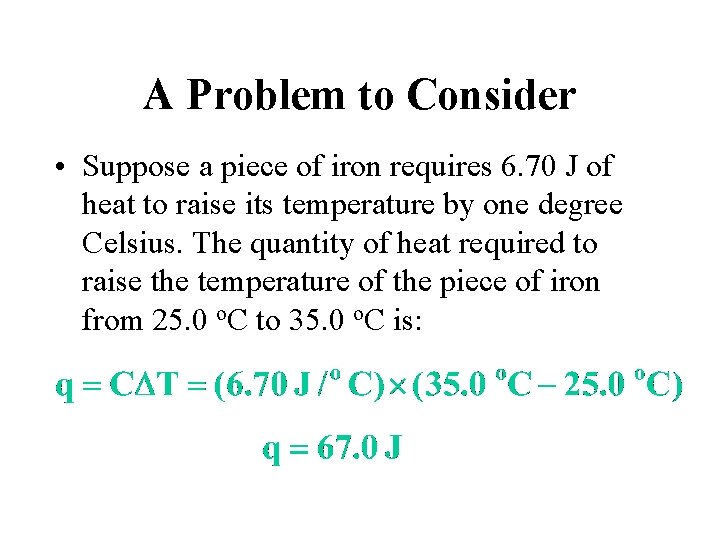
A Problem to Consider • Suppose a piece of iron requires 6. 70 J of heat to raise its temperature by one degree Celsius. The quantity of heat required to raise the temperature of the piece of iron from 25. 0 o. C to 35. 0 o. C is:

A Problem to Consider • Calculate the heat absorbed when the temperature of 15. 0 grams of water is raised from 20. 0 o. C to 50. 0 o. C. (The specific heat of water is 4. 184 J/g. o. C. )

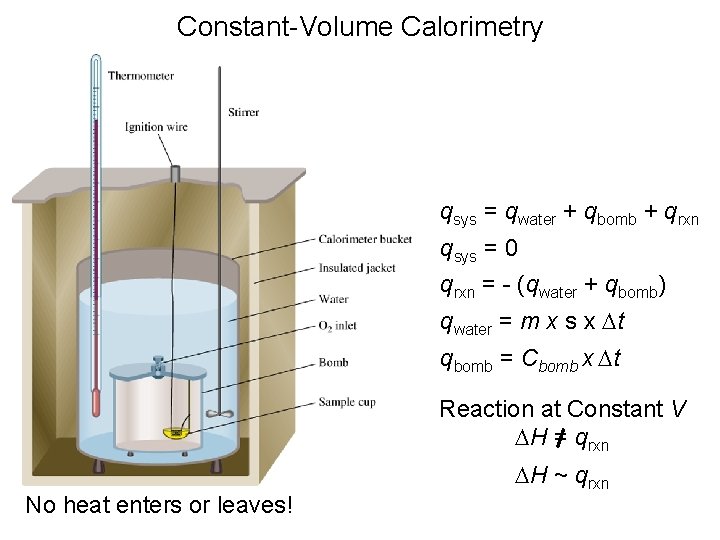
Constant-Volume Calorimetry qsys = qwater + qbomb + qrxn qsys = 0 qrxn = - (qwater + qbomb) qwater = m x s x Dt qbomb = Cbomb x Dt Reaction at Constant V DH = qrxn No heat enters or leaves! DH ~ qrxn

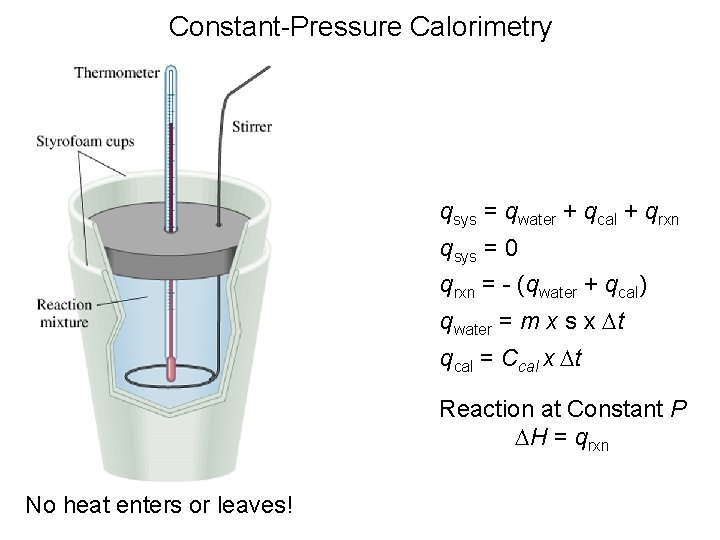
Constant-Pressure Calorimetry qsys = qwater + qcal + qrxn qsys = 0 qrxn = - (qwater + qcal) qwater = m x s x Dt qcal = Ccal x Dt Reaction at Constant P DH = qrxn No heat enters or leaves!

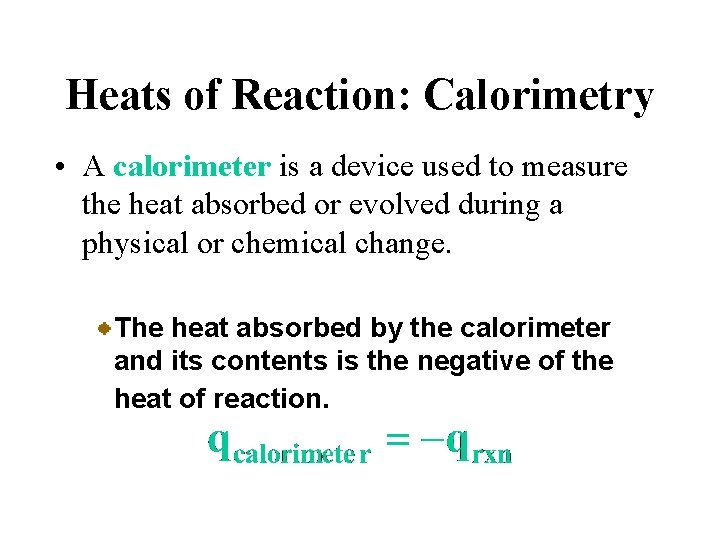
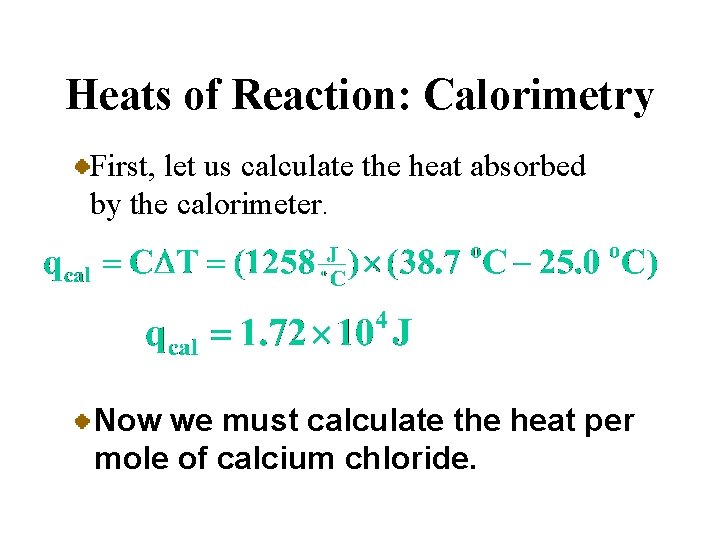
Heats of Reaction: Calorimetry • A calorimeter is a device used to measure the heat absorbed or evolved during a physical or chemical change. The heat absorbed by the calorimeter and its contents is the negative of the heat of reaction.

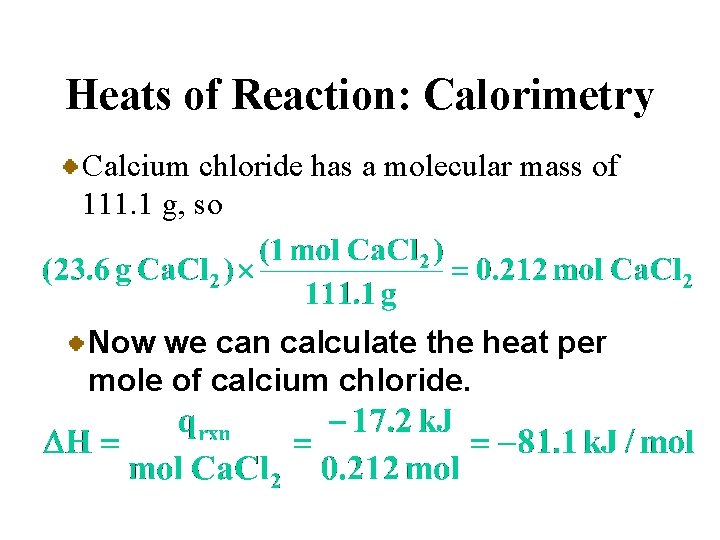
A Problem to Consider • When 23. 6 grams of calcium chloride, Ca. Cl 2, was dissolved in water in a calorimeter, the temperature rose from 25. 0 o. C to 38. 7 o. C. If the heat capacity of the solution and the calorimeter is 1258 J/o. C, what is the enthalpy change per mole of calcium chloride?

Heats of Reaction: Calorimetry First, let us calculate the heat absorbed by the calorimeter. Now we must calculate the heat per mole of calcium chloride.

Heats of Reaction: Calorimetry Calcium chloride has a molecular mass of 111. 1 g, so Now we can calculate the heat per mole of calcium chloride.


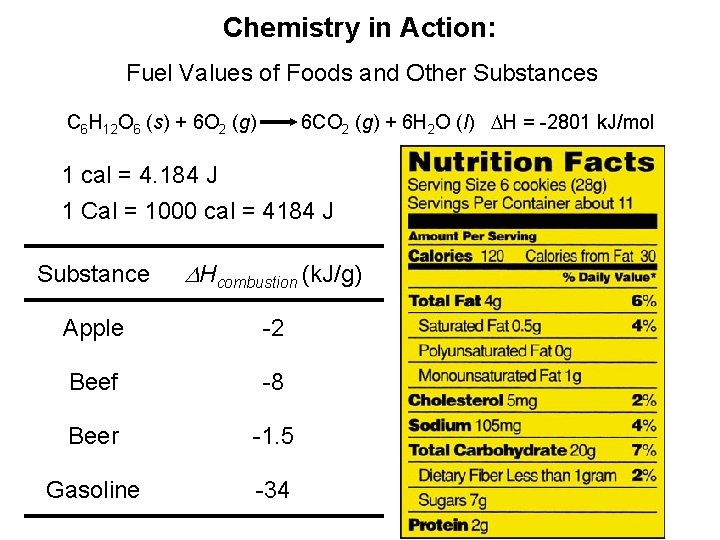
Chemistry in Action: Fuel Values of Foods and Other Substances 6 CO 2 (g) + 6 H 2 O (l) DH = -2801 k. J/mol C 6 H 12 O 6 (s) + 6 O 2 (g) 1 cal = 4. 184 J 1 Cal = 1000 cal = 4184 J Substance DHcombustion (k. J/g) Apple -2 Beef -8 Beer -1. 5 Gasoline -34

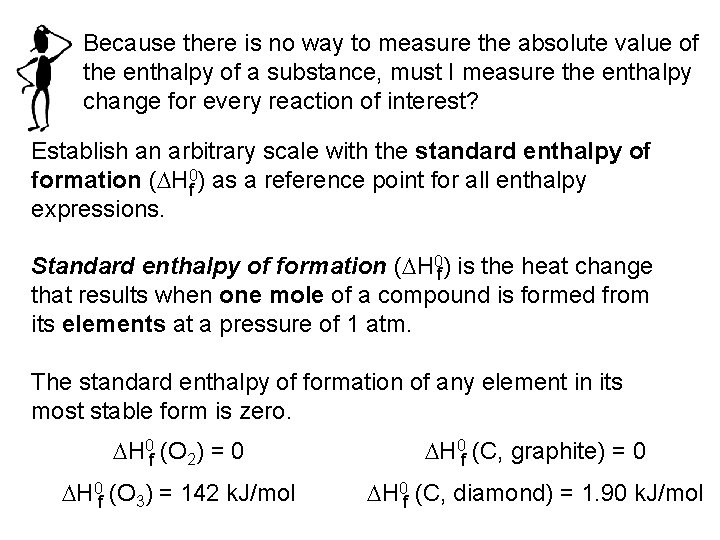
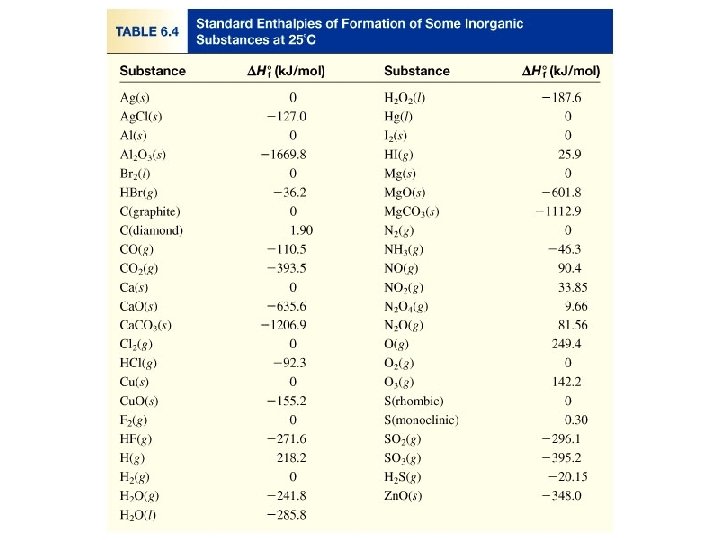
Because there is no way to measure the absolute value of the enthalpy of a substance, must I measure the enthalpy change for every reaction of interest? Establish an arbitrary scale with the standard enthalpy of formation (DH 0 f ) as a reference point for all enthalpy expressions. Standard enthalpy of formation (DH 0 f) is the heat change that results when one mole of a compound is formed from its elements at a pressure of 1 atm. The standard enthalpy of formation of any element in its most stable form is zero. DH 0 f (O 2) = 0 DH 0 f (C, graphite) = 0 DH 0 f (O 3) = 142 k. J/mol DH 0 f (C, diamond) = 1. 90 k. J/mol


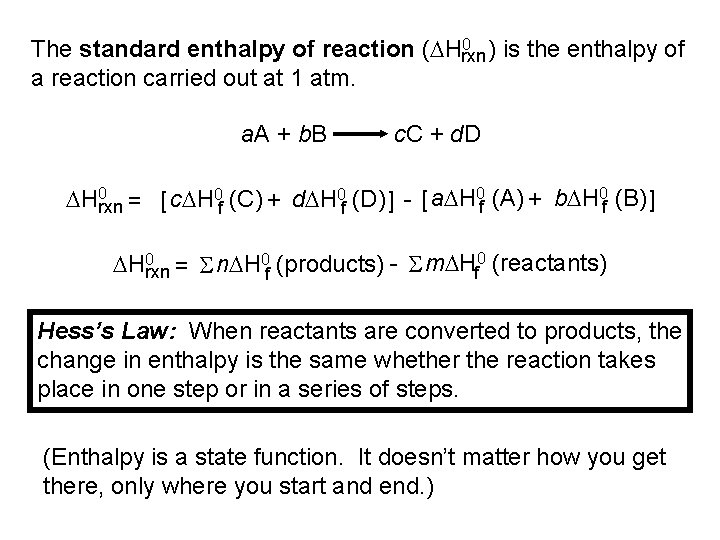
0 ) is the enthalpy of The standard enthalpy of reaction (DHrxn a reaction carried out at 1 atm. a. A + b. B c. C + d. D DH 0 rxn = [ c. DH 0 f (C) + d. DH 0 f (D) ] - [ a. DH 0 f (A) + b. DH 0 f (B) ] DH 0 rxn = S n. DH 0 f (products) - S m. DHf 0 (reactants) Hess’s Law: When reactants are converted to products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps. (Enthalpy is a state function. It doesn’t matter how you get there, only where you start and end. )

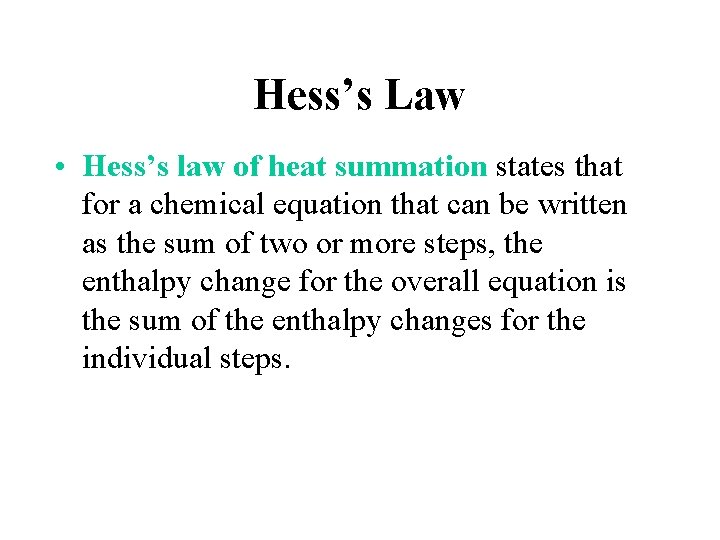
Hess’s Law • Hess’s law of heat summation states that for a chemical equation that can be written as the sum of two or more steps, the enthalpy change for the overall equation is the sum of the enthalpy changes for the individual steps.

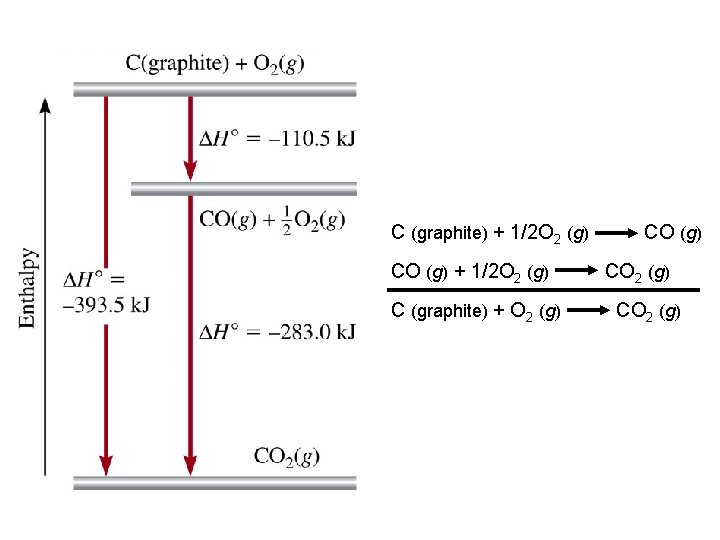
C (graphite) + 1/2 O 2 (g) CO (g) + 1/2 O 2 (g) C (graphite) + O 2 (g) CO 2 (g)

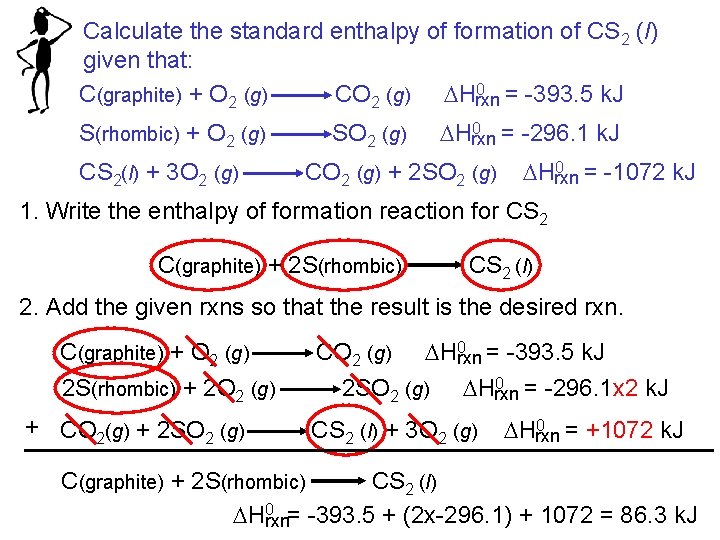
Calculate the standard enthalpy of formation of CS 2 (l) given that: 0 = -393. 5 k. J C(graphite) + O 2 (g) CO 2 (g) DHrxn S(rhombic) + O 2 (g) CS 2(l) + 3 O 2 (g) SO 2 (g) 0 = -296. 1 k. J DHrxn CO 2 (g) + 2 SO 2 (g) 0 = -1072 k. J DHrxn 1. Write the enthalpy of formation reaction for CS 2 C(graphite) + 2 S(rhombic) CS 2 (l) 2. Add the given rxns so that the result is the desired rxn. C(graphite) + O 2 (g) 2 S(rhombic) + 2 O 2 (g) + CO 2(g) + 2 SO 2 (g) 0 = -393. 5 k. J CO 2 (g) DHrxn 0 = -296. 1 x 2 k. J 2 SO 2 (g) DHrxn CS 2 (l) + 3 O 2 (g) 0 = +1072 k. J DHrxn C(graphite) + 2 S(rhombic) CS 2 (l) DH 0 rxn= -393. 5 + (2 x-296. 1) + 1072 = 86. 3 k. J

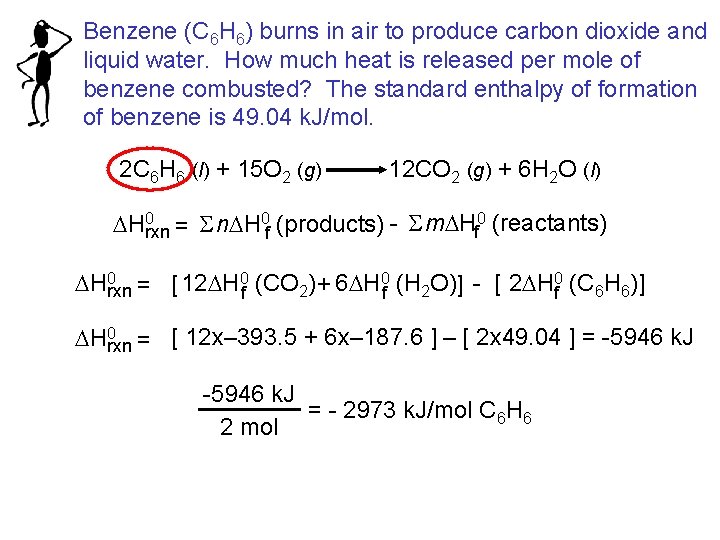
Benzene (C 6 H 6) burns in air to produce carbon dioxide and liquid water. How much heat is released per mole of benzene combusted? The standard enthalpy of formation of benzene is 49. 04 k. J/mol. 2 C 6 H 6 (l) + 15 O 2 (g) 12 CO 2 (g) + 6 H 2 O (l) DH 0 rxn = S n. DH 0 f (products) - S m. DHf 0 (reactants) DH 0 rxn = [ 12 DH 0 f (CO 2) + 6 DH 0 f (H 2 O)] - [ 2 DH 0 f (C 6 H 6)] DH 0 rxn = [ 12 x– 393. 5 + 6 x– 187. 6 ] – [ 2 x 49. 04 ] = -5946 k. J = - 2973 k. J/mol C 6 H 6 2 mol

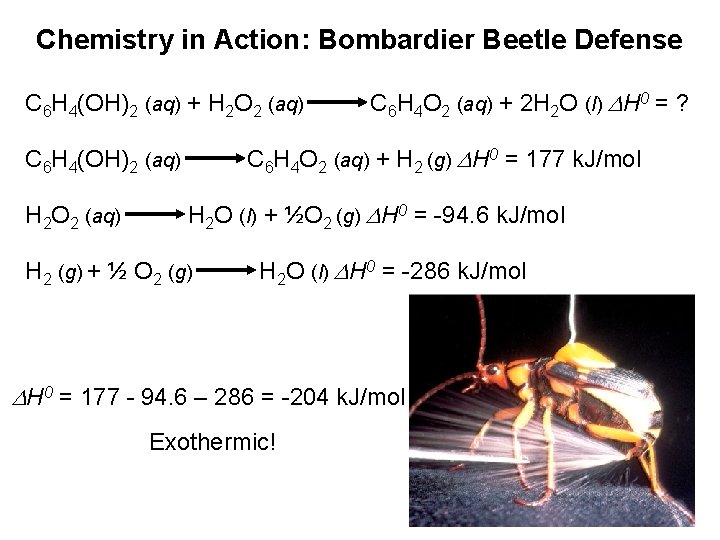
Chemistry in Action: Bombardier Beetle Defense C 6 H 4(OH)2 (aq) + H 2 O 2 (aq) C 6 H 4 O 2 (aq) + H 2 (g) DH 0 = 177 k. J/mol C 6 H 4(OH)2 (aq) H 2 O 2 (aq) C 6 H 4 O 2 (aq) + 2 H 2 O (l) DH 0 = ? H 2 O (l) + ½O 2 (g) DH 0 = -94. 6 k. J/mol H 2 (g) + ½ O 2 (g) H 2 O (l) DH 0 = -286 k. J/mol DH 0 = 177 - 94. 6 – 286 = -204 k. J/mol Exothermic!

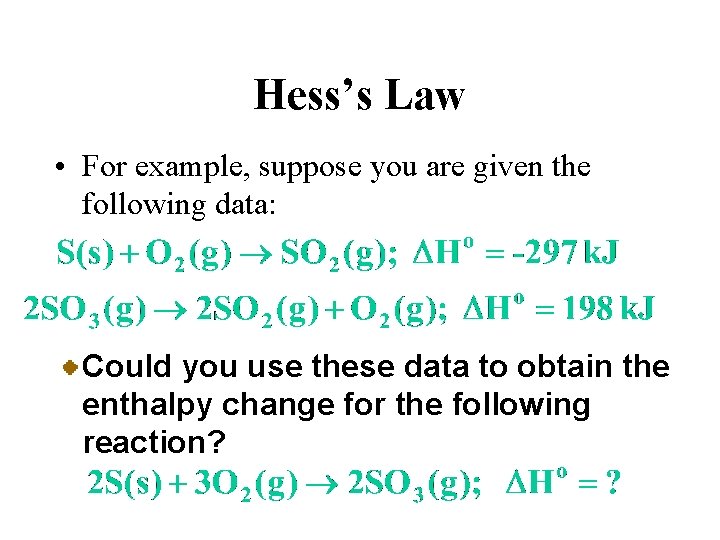
Hess’s Law • For example, suppose you are given the following data: Could you use these data to obtain the enthalpy change for the following reaction?

Hess’s Law • If we multiply the first equation by 2 and reverse the second equation, they will sum together to become third.

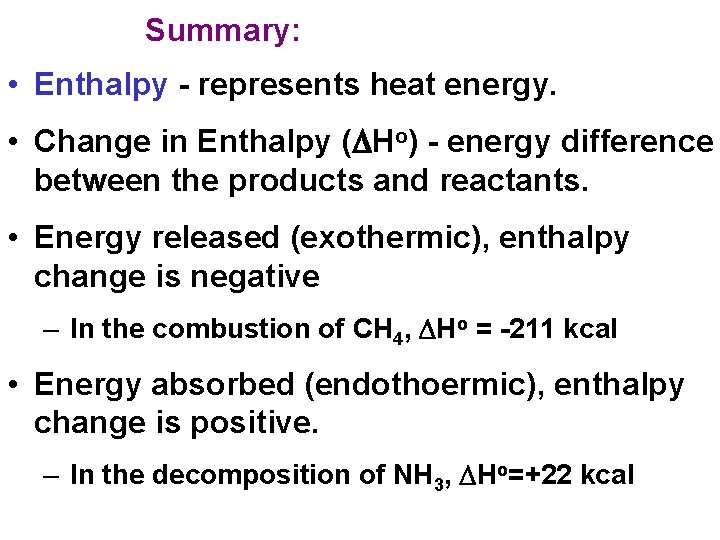
Summary: • Enthalpy - represents heat energy. • Change in Enthalpy (DHo) - energy difference between the products and reactants. • Energy released (exothermic), enthalpy change is negative – In the combustion of CH 4, DHo = -211 kcal • Energy absorbed (endothoermic), enthalpy change is positive. – In the decomposition of NH 3, DHo=+22 kcal

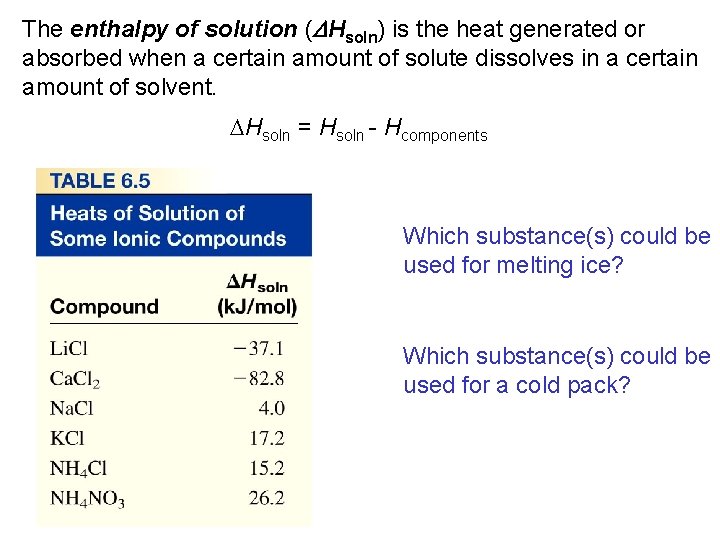
The enthalpy of solution (DHsoln) is the heat generated or absorbed when a certain amount of solute dissolves in a certain amount of solvent. DHsoln = Hsoln - Hcomponents Which substance(s) could be used for melting ice? Which substance(s) could be used for a cold pack?

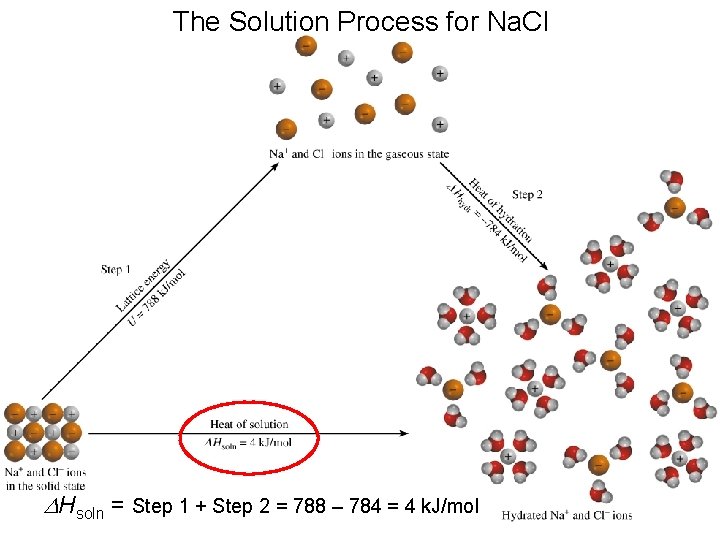
The Solution Process for Na. Cl DHsoln = Step 1 + Step 2 = 788 – 784 = 4 k. J/mol

So enthalpy explains heat transfer in a reaction…. . • What about predicting if a reaction will occur spontaneously at a given temperature?

Spontaneous and Nonspontaneous Reactions • Spontaneous reaction - occurs without any external energy input. • Often, but not always, exothermic reactions are spontaneous. • Thermodynamics is used to help predict if a reaction will occur. • Another factor is needed.

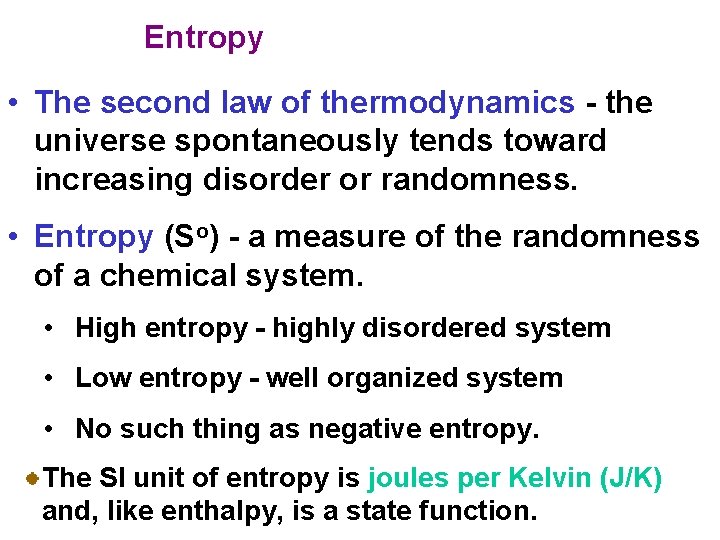
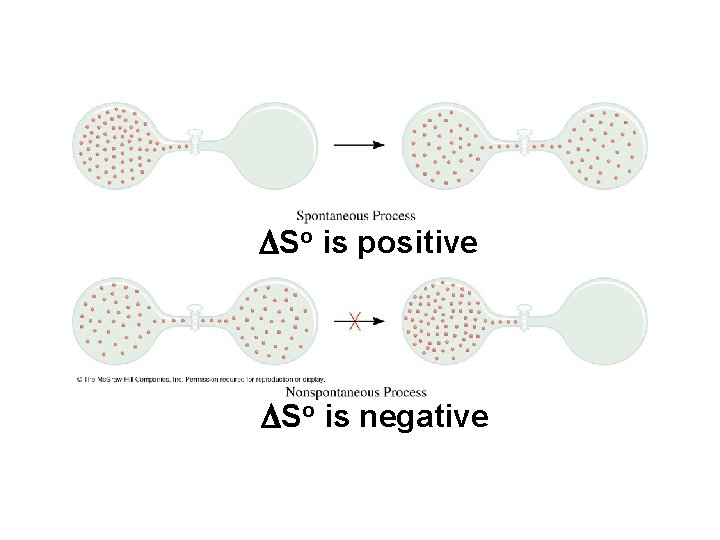
Entropy • The second law of thermodynamics - the universe spontaneously tends toward increasing disorder or randomness. • Entropy (So) - a measure of the randomness of a chemical system. • High entropy - highly disordered system • Low entropy - well organized system • No such thing as negative entropy. The SI unit of entropy is joules per Kelvin (J/K) and, like enthalpy, is a state function.

DSo of a reaction = So(products) - So(reactants) • A positive DSo means an increase in disorder for the reaction. • A negative DSo means a decrease in disorder for the reaction.

DSo is positive DSo is negative

• If exothermic and positive DSo… SPONTANEOUS • If endothermic and negative DSo… NONSPONTANEOUS • For any other situations, it depends on the relative size of DHo and DSo.

Free Energy Concept • The American physicist J. Willard Gibbs introduced the concept of free energy (sometimes called the Gibbs free energy), G, which is a thermodynamic quantity defined by the equation G=H-TS. This quantity gives a direct criterion for spontaneity of reaction.

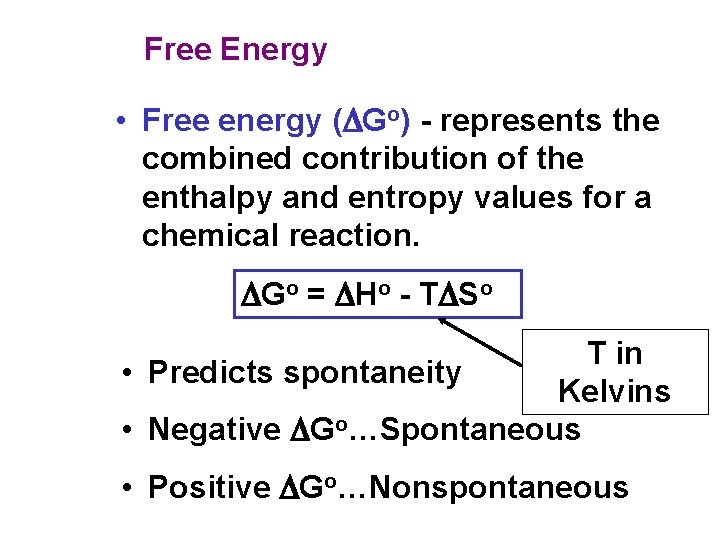
Free Energy • Free energy (DGo) - represents the combined contribution of the enthalpy and entropy values for a chemical reaction. DGo = DHo - TDSo T in • Predicts spontaneity Kelvins • Negative DGo…Spontaneous • Positive DGo…Nonspontaneous

Further summary: • Thermodynamics determines if a reaction will occur but tells us nothing about the time it will take • Enthalpy - represents heat energy. • Entropy (So) - a measure of the randomness of a chemical system. • Free energy (DGo) - represents the combined contribution of the enthalpy and entropy values for a chemical reaction. ∆G predicts spontaneity of a chemical reaction at a given temperature. More in next semester.

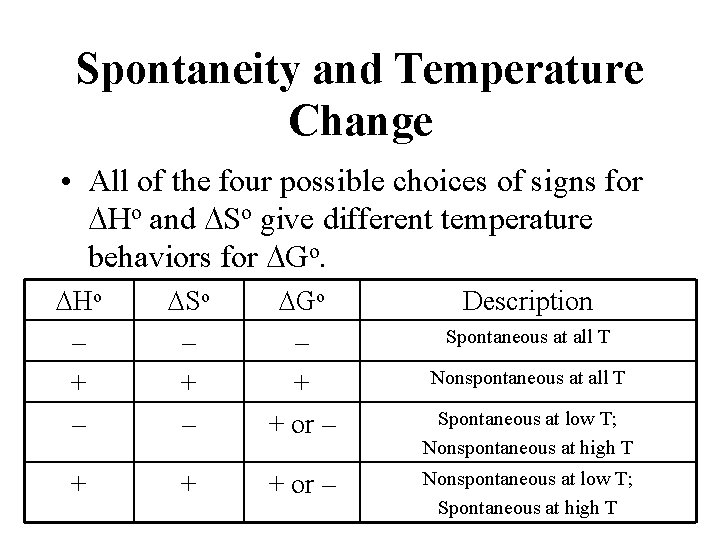
Spontaneity and Temperature Change • All of the four possible choices of signs for DHo and DSo give different temperature behaviors for DGo. DHo – + – DSo – + – DGo – + + or – + + + or – Description Spontaneous at all T Nonspontaneous at all T Spontaneous at low T; Nonspontaneous at high T Nonspontaneous at low T; Spontaneous at high T

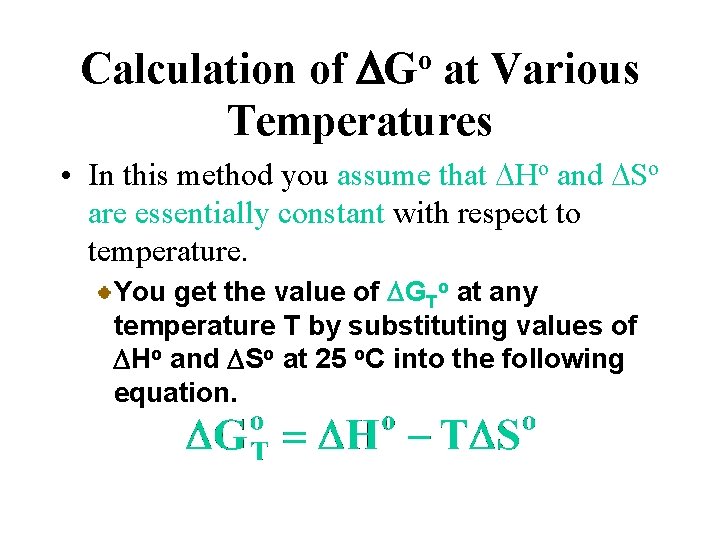
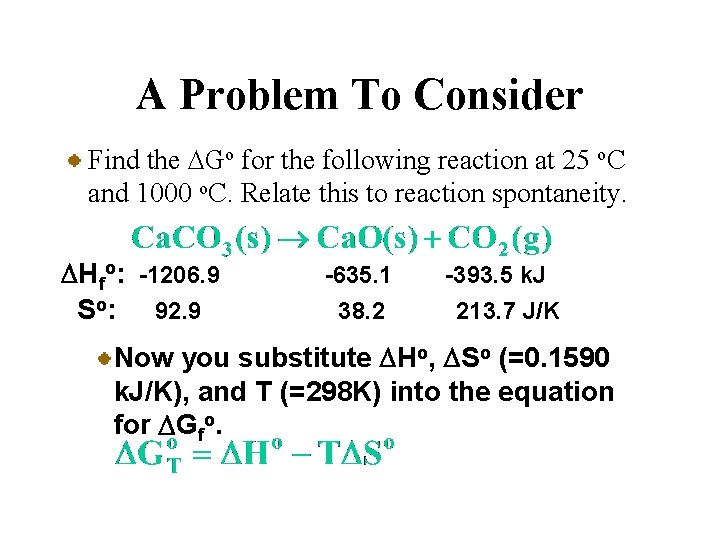
Calculation of DGo at Various Temperatures • In this method you assume that DHo and DSo are essentially constant with respect to temperature. You get the value of DGTo at any temperature T by substituting values of DHo and DSo at 25 o. C into the following equation.

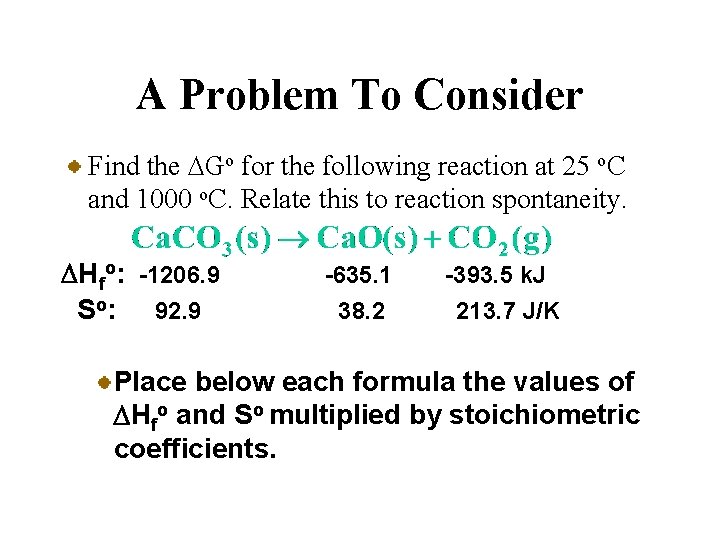
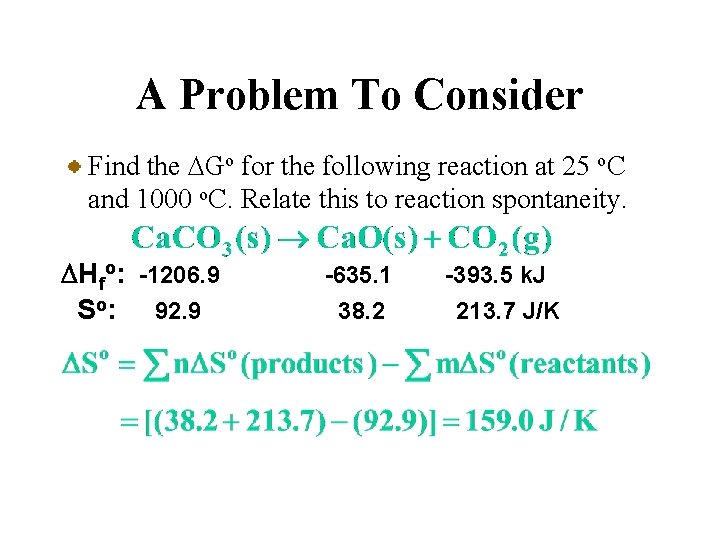
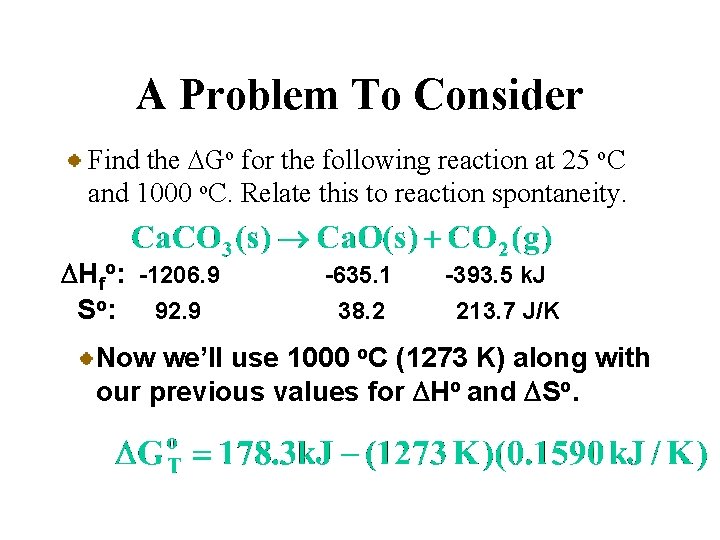
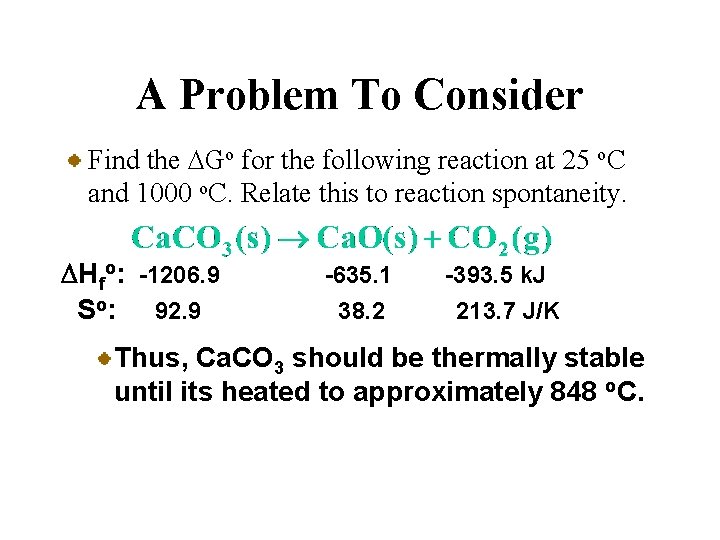
A Problem To Consider Find the DGo for the following reaction at 25 o. C and 1000 o. C. Relate this to reaction spontaneity. DHfo: -1206. 9 So: 92. 9 -635. 1 38. 2 -393. 5 k. J 213. 7 J/K Place below each formula the values of DHfo and So multiplied by stoichiometric coefficients.

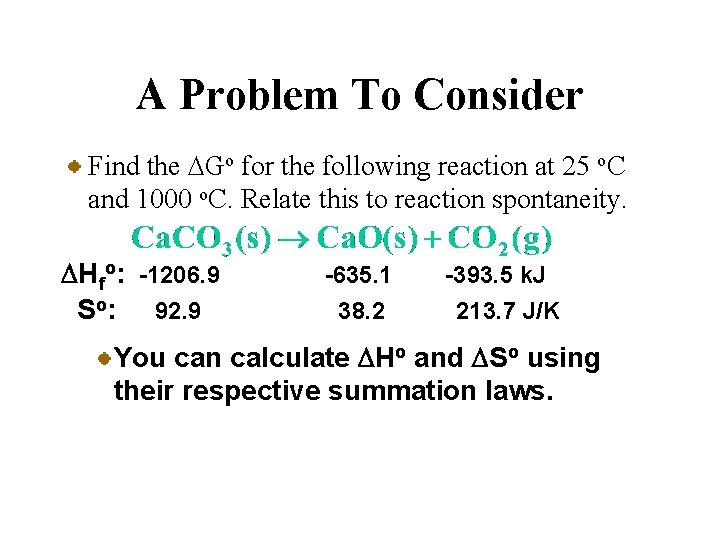
A Problem To Consider Find the DGo for the following reaction at 25 o. C and 1000 o. C. Relate this to reaction spontaneity. DHfo: -1206. 9 So: 92. 9 -635. 1 38. 2 -393. 5 k. J 213. 7 J/K You can calculate DHo and DSo using their respective summation laws.

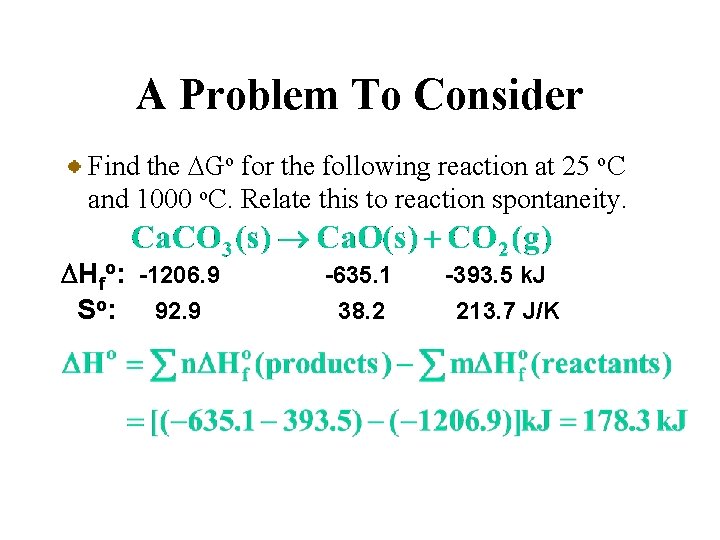
A Problem To Consider Find the DGo for the following reaction at 25 o. C and 1000 o. C. Relate this to reaction spontaneity. DHfo: -1206. 9 So: 92. 9 -635. 1 38. 2 -393. 5 k. J 213. 7 J/K

A Problem To Consider Find the DGo for the following reaction at 25 o. C and 1000 o. C. Relate this to reaction spontaneity. DHfo: -1206. 9 So: 92. 9 -635. 1 38. 2 -393. 5 k. J 213. 7 J/K

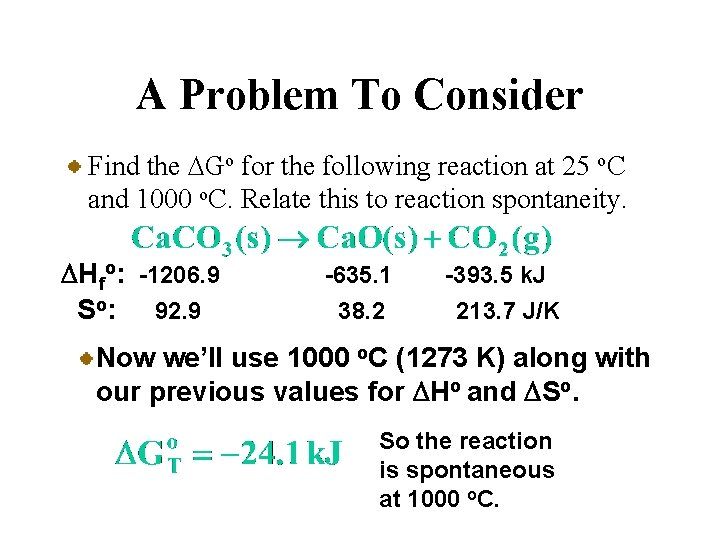
A Problem To Consider Find the DGo for the following reaction at 25 o. C and 1000 o. C. Relate this to reaction spontaneity. DHfo: -1206. 9 So: 92. 9 -635. 1 38. 2 -393. 5 k. J 213. 7 J/K Now you substitute DHo, DSo (=0. 1590 k. J/K), and T (=298 K) into the equation for DGfo.

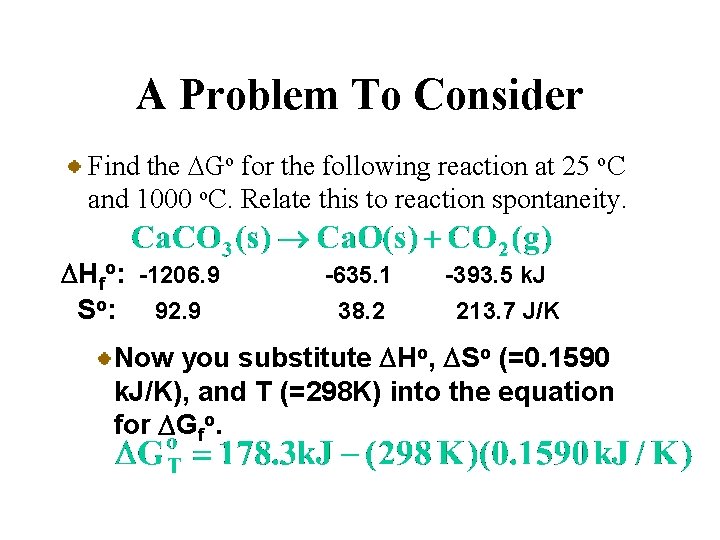
A Problem To Consider Find the DGo for the following reaction at 25 o. C and 1000 o. C. Relate this to reaction spontaneity. DHfo: -1206. 9 So: 92. 9 -635. 1 38. 2 -393. 5 k. J 213. 7 J/K Now you substitute DHo, DSo (=0. 1590 k. J/K), and T (=298 K) into the equation for DGfo.

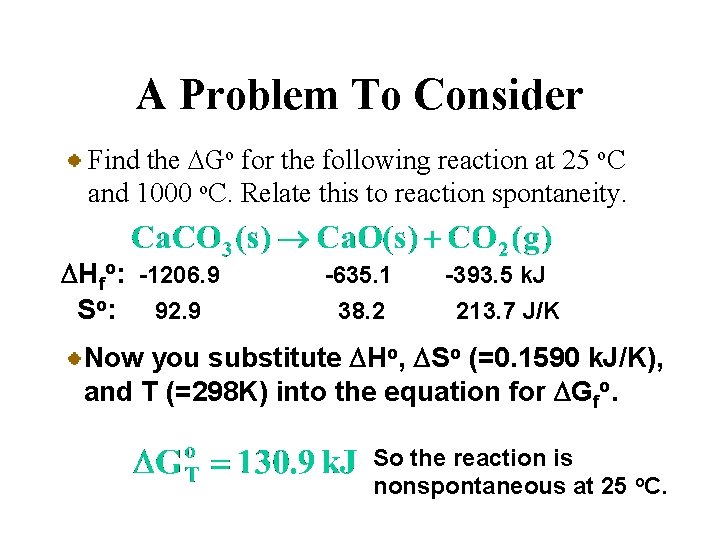
A Problem To Consider Find the DGo for the following reaction at 25 o. C and 1000 o. C. Relate this to reaction spontaneity. DHfo: -1206. 9 So: 92. 9 -635. 1 38. 2 -393. 5 k. J 213. 7 J/K Now you substitute DHo, DSo (=0. 1590 k. J/K), and T (=298 K) into the equation for DGfo. So the reaction is nonspontaneous at 25 o. C.

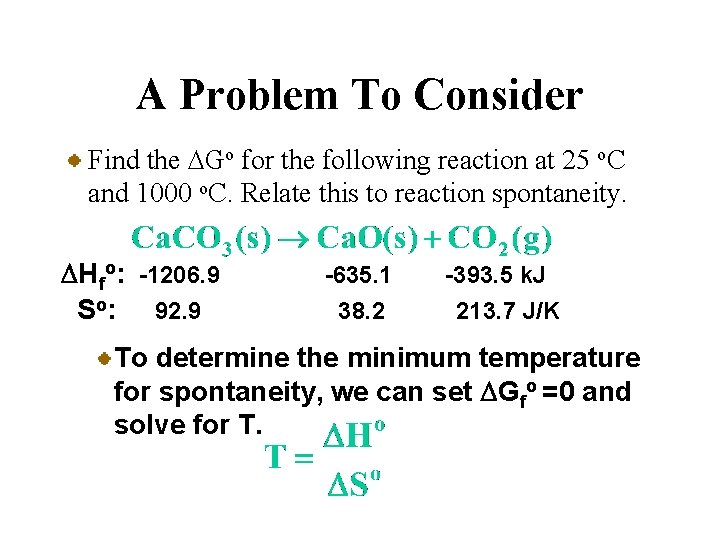
A Problem To Consider Find the DGo for the following reaction at 25 o. C and 1000 o. C. Relate this to reaction spontaneity. DHfo: -1206. 9 So: 92. 9 -635. 1 38. 2 -393. 5 k. J 213. 7 J/K Now we’ll use 1000 o. C (1273 K) along with our previous values for DHo and DSo.

A Problem To Consider Find the DGo for the following reaction at 25 o. C and 1000 o. C. Relate this to reaction spontaneity. DHfo: -1206. 9 So: 92. 9 -635. 1 38. 2 -393. 5 k. J 213. 7 J/K Now we’ll use 1000 o. C (1273 K) along with our previous values for DHo and DSo. So the reaction is spontaneous at 1000 o. C.

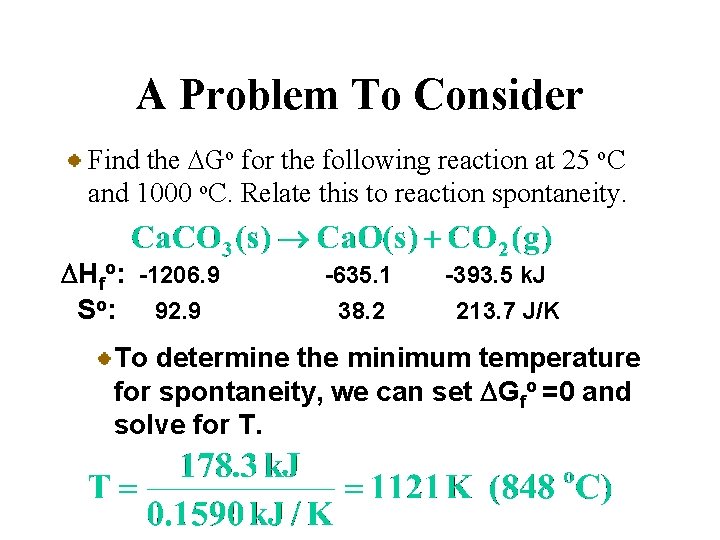
A Problem To Consider Find the DGo for the following reaction at 25 o. C and 1000 o. C. Relate this to reaction spontaneity. DHfo: -1206. 9 So: 92. 9 -635. 1 38. 2 -393. 5 k. J 213. 7 J/K To determine the minimum temperature for spontaneity, we can set DGfo =0 and solve for T.

A Problem To Consider Find the DGo for the following reaction at 25 o. C and 1000 o. C. Relate this to reaction spontaneity. DHfo: -1206. 9 So: 92. 9 -635. 1 38. 2 -393. 5 k. J 213. 7 J/K To determine the minimum temperature for spontaneity, we can set DGfo =0 and solve for T.

A Problem To Consider Find the DGo for the following reaction at 25 o. C and 1000 o. C. Relate this to reaction spontaneity. DHfo: -1206. 9 So: 92. 9 -635. 1 38. 2 -393. 5 k. J 213. 7 J/K Thus, Ca. CO 3 should be thermally stable until its heated to approximately 848 o. C.

Fuels • A fuel is any substance that is burned to provide heat or other forms of energy. In this section we will look at: Foods as fuels Fossil fuels Coal gasification and liquefaction

Fuels • Food fills three needs of the body: It supplies substances for the growth and repair of tissue. It supplies substances for the synthesis of compounds used in the regulation of body processes. It supplies energy. About 80% of the energy we need is for heat. The rest is used for muscular action and other body processes

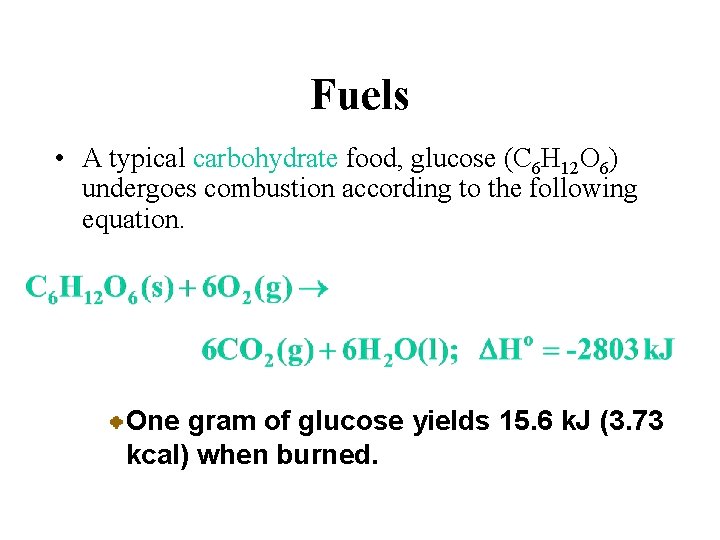
Fuels • A typical carbohydrate food, glucose (C 6 H 12 O 6) undergoes combustion according to the following equation. One gram of glucose yields 15. 6 k. J (3. 73 kcal) when burned.

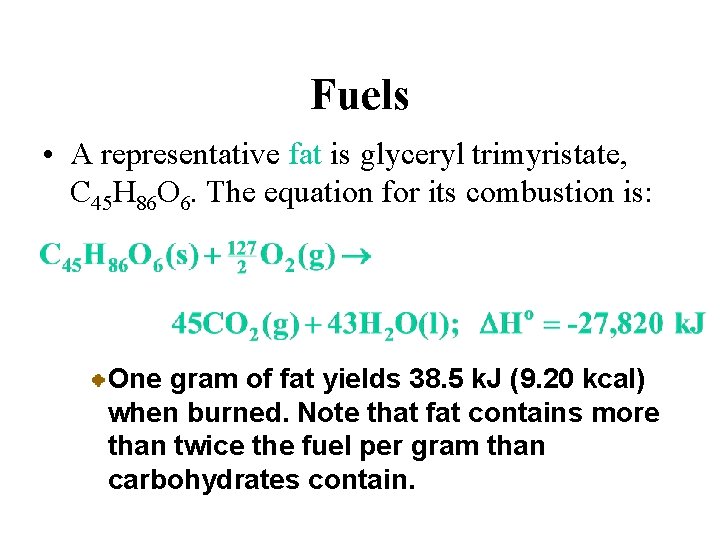
Fuels • A representative fat is glyceryl trimyristate, C 45 H 86 O 6. The equation for its combustion is: One gram of fat yields 38. 5 k. J (9. 20 kcal) when burned. Note that fat contains more than twice the fuel per gram than carbohydrates contain.

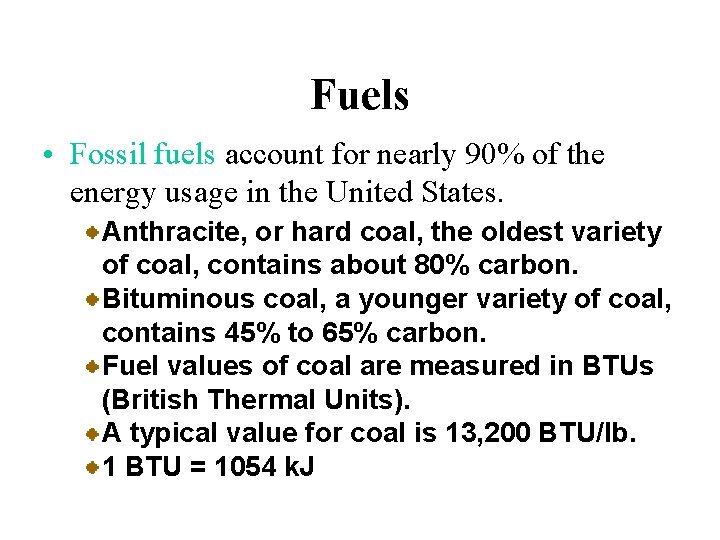
Fuels • Fossil fuels account for nearly 90% of the energy usage in the United States. Anthracite, or hard coal, the oldest variety of coal, contains about 80% carbon. Bituminous coal, a younger variety of coal, contains 45% to 65% carbon. Fuel values of coal are measured in BTUs (British Thermal Units). A typical value for coal is 13, 200 BTU/lb. 1 BTU = 1054 k. J

Fuels • Natural gas and petroleum account for nearly three -quarters of the fossil fuels consumed per year. Purified natural gas is primarily methane, CH 4, but also contains small quantities of ethane, C 2 H 6, propane, C 3 H 8, and butane, C 4 H 10. We would expect the fuel value of natural gas to be close to that for the combustion of methane.

Fuels • Petroleum is a very complicated mixture of compounds. Gasoline, obtained from petroleum, contains many different hydrocarbons, one of which is octane, C 8 H 18. This value of DHo is equivalent to 44. 4 k. J/gram.

Fuels • With supplies of petroleum estimated to be 80% depleted by the year 2030, the gasification of coal has become a possible alternative. First, coal is converted to carbon monoxide using steam. The carbon monoxide can then be used to produce a variety of other fuels, such as methane.
 Science form 3 chapter 5 exercise with answers
Science form 3 chapter 5 exercise with answers What is her favourite subject?
What is her favourite subject? Thermodynamics is a branch of science which deals with
Thermodynamics is a branch of science which deals with Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Chapter 17 thermochemistry
Chapter 17 thermochemistry Chapter 17 thermochemistry answer key
Chapter 17 thermochemistry answer key Quiz 2: heat flow and technology
Quiz 2: heat flow and technology Chapter 6 thermochemistry
Chapter 6 thermochemistry Chapter 17 thermochemistry
Chapter 17 thermochemistry Reschual
Reschual Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Thermodynamics for chemical engineering
Thermodynamics for chemical engineering Chemical engineering thermodynamics 8th solution chapter 6
Chemical engineering thermodynamics 8th solution chapter 6 Control mass
Control mass Chemical engineering thermodynamics 8th solution chapter 10
Chemical engineering thermodynamics 8th solution chapter 10 Thermodynamics chapter 4
Thermodynamics chapter 4 Thermodynamics for chemical engineering
Thermodynamics for chemical engineering Specific heat capacity
Specific heat capacity Thermodynamics chapter 3
Thermodynamics chapter 3 Thermodynamics chapter 2
Thermodynamics chapter 2 Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Chúa yêu trần thế
Chúa yêu trần thế Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc cc
V cc cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Thế nào là số nguyên tố
Thế nào là số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Chemistry: the central science chapter 14 answers
Chemistry: the central science chapter 14 answers Thermochemistry
Thermochemistry Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is study of
Thermochemistry is study of Thermochemistry equations
Thermochemistry equations Thermochemistry
Thermochemistry Thermochemistry gaussian
Thermochemistry gaussian Thermochemistry formulas
Thermochemistry formulas Thermochemistry cartoon
Thermochemistry cartoon Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Thermochemistry is the study of...
Thermochemistry is the study of... Thermochemistry equations
Thermochemistry equations Symbolic border one pager
Symbolic border one pager Thermochemical equation
Thermochemical equation Kirchhoff's law of thermochemistry
Kirchhoff's law of thermochemistry Thermochemistry equations
Thermochemistry equations Thermochemistry cartoon
Thermochemistry cartoon Ap chemistry thermodynamics
Ap chemistry thermodynamics Ap chemistry thermochemistry frq
Ap chemistry thermochemistry frq Thermochemistry is study of
Thermochemistry is study of General chemistry thermochemistry
General chemistry thermochemistry Study of energy transformations
Study of energy transformations Kimia adalah
Kimia adalah What is thermochemistry
What is thermochemistry