Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry

























































































- Slides: 134

Chapter 6 Thermochemistry Chapter 6

Chapter 6 Thermochemistry 6. 1 6. 2 6. 3 6. 4 6. 5 6. 6 6. 7 6. 8 6. 9 6. 10 Chemical Hand Warmers The Nature of Energy: Key Definitions The First Law of Thermodynamics: There is no Free Lunch Quantifying Heat and Work Measuring DE for Chemical Reactions: Constant – Volume Calorimetry Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Constant – Pressure Calorimetry: Measuring DHrxn Relationships Involving DHrxn Determining Enthalpies of Reaction from Standard Enthalpies of Formation Energy Use and the Environment 2

Chapter 6 Thermochemistry Matter vs Energy • Chapters 1 – 5 have mainly been concerned with matter – – • How we describe it How we measure it This chapter describes energy – – How we describe it How we measure it 3

Section 6. 1 Chemical Hand Warmers Thermochemistry • The study of the relationships between matter and energy is a field called thermochemistry • 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) + heat – Oxidation of iron • This reaction takes place inside chemical heat packs • Notice heat is written as a product 4

Section 6. 1 Chemical Hand Warmers Thermochemistry • Another familiar reaction we have seen • 2 C 8 H 18 (l) + 25 O 2 (g) → 16 CO 2 (g) + 18 H 2 O (g) + heat – Oxidation of octane • This reaction also generates heat 5

Section 6. 2 The Nature of Energy: Key Definitions Potential vs Kinetic Energy in Chemistry • Chemical Potential energy is stored energy – the energy stored in chemical bonds – – C 8 H 18 Hi Ep Less stable Higher energy CO 2 + H 2 O Lo Ep More stable Lower energy • Chemical Kinetic energy is the energy of motion of the particles – what we measure when we measure temperature 6

Section 6. 1 Chemical Hand Warmers Thermochemistry • Another familiar reaction we have seen • 2 C 8 H 18 (l) + 25 O 2 (g) → 16 CO 2 (g) + 18 H 2 O (g) + heat – Oxidation of octane • This reaction generates heat – release of Ep • The heat is used to do work – we will talk about this in this chapter 7

Section 6. 2 The Nature of Energy: Key Definitions The Transfer of Energy: Heat and Work • Energy is something an object has • Heat and work are ways energy is transferred • When you hold a warm object in you hand, energy is transferred from the object to your hand in the form of heat • When a ball on a pool table hits another ball energy is transferred from the first ball to the second ball in the form of work 8

Section 6. 2 The Nature of Energy: Key Definitions The Law of Conservation of Energy • Law of conservation of energy – energy can be neither created nor destroyed – Can assume different forms • Energy from iron and oxygen in our heat pack example becomes heat • Energy from octane becomes heat that is converted to work • Energy from the sun is converted to complex sugars in plants 9

Section 6. 2 The Nature of Energy: Key Definitions The Law of Conservation of Energy • Law of conservation of energy – energy can be neither created nor destroyed – Can be transferred from one object to another • In this chapter we are going to learn how to quantitatively measure the transfer of energy as either heat or work 10

Section 6. 2 The Nature of Energy: Key Definitions System vs Surroundings • Energy is exchanged between the system under investigation and the surroundings • System – part of the universe on which we wish to focus attention – Kind of a weird definition can be just the chemicals reacting or can include the beaker and liquids in it • Surroundings – everything with which the system exchanges energy • The definition isn’t really the most important thing – Understanding how they exchange Energy is important 11

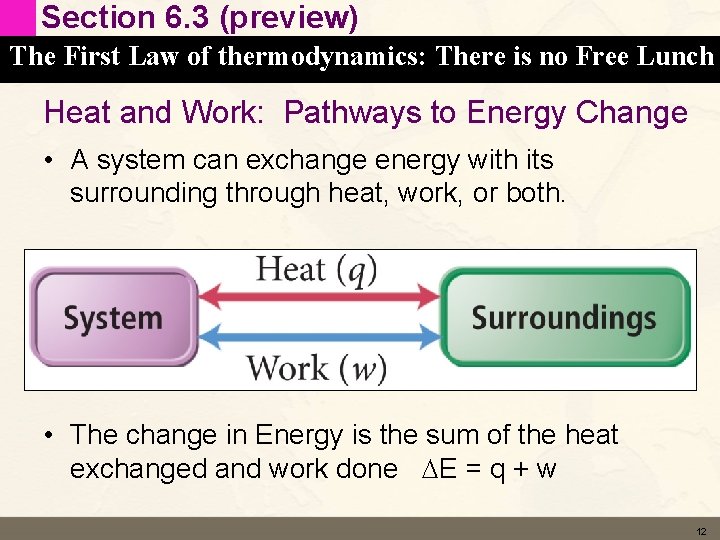
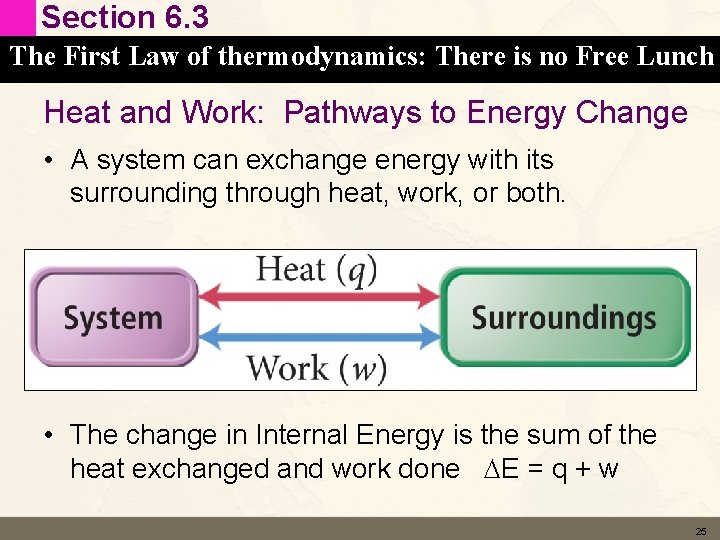
Section 6. 3 (preview) The First Law of thermodynamics: There is no Free Lunch Heat and Work: Pathways to Energy Change • A system can exchange energy with its surrounding through heat, work, or both. • The change in Energy is the sum of the heat exchanged and work done DE = q + w 12

Section 6. 2 The Nature of Energy: Key Definitions Units of Energy • In Chapter 5 we saw that Kinetic Energy was defined as ½ mv 2 where m = mass and v = velocity • 1 J = the joule • Another common unit is the calorie (cal) – 1 cal = 4. 184 Joules • The Calorie we see on nutritional labels = 1000 cal = kilocal 13

Section 6. 2 The Nature of Energy: Key Definitions Energy Conversion Factors • Don’t memorize these! • Be able to use them. 14

Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Internal Energy • The Internal Energy of a system is the sum of all the kinetic and potential energies of the particles that compose the system. – Potential energy is the energy stored in the chemical bonds – Kinetic energy is the energy of the motion of the particles • E = Ek + Ep 15

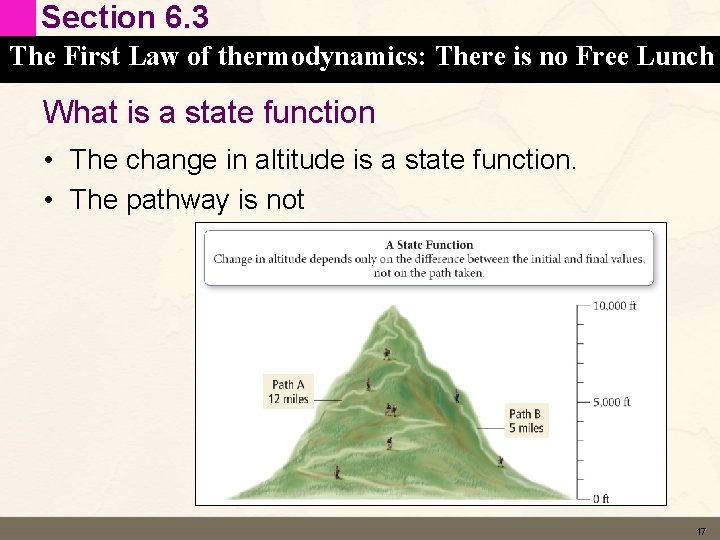
Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Internal Energy is a State Function • A state function is a quantity whose value depends only on the state of the system not on the pathway the system arrived in that state. • What does this mean? 16

Section 6. 3 The First Law of thermodynamics: There is no Free Lunch What is a state function • The change in altitude is a state function. • The pathway is not 17

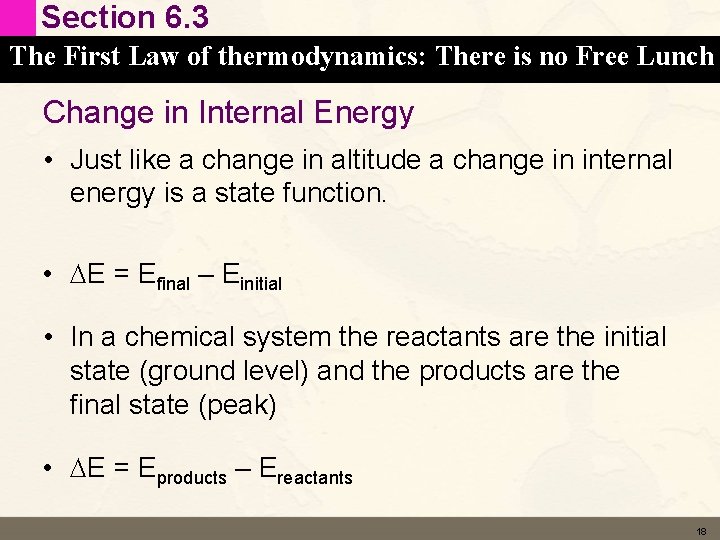
Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Change in Internal Energy • Just like a change in altitude a change in internal energy is a state function. • DE = Efinal – Einitial • In a chemical system the reactants are the initial state (ground level) and the products are the final state (peak) • DE = Eproducts – Ereactants 18

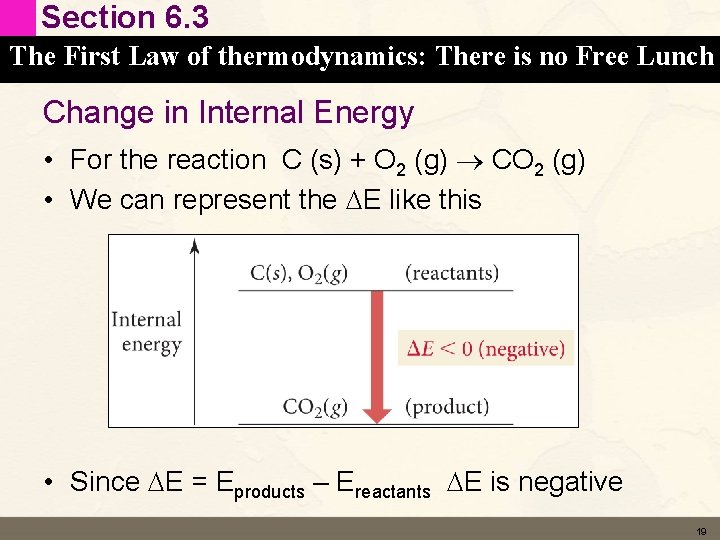
Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Change in Internal Energy • For the reaction C (s) + O 2 (g) CO 2 (g) • We can represent the DE like this • Since DE = Eproducts – Ereactants DE is negative 19

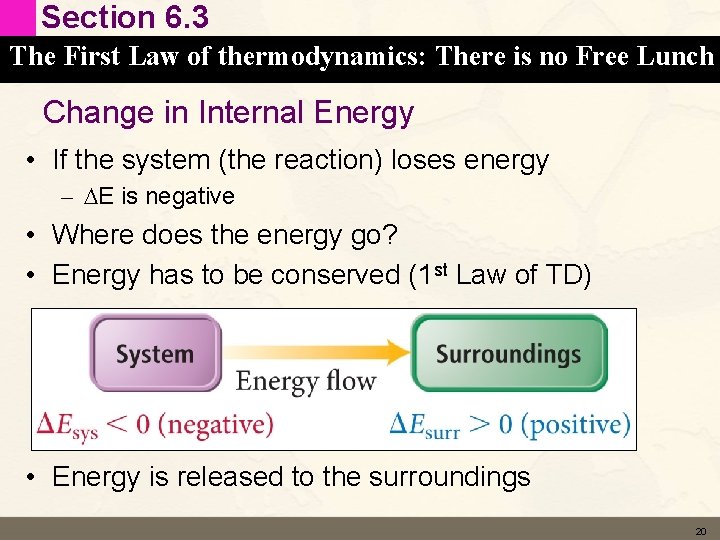
Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Change in Internal Energy • If the system (the reaction) loses energy – DE is negative • Where does the energy go? • Energy has to be conserved (1 st Law of TD) • Energy is released to the surroundings 20

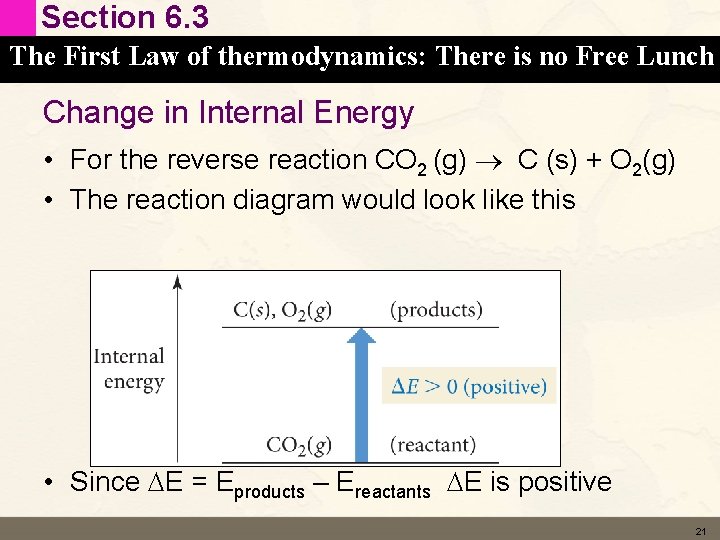
Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Change in Internal Energy • For the reverse reaction CO 2 (g) C (s) + O 2(g) • The reaction diagram would look like this • Since DE = Eproducts – Ereactants DE is positive 21

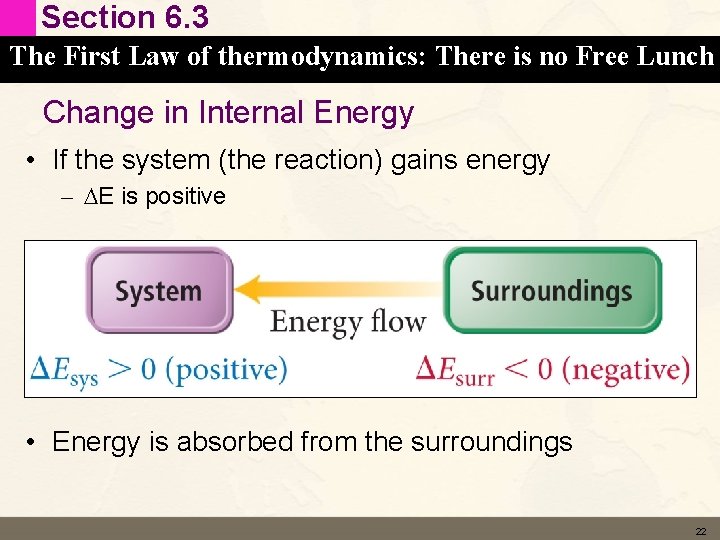
Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Change in Internal Energy • If the system (the reaction) gains energy – DE is positive • Energy is absorbed from the surroundings 22

Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Concept Check Hydrogen gas and oxygen gas react explosively to form water. § Which is lower in energy: a mixture of hydrogen and oxygen gases, or water? 23

Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Solution Hydrogen gas and oxygen gas react explosively to form water. § Which is lower in energy: a mixture of hydrogen and oxygen gases, or water? Water is lower in energy because a lot of energy was released when hydrogen and oxygen gases reacted. 24

Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Heat and Work: Pathways to Energy Change • A system can exchange energy with its surrounding through heat, work, or both. • The change in Internal Energy is the sum of the heat exchanged and work done DE = q + w 25

Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Heat and Work: Pathways to Energy Change • When we looked at the change in altitude on the mountain we saw that the two pathways were very different. – The change in altitude was a state function – The pathways were not state functions • Same situation for heat and work • DE = heat + work = q + w – DE is a state function – q and w are not 26

Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Heat and Work: Pathways to Energy Change • DE is a state function – q and w are not • Think about a gallon of gas – Burning in an open container (lots of heat, not much work) – Moving a car 40 miles (lots of work, less heat) • Same change in DE but q and w are completely different depending on the path 27

Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Sign conventions for DE, q and w • DE with no subscript always refers to DE of the system – Negative sign means the system loses energy • What about heat and work? • Signs are defined from the point of view of the system. – – If the system does work w is negative If the system loses heat q is negative If work on done on the system w is positive If the system gains heat q is positive 28

Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Concept Check Determine the value of DE for the combustion of 1 gallon of gasoline in a car engine q = – 8. 89 x 104 k. J w = – 3. 81 x 103 k. J 29

Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Solution Determine the value of DE for the combustion of 1 gallon of gasoline in a car engine DE = q + w = – 8. 89 x 104 k. J + – 3. 81 x 103 k. J DE = – 9. 27 x 104 k. J 30

Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Concept Check In this problem for the determination of the value of DE for the combustion of 1 gallon of gasoline in a car engine, why are the values of q and w both negative. q = – 8. 89 x 104 k. J w = – 3. 81 x 103 k. J 31

Section 6. 3 The First Law of thermodynamics: There is no Free Lunch Solution In this problem for the determination of the value of DE for the combustion of 1 gallon of gasoline in a car engine, why are the values of q and w both negative. q = – 8. 89 x 104 k. J combustion produces heat which leaves the system w = – 3. 81 x 103 k. J work is done on the surroundings 32

Section 6. 4 Quantifying Heat and Work How do we measure Heat and Work • How do we measure the amount of heat gained or released or the amount of work done on a system, or performed by a system? • We measure changes in temperature or volume. • Then use a bunch of equations. • Lets do heat first. 33

Section 6. 4 Quantifying Heat and Work Heat • Heat is the exchange of thermal energy between a system and its surroundings caused by a temperature difference. • Heat and temperature are not the same thing. • Although sometimes we talk about them as if they were. • Temperature is a measure of thermal Energy. • Heat is a transfer of thermal Energy – Heat is not a substance contained by an object, although we often talk of heat as if this were true. 34

Section 6. 4 Quantifying Heat and Work Temperature Change and Heat Capacity • Substances respond differently to being heated § Think of a pool vs a car door on a hot day § Metal absorbs heat fast § Water absorbs heat slowly • The Heat capacity (C) is a measure of this property 35

Section 6. 4 Quantifying Heat and Work Temperature Change and Heat Capacity • Metal absorbs heat fast • Has a low heat capacity • Water absorbs heat slowly • Has a high heat capacity 36

Section 6. 4 Quantifying Heat and Work Heat Capacity • Specific heat capacity (Cs): § The energy required to raise the temperature of one gram of a substance by one degree Celsius. • Molar heat capacity (Cm): § The energy required to raise the temperature of one mole of substance by one degree Celsius. 37

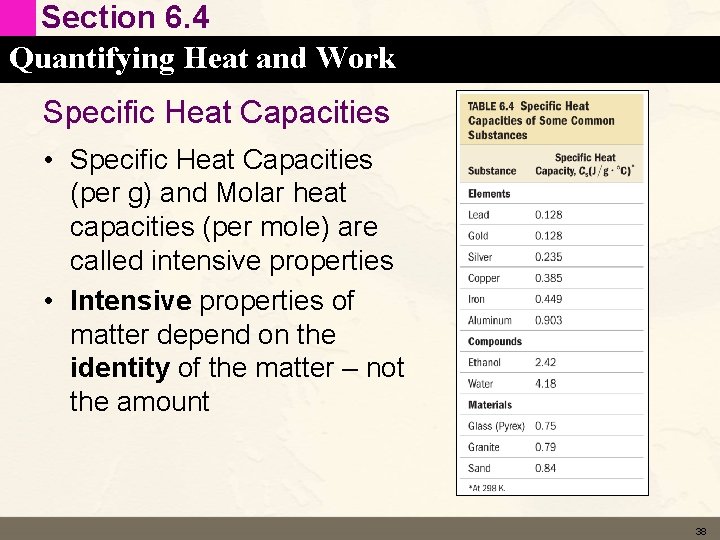
Section 6. 4 Quantifying Heat and Work Specific Heat Capacities • Specific Heat Capacities (per g) and Molar heat capacities (per mole) are called intensive properties • Intensive properties of matter depend on the identity of the matter – not the amount 38

Section 6. 4 Quantifying Heat and Work Heat Calculations • Specific Heat Capacity is used to determine the relationship between the amount of heat added to a system and the corresponding change in temperature. • DT is always Tfinal - Tinitial 39

Section 6. 4 Quantifying Heat and Work Learning Check A piece of iron with a mass of 75. 0 g at 125. 0 °C is allowed to cool to room temperature of 25. 0 °C. Determine the magnitude and sign of q. The specific heat capacity of iron is 0. 449 J/°C g. 40

Section 6. 4 Quantifying Heat and Work Solution A piece of iron with a mass of 75. 0 g at 125. 0 °C is allowed to cool to room temperature of 25. 0 °C. Determine the magnitude and sign of q. The specific heat capacity of iron is 0. 449 J/°C g. q = m × Cs × ΔT m = 75. 0 g Cs = 0. 449 J/°C g. DT = 25. 0 – 125. 0 = -100. 0°C 41

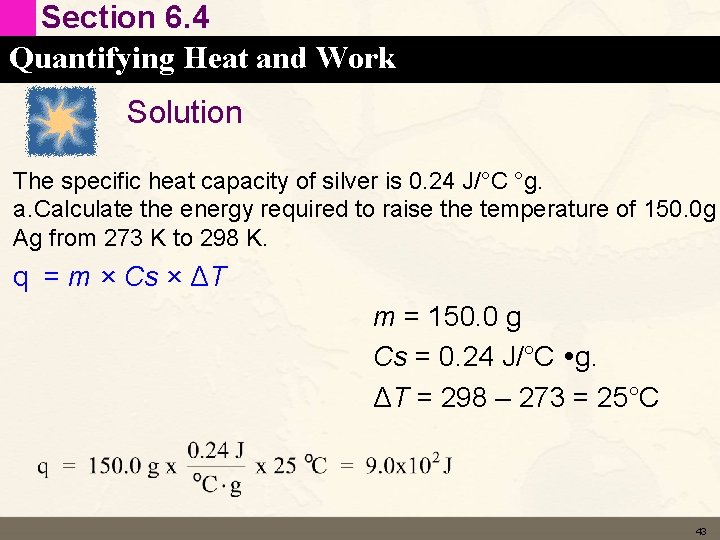
Section 6. 4 Quantifying Heat and Work Learning Check The specific heat capacity of silver is 0. 24 J/°C g. a. Calculate the energy required to raise the temperature of 150. 0 g Ag from 273 K to 298 K. b. Calculate the energy required to raise the temperature of 1. 0 mol Ag by 1. 0°C (called the molar heat capacity of silver). 42

Section 6. 4 Quantifying Heat and Work Solution The specific heat capacity of silver is 0. 24 J/°C °g. a. Calculate the energy required to raise the temperature of 150. 0 g Ag from 273 K to 298 K. q = m × Cs × ΔT m = 150. 0 g Cs = 0. 24 J/°C g. ΔT = 298 – 273 = 25°C 43

Section 6. 4 Quantifying Heat and Work Solution The specific heat capacity of silver is 0. 24 J/°C °g. b. Calculate the energy required to raise the temperature of 1. 0 mol Ag by 1. 0°C (called the molar heat capacity of silver). 44

Section 6. 4 Quantifying Heat and Work Thermal Energy Transfer • If two substances at different temperatures are combined the heat lost by one substance is absorbed by the other • – qsys = qsurr • For example if we place a piece of hot metal in a beaker of water • – qmetal = qwater 45

Section 6. 4 Quantifying Heat and Work Thermal Energy Transfer • How do we use this in calculations • – qmetal = – qwater • – mmetal × Csmetal × ΔTmetal = mwater × Cswater × Δtwater • Heat lost by metal = - heat gained by water 46

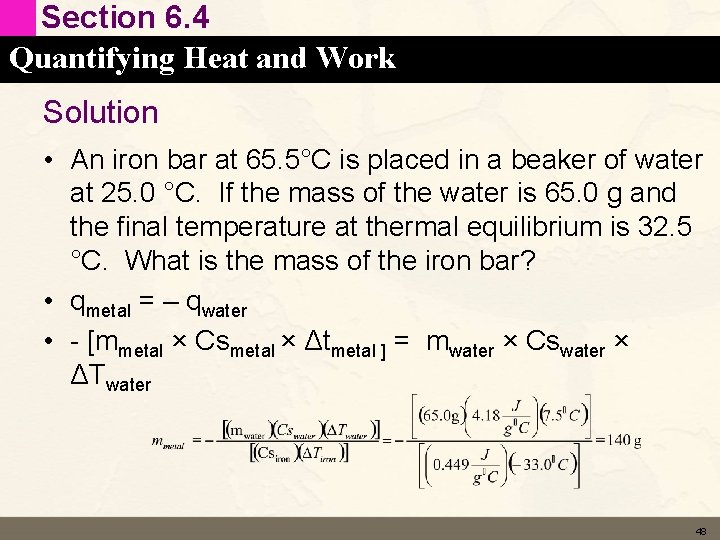
Section 6. 4 Quantifying Heat and Work Learning Check • An iron bar at 65. 5°C is placed in a beaker of water at 25. 0 °C. If the mass of the water is 65. 0 g and the final temperature at thermal equilibrium is 32. 5 °C. What is the mass of the iron bar? The specific heat capacity of water is 4. 184 J/ °C g. 47

Section 6. 4 Quantifying Heat and Work Solution • An iron bar at 65. 5°C is placed in a beaker of water at 25. 0 °C. If the mass of the water is 65. 0 g and the final temperature at thermal equilibrium is 32. 5 °C. What is the mass of the iron bar? • qmetal = – qwater • - [mmetal × Csmetal × Δtmetal ] = mwater × Cswater × ΔTwater 48

Section 6. 4 Quantifying Heat and Work • We know that energy transfer can occur via heat or work • We have seen how to determine the amount of heat transfer by measuring the change in temperature • Now we will look at how to determine the amount of work by measuring the change in volume 49

Section 6. 4 Quantifying Heat and Work: Pressure-Volume Work • Chemical reactions can do several different types of work • We are only going to consider pressure-volume (or PV) work. • PV work occurs when there is a volume change against a constant pressure. – Like in the engine of a car – when the pistons are pushed outward against the external atmospheric pressure. 50

Section 6. 4 Quantifying Heat and Work: Pressure-Volume Work • w = PDV (where A x Dh = DV) • What about the sign of this work 51

Section 6. 4 Quantifying Heat and Work: Pressure-Volume Work • As the volume of the cylinder expands, work is done on the surroundings, so the sign of the work must be negative according to the way we defined work and heat, the only way to accomplish this is to include the negative sign in the formula • w = – PDV 52

Section 6. 4 Quantifying Heat and Work Concept Check Which of the following performs more work? a) A gas expanding against a pressure of 2 atm from 1. 0 L to 4. 0 L. b) A gas expanding against a pressure of 3 atm from 1. 0 L to 3. 0 L. 53

Section 6. 4 Quantifying Heat and Work Solution Which of the following performs more work? a)A gas expanding against a pressure of 2 atm from 1. 0 L to 4. 0 L. w = – (2 atm)(4. 0 – 1. 0) = – 6 L·atm b) A gas expanding against a pressure of 3 atm from 1. 0 L to 3. 0 L w = – (3 atm)(3. 0 – 1. 0) = – 6 L·atm They both perform the same amount of work. 54

Section 6. 4 Quantifying Heat and Work Concept Check A balloon is being inflated to its full extent by heating the air inside it. The volume of the balloon changes from 4. 00 x 106 L to 4. 50 x 106 L. Assuming that the balloon expands against a constant pressure of 1. 0 atm, calculate the work done in this process in Joules. (1 L atm =101. 3 J. ) 55

Section 6. 4 Quantifying Heat and Work Solution A balloon is being inflated to its full extent by heating the air inside it. The volume of the balloon changes from 4. 00 x 106 L to 4. 50 x 106 L. Assuming that the balloon expands against a constant pressure of 1. 0 atm, calculate the work done in this process in Joules. (To convert between L atm and J, use 1 L atm =101. 3 J. ) 56

Section 6. 4 Quantifying Heat and Work Concept Check The balloon in the previous example was expanded by heating the air inside it by the addition of 1. 3 x 108 J of energy as heat. Calculate DE for the process. 57

Section 6. 4 Quantifying Heat and Work Solution DE = q+w Heat = 1. 3 x 108 J (given in problem) Work = – 5. 1 x 107 J (previous problem) 58

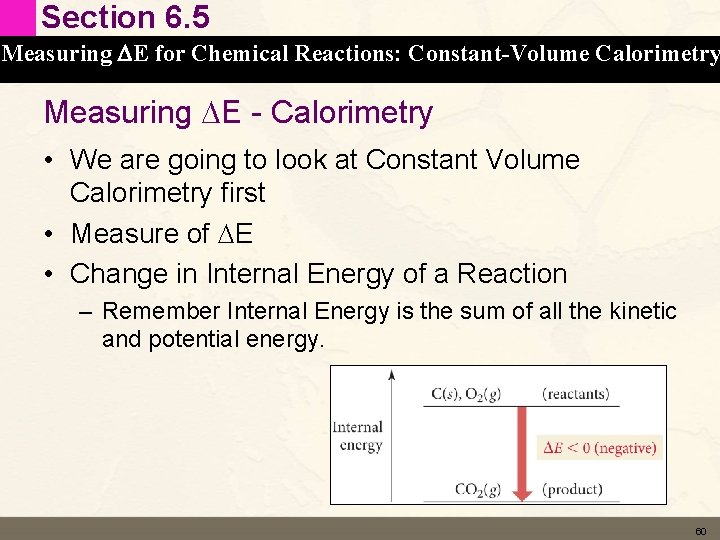
Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry Measuring DE - Calorimetry • Measures thermal energy (heat) exchanged between the reaction (the system) and the surroundings by measuring the change in temperature of water • Two types of Calorimetry – Constant Volume – measures DE – Constant Pressure – measures DH (see slide 86) • Calorimetry - Crash Course 59

Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry Measuring DE - Calorimetry • We are going to look at Constant Volume Calorimetry first • Measure of DE • Change in Internal Energy of a Reaction – Remember Internal Energy is the sum of all the kinetic and potential energy. 60

Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry Measuring DE • So we know that systems exchange energy with their surroundings via heat and work. 61

Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry Measuring DE • Change in internal energy during a reaction is a sum of both the heat and work. DE = q + w • We could determine q and w separately and then add them to determine DE. • It would be easier though if we could force all the change in internal energy to manifest as heat flow (q) with no work done. Only 1 measurement. 62

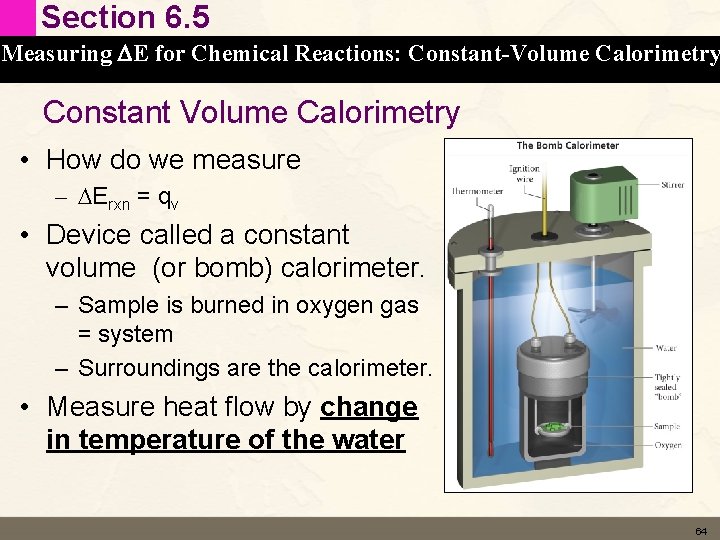
Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry Constant Volume Calorimetry • • • How do we force all of the DE to be heat flow (q) Well DE = q + w And w = – PDV So DE = q – PDV So if we carry out the reaction at constant volume then DV = 0 and w = 0 so • DE = q • This is written as • DErxn = qv 63

Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry Constant Volume Calorimetry • How do we measure – DErxn = qv • Device called a constant volume (or bomb) calorimeter. – Sample is burned in oxygen gas = system – Surroundings are the calorimeter. • Measure heat flow by change in temperature of the water 64

Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry Constant Volume Calorimetry • DErxn = qv • Temperature change measured in the bomb calorimeter is converted to DE using • qcal = Ccal x ΔT C = heat capacity of the calorimeter (J/°C) DT = change in temperature (°C) The calorimeter absorbs heat so the sign of the qrxn is reversed to reflect that the system (the sample) released heat • qrxn = – qcal 65

Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry Learning Check When a 1. 50 g sample of methane or hydrogen gas was burned with excess oxygen in the calorimeter, the temperature increased by 7. 3°C for methane and 14. 3°C for hydrogen. Calculate qrxn for methane and hydrogen. The bomb calorimeter has heat capacity of 11. 3 k. J/°C. 66

Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry Solution When a 1. 50 g sample of methane or hydrogen gas was burned with excess oxygen in the calorimeter, the temperature increased by 7. 3°C for methane and 14. 3°C for hydrogen. Calculate qrxn for methane and hydrogen. The bomb calorimeter has heat capacity of 11. 3 k. J/°C. 67

Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry DErxn per mole • We just calculated the heat of reaction (in Joules) based on temperature change and heat capacity of the calorimeter • DErxn can also be expressed per mole of reactant • Divide qrxn/moles of reactant 68

Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry Learning Check Calculate DErxn/mole for methane and hydrogen from the last problem. 69

Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry Solution Calculate DErxn/mole for methane and hydrogen from the last problem. 70

Section 6. 5 Measuring DE for Chemical Reactions: Constant-Volume Calorimetry Two Types of Calorimetry • Two types of Calorimetry – Constant Volume – measures DE – did this – Constant Pressure – measures DH – what is this • DH is a change in Enthalpy • Energy, Enthaply and the First Law of Thermodynamics • Enthalpy - Crash Course • Enthalpy (Khan Academy) 71

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure What is Enthalpy • • Enthalpy (H) is the total energy of a system Enthalpy is the sum of internal energy (E) and a quantity PV – – • • Internal energy (E) - energy required to create a system PV - energy required to make room for the system it by displacing its environment and establishing its volume and pressure. So H = E + PV Enthalpy is a State function just like Internal Energy 72

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure What is Enthalpy • • So H = E + PV Enthalpy represents both internal energy and a quantity PV So a change in Enthalpy represents a change in internal energy and a change in PV DH = DE +D(PV) How do we measure Enthalpy? 73

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure How do we measure Enthalpy? • • • When a reaction occurs in a sealed bomb calorimeter all the energy exchanged is in the form of heat Most chemical reactions in the lab don’t occur this way – they occur in open containers on the bench. Energy is exchanged as both heat and work In reality though, most of the time we are not really interested in the small amount of work the reaction does expanding against the atmosphere What we are really interested in is the heat exchanged How do we determine this value? 74

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Change in Enthalpy is the Heat Flow at Constant Pressure • If H = E + PV • And DH = DE +D(PV) – • So DH = DE +PDV – • • At constant P – the only change is volume And we have already defined ΔE = q + w So DH = q + w +PDV and –PDV = w so So DH = q + w – w And DH = qp So Enthalpy is a measure of heat exchange only! 75

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Enthalpy vs Internal Energy DH and DE can seem quite similar • They both represent state functions DE is a measure of all the energy (heat and work) exchanged with the surroundings DH is a measure of only the heat exchanged under conditions of constant pressure 76

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Summarizing Enthalpy • DH is the amount of heat absorbed or released under conditions of constant pressure – Endothermic Reaction • DH will be positive – heat absorbed • Reaction absorbs heat from the surroundings • Feels cool – Exothermic Reaction • DH will be negative – heat released • Reaction gives off heat to the surroundings • Feels warm 77

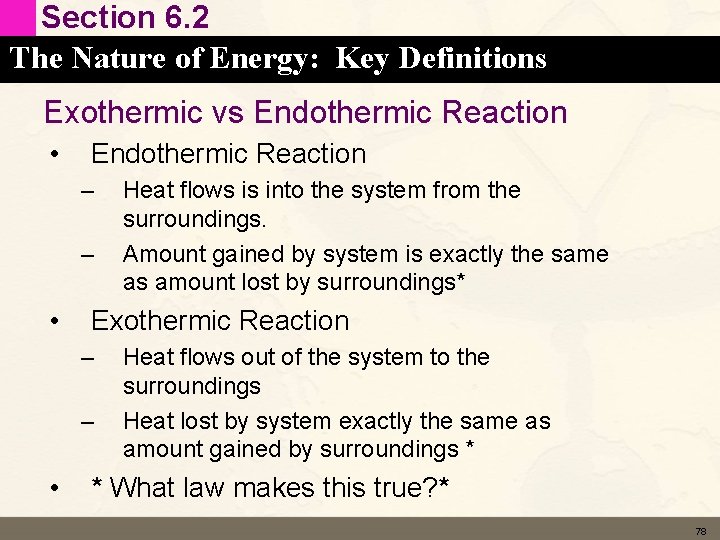
Section 6. 2 The Nature of Energy: Key Definitions Exothermic vs Endothermic Reaction • Endothermic Reaction – – • Exothermic Reaction – – • Heat flows is into the system from the surroundings. Amount gained by system is exactly the same as amount lost by surroundings* Heat flows out of the system to the surroundings Heat lost by system exactly the same as amount gained by surroundings * * What law makes this true? * 78

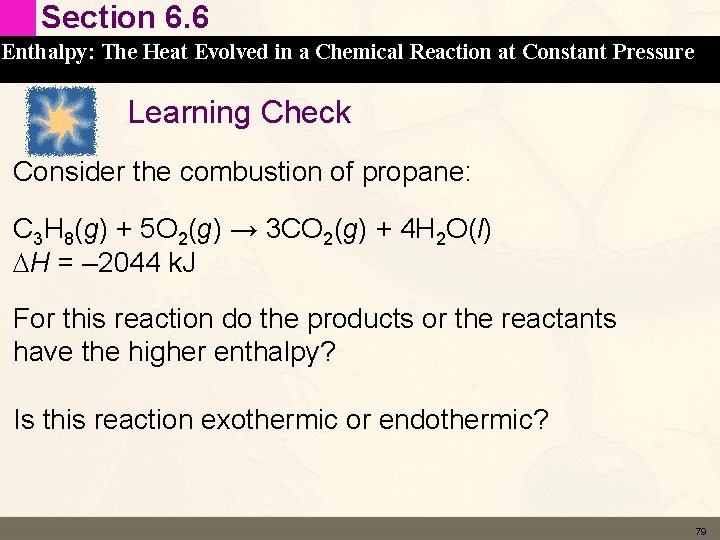
Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Learning Check Consider the combustion of propane: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l) DH = – 2044 k. J For this reaction do the products or the reactants have the higher enthalpy? Is this reaction exothermic or endothermic? 79

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Solution Consider the combustion of propane: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l) DH = – 2044 k. J For this reaction do the products or the reactants have the higher enthalpy? The ΔH is negative therefore the products have less enthalpy than the reactants. Is this reaction exothermic or endothermic? Exothermic 80

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Concept Check • An exothermic reaction releases energy as heat • Where does that energy come from? • Two forms of energy kinetic and potential. 81

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Solution • Kinetic is energy of motion – reflected in temperature. • Exothermic reaction can’t be drawing from the pool of kinetic energy – otherwise the temperature would go down. • In an exothermic reaction the reaction feels warm • So the energy must be coming from the stored potential energy in the bonds of the reactants 82

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure How do we measure Enthalpy • • • Total enthalpy (H) cannot be measured directly We can only measure a change in Enthalpy (DH) And we do this by measuring heat flow (DT) We measure a change in enthalpy by measuring DT at constant pressure Then we convert the change in temperature to an amount of heat 83

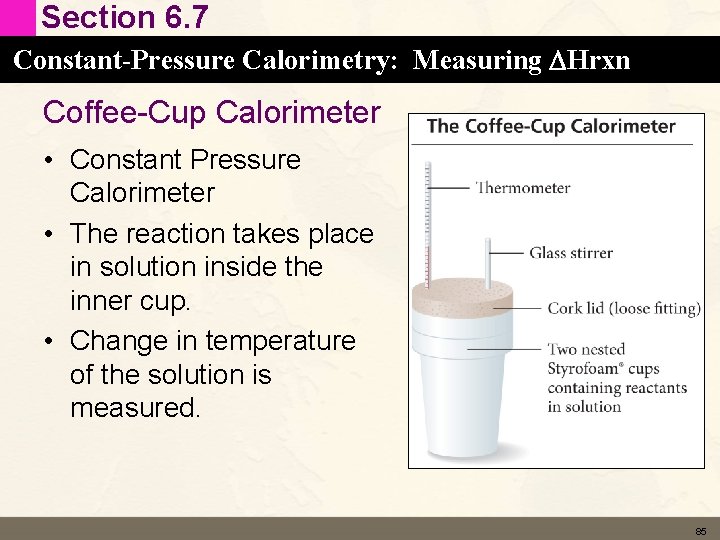
Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure How do we measure Enthalpy vs Internal Energy • The value of DE for a reaction is the amount of heat absorbed or released under constant volume. – Bomb Calorimeter DE = qv • The value of DH for a reaction is the amount of heat absorbed or released under constant pressure. – Coffee Cup Calorimeter DH = qp 84

Section 6. 7 Constant-Pressure Calorimetry: Measuring DHrxn Coffee-Cup Calorimeter • Constant Pressure Calorimeter • The reaction takes place in solution inside the inner cup. • Change in temperature of the solution is measured. 85

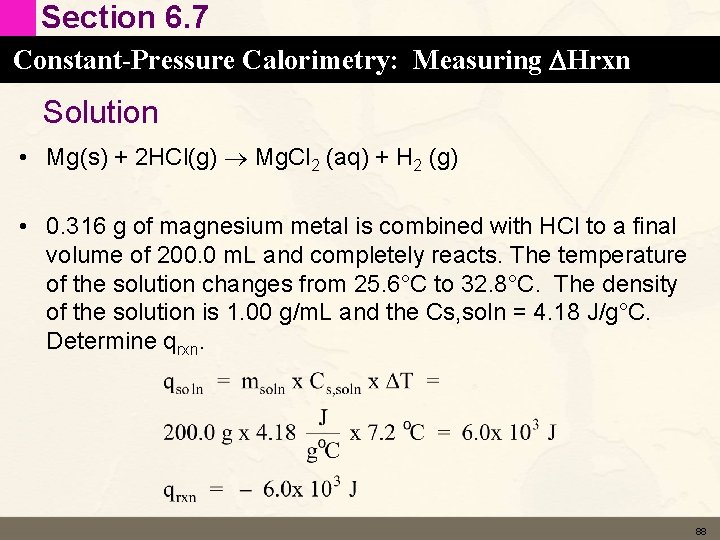
Section 6. 7 Constant-Pressure Calorimetry: Measuring DHrxn Coffee-Cup Calorimeter • Temperature change measured in the coffee-cup calorimeter is converted to DH using • qsoln = msoln x Cs, soln x ΔT msoln = mass of solution Cs, soln = heat capacity of the solution inside the calorimeter (J/g°C) DT = change in temperature (°C) • qrxn = – qsoln 86

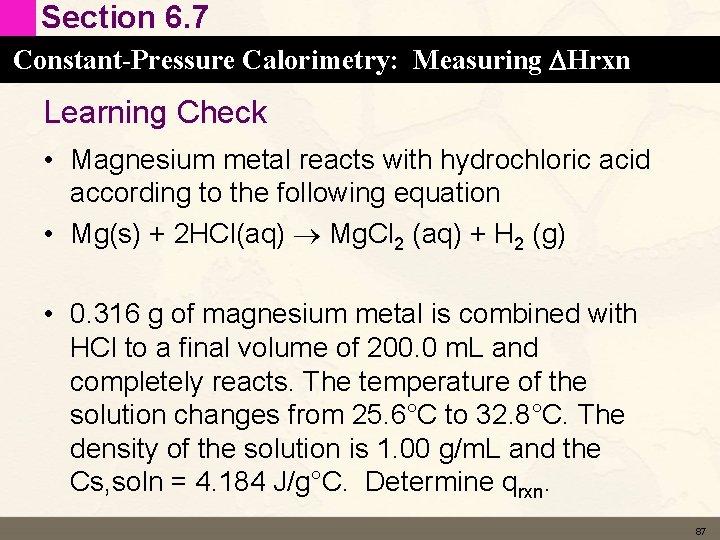
Section 6. 7 Constant-Pressure Calorimetry: Measuring DHrxn Learning Check • Magnesium metal reacts with hydrochloric acid according to the following equation • Mg(s) + 2 HCl(aq) Mg. Cl 2 (aq) + H 2 (g) • 0. 316 g of magnesium metal is combined with HCl to a final volume of 200. 0 m. L and completely reacts. The temperature of the solution changes from 25. 6°C to 32. 8°C. The density of the solution is 1. 00 g/m. L and the Cs, soln = 4. 184 J/g°C. Determine qrxn. 87

Section 6. 7 Constant-Pressure Calorimetry: Measuring DHrxn Solution • Mg(s) + 2 HCl(g) Mg. Cl 2 (aq) + H 2 (g) • 0. 316 g of magnesium metal is combined with HCl to a final volume of 200. 0 m. L and completely reacts. The temperature of the solution changes from 25. 6°C to 32. 8°C. The density of the solution is 1. 00 g/m. L and the Cs, soln = 4. 18 J/g°C. Determine qrxn. 88

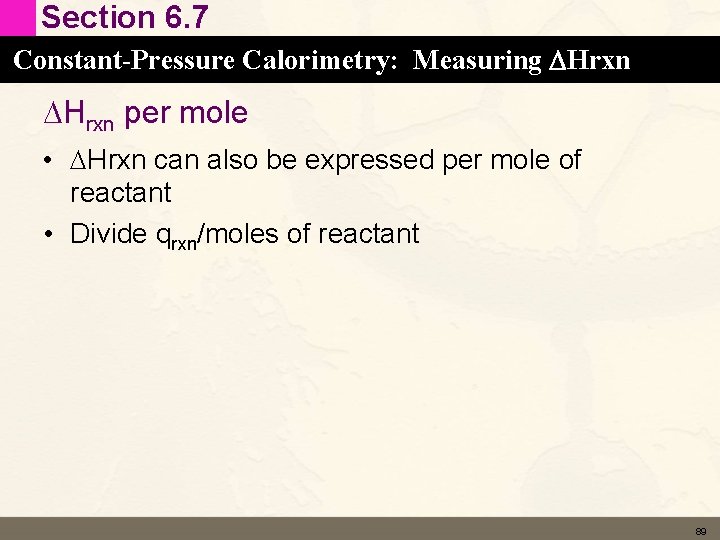
Section 6. 7 Constant-Pressure Calorimetry: Measuring DHrxn per mole • DHrxn can also be expressed per mole of reactant • Divide qrxn/moles of reactant 89

Section 6. 7 Constant-Pressure Calorimetry: Measuring DHrxn Learning Check • Find DHrxn/mole for the previous problem. 90

Section 6. 7 Constant-Pressure Calorimetry: Measuring DHrxn Solution • Find DHrxn/mole for the previous problem. 91

Section 6. 7 Constant-Pressure Calorimetry: Measuring DHrxn Conceptual Connection • Lighters are usually fueled by butane (C 4 H 10). When 1 mole of butane burns at constant pressure it produces 2658 k. J of heat and does 3 k. J of work. What are the values of DH and DE for the combustion of one mole of butane. 92

Section 6. 7 Constant-Pressure Calorimetry: Measuring DHrxn Solution • Lighters are usually fueled by butane (C 4 H 10). When 1 mole of butane burns at constant pressure it produces 2658 k. J of heat and does 3 k. J of work. What are the values of DH and DE for the combustion of one mole of butane. • w and q are both negative – work is done on the surroundings and heat is lost to surroundings • DE = q + w = – 2661 k. J • DH = q = – 2658 k. J 93

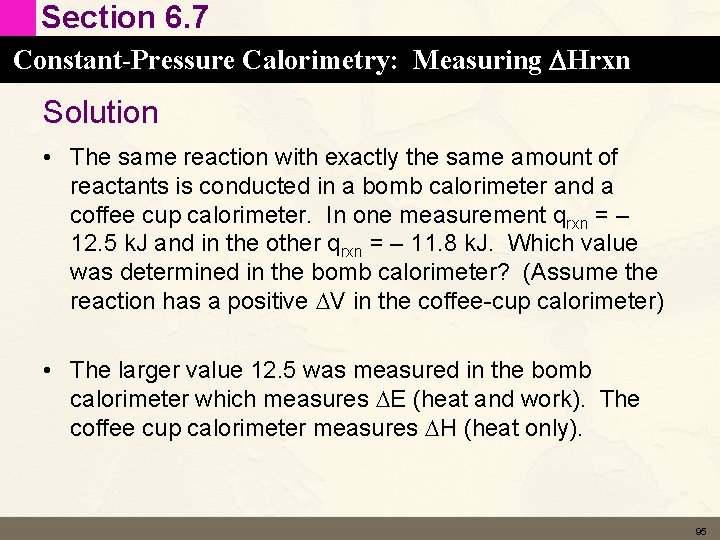
Section 6. 7 Constant-Pressure Calorimetry: Measuring DHrxn Conceptual Connection • The same reaction with exactly the same amount of reactants is conducted in a bomb calorimeter and a coffee cup calorimeter. In one measurement qrxn = – 12. 5 k. J and in the other qrxn = – 11. 8 k. J. Which value was determined in the bomb calorimeter? (Assume the reaction has a positive DV in the coffee-cup calorimeter) 94

Section 6. 7 Constant-Pressure Calorimetry: Measuring DHrxn Solution • The same reaction with exactly the same amount of reactants is conducted in a bomb calorimeter and a coffee cup calorimeter. In one measurement qrxn = – 12. 5 k. J and in the other qrxn = – 11. 8 k. J. Which value was determined in the bomb calorimeter? (Assume the reaction has a positive DV in the coffee-cup calorimeter) • The larger value 12. 5 was measured in the bomb calorimeter which measures DE (heat and work). The coffee cup calorimeter measures DH (heat only). 95

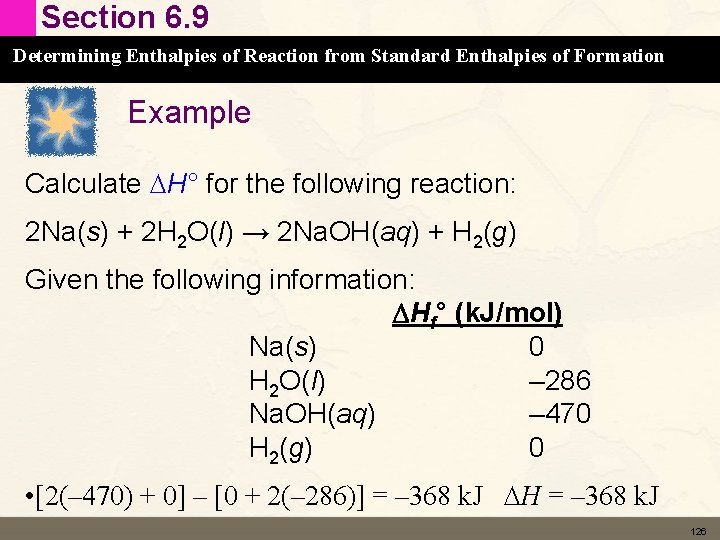
Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Stoichiometry Involving DH: Thermochemical Equations • Enthalpy change is also called enthalpy of reaction or heat of reaction. • Extensive property – depends on amount of material undergoing the reaction – The amount of heat generated depends on the amount of reactant or product • Consider the combustion of propane • Stoichiometry Calculations Using Enthaply 96

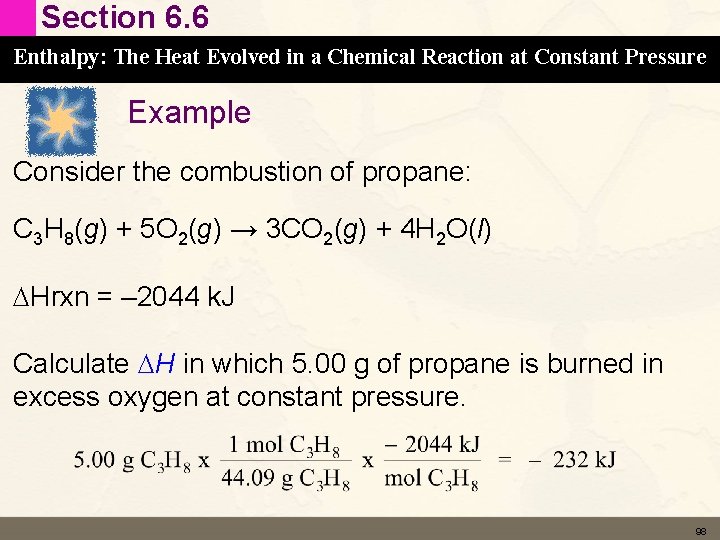
Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Example Consider the combustion of propane: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l) DHrxn = – 2044 k. J This means that 2044 k. J of energy is released for every mol of propane or every 5 moles of oxygen reacted 97

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Example Consider the combustion of propane: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l) DHrxn = – 2044 k. J Calculate DH in which 5. 00 g of propane is burned in excess oxygen at constant pressure. 98

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Learning Check Consider the combustion of propane: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l) DHrxn = – 2044 k. J Calculate DH in which 25. 0 g of water is released when propane is burned in excess oxygen at constant pressure. 99

Section 6. 6 Enthalpy: The Heat Evolved in a Chemical Reaction at Constant Pressure Solution Consider the combustion of propane: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l) DHrxn = – 2044 k. J Calculate DH in which 25. 0 g of water is released when propane is burned in excess oxygen at constant pressure. 100

Section 6. 8 Relationships Involving DHrxn Characteristics of Enthalpy Changes DHrxn • The change in enthalpy for a reaction (DHrxn) is associated with a particular reaction • If we change the characteristics of the reaction then DHrxn changes too • Lets look at a couple of examples 101

Section 6. 8 Relationships Involving DHrxn Characteristics of Enthalpy Changes 1. If a chemical reaction is multiplied by some factor then DHrxn is multiplied by the dame factor. A + 2 B C DH 1 Multiply by 2 2 A + 4 B 2 C DH 2 = DH 1 x 2 102

Section 6. 8 Relationships Involving DHrxn Characteristics of Enthalpy Changes 2. If a reaction is reversed, then DHrxn changes sign. A + 2 B C DH 1 Reverse reaction C A + 2 B DH 2 = – DH 1 103

Section 6. 8 Relationships Involving DHrxn 3. If a chemical reaction can expressed as the sum of a series of steps, then DHrxn for the overall equation is the sum of the heats of reactions for each step. A + 2 B C DH 1 C 2 D DH 2 –––––––––– A + 2 B 2 D DH 3 = DH 1 + DH 2 104

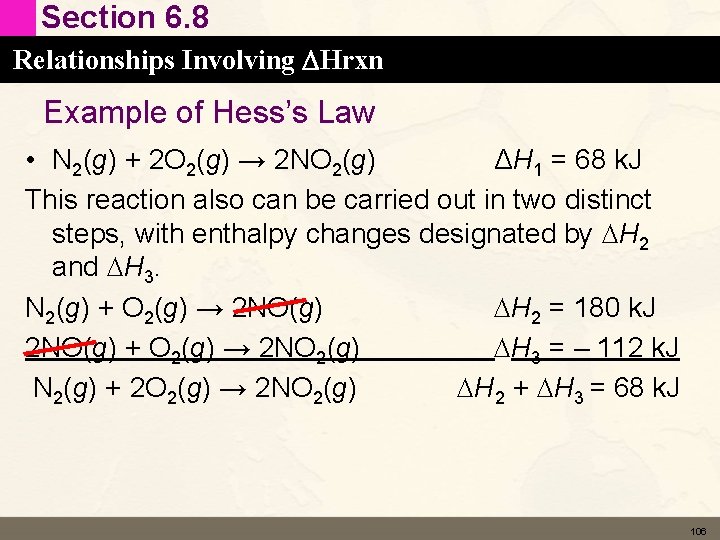
Section 6. 8 Relationships Involving DHrxn Hess’s Law • This last relationship is called Hess’s Law 105

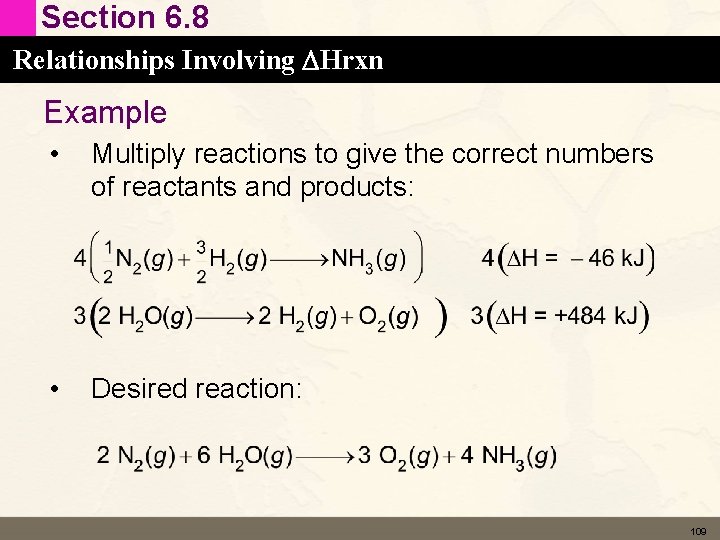
Section 6. 8 Relationships Involving DHrxn Example of Hess’s Law • N 2(g) + 2 O 2(g) → 2 NO 2(g) ΔH 1 = 68 k. J This reaction also can be carried out in two distinct steps, with enthalpy changes designated by DH 2 and DH 3. N 2(g) + O 2(g) → 2 NO(g) DH 2 = 180 k. J 2 NO(g) + O 2(g) → 2 NO 2(g) DH 3 = – 112 k. J N 2(g) + 2 O 2(g) → 2 NO 2(g) DH 2 + DH 3 = 68 k. J 106

Section 6. 8 Relationships Involving DHrxn Example • Consider the following data: • Calculate ΔH for the reaction 107

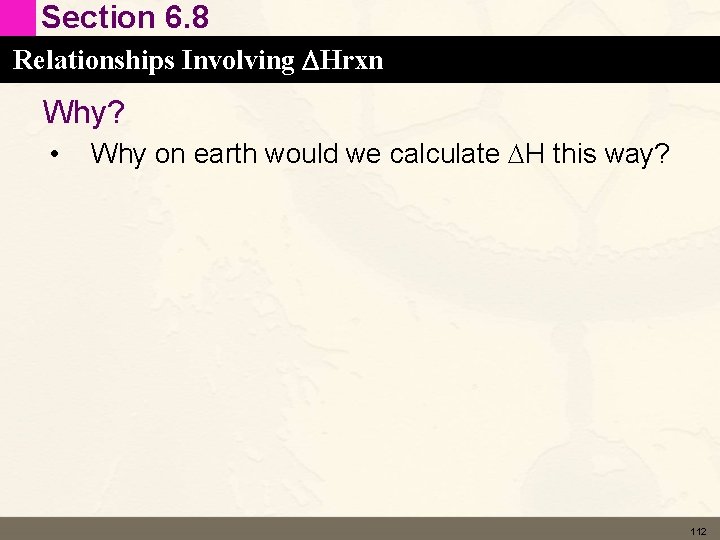
Section 6. 8 Relationships Involving DHrxn Example Desired reaction: Reverse the two reactions: 108

Section 6. 8 Relationships Involving DHrxn Example • Multiply reactions to give the correct numbers of reactants and products: • Desired reaction: 109

Section 6. 8 Relationships Involving DHrxn Example • Final reactions: • Desired reaction: DH = +1268 k. J 110

Section 6. 8 Relationships Involving DHrxn Problem-Solving Strategy • • • Work backward from the required reaction, using the reactants and products to decide how to manipulate the other given reactions at your disposal. Reverse any reactions as needed to give the required reactants and products. Multiply reactions to give the correct numbers of reactants and products. 111

Section 6. 8 Relationships Involving DHrxn Why? • Why on earth would we calculate DH this way? 112

Section 6. 8 Links • • Hess's Law (Socratic) Hess's Law and Enthalpy Change (Khan Academy) Hess's Law Examples (Khan Academy) Hess's Law Practice 113

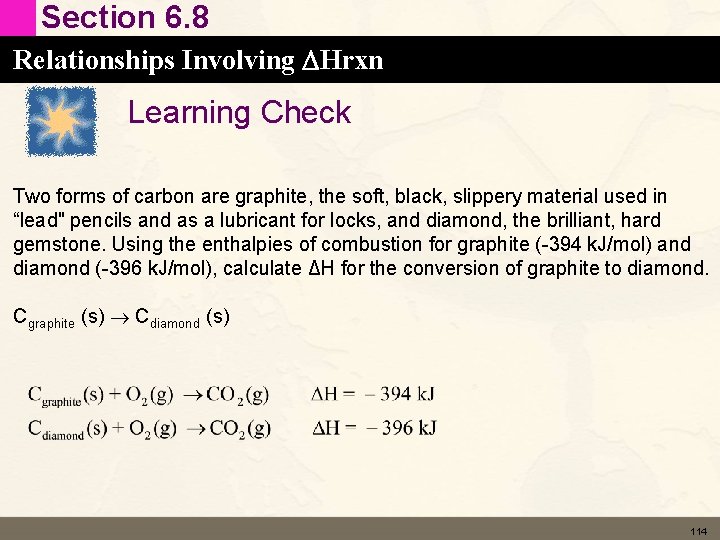
Section 6. 8 Relationships Involving DHrxn Learning Check Two forms of carbon are graphite, the soft, black, slippery material used in “lead" pencils and as a lubricant for locks, and diamond, the brilliant, hard gemstone. Using the enthalpies of combustion for graphite (-394 k. J/mol) and diamond (-396 k. J/mol), calculate ΔH for the conversion of graphite to diamond. Cgraphite (s) Cdiamond (s) 114

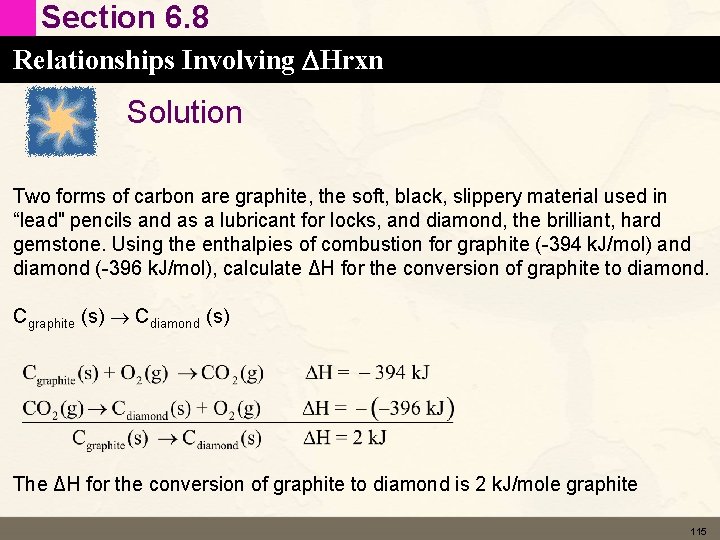
Section 6. 8 Relationships Involving DHrxn Solution Two forms of carbon are graphite, the soft, black, slippery material used in “lead" pencils and as a lubricant for locks, and diamond, the brilliant, hard gemstone. Using the enthalpies of combustion for graphite (-394 k. J/mol) and diamond (-396 k. J/mol), calculate ΔH for the conversion of graphite to diamond. Cgraphite (s) Cdiamond (s) The ΔH for the conversion of graphite to diamond is 2 k. J/mole graphite 115

Section 6. 8 Relationships Involving DHrxn Learning Check • Find DHrxn for the reaction • N 2 O(g) + NO 2(g) 3 NO(g) • Using • 2 NO(g) + O 2(g) 2 NO 2(g) • N 2(g) + O 2(g) 2 NO(g) • 2 N 2 O(g) 2 N 2(g) + O 2 (g) DH = – 113. 1 k. J DH = 182. 6 k. J DH = – 163. 2 k. J 116

Section 6. 8 Relationships Involving DHrxn Solution • Desired Reaction N 2 O(g) + NO 2(g) 3 NO(g) • Reverse eq 1, multiply eq 1 and 3 by 1/2 • ½[2 NO 2(g) 2 NO(g) + O 2(g)] ½[DH = + 113. 1 k. J] • N 2(g) + O 2(g) 2 NO(g) DH = 182. 6 k. J • ½[2 N 2 O(g) 2 N 2(g) + O 2(g)] ½[DH = – 163. 2 k. J] 117

Section 6. 8 Relationships Involving DHrxn Solution • Desired Reaction N 2 O(g) + NO 2(g) 3 NO(g) • Reverse eq 1, multiply eq 1 and 3 by 1/2 • NO 2(g) NO(g) + ½ O 2(g) • N 2(g) + O 2(g) 2 NO(g) • N 2 O(g) N 2(g) + ½ O 2(g) ½[DH = + 113. 1 k. J] DH = 182. 6 k. J ½[DH = – 163. 2 k. J] 118

Section 6. 8 Relationships Involving DHrxn Solution • Desired Reaction N 2 O(g) + NO 2(g) 3 NO(g) • Reverse eq 1, multiply eq 1 and 3 by 1/2 • NO 2(g) NO(g) + ½ O 2(g) ½ + 113. 1 = 56. 55 k. J • N 2(g) + O 2(g) 2 NO(g) 182. 6 k. J • N 2 O(g) N 2(g) + ½ O 2(g) ½ – 163. 2 k. J = – 81. 6 k. J • 157. 6 k. J 119

Section 6. 9 Determining Enthalpies of Reaction from Standard Enthalpies of Formation How many ways are there to determine DHrxn? • So far we have looked at two different ways to determine DHrxn • Calorimetry – coffee cup calorimeter – qp = DHrxn • Hess’s Law – where we infer DHrxn by knowing the values of DHrxn of our reaction from other reactions with known values • Now we will look at a third way – Standard Enthalpies of Formation 120

Section 6. 9 Determining Enthalpies of Reaction from Standard Enthalpies of Formation Standard States and Standard Enthalpy Changes • The third method to determine DHrxn uses tables of Enthalpies (or Heats of Formation) • This method determines the amount of heat required to make all the reactants and products in something called the “standard state” and then compares them to each other. 121

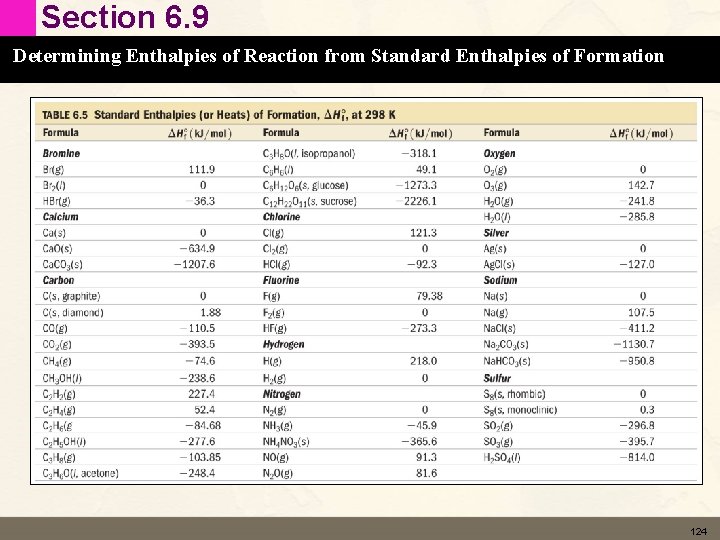
Section 6. 9 Determining Enthalpies of Reaction from Standard Enthalpies of Formation Standard States and Standard Enthalpy Changes • Standard State • For a gas – pure gas at 1 atm of pressure • For a liquid or solid – pure substance in its most stable form at 1 atm of pressure and 298 K (25 °C) • For a solution – 1 M solution 122

Section 6. 9 Determining Enthalpies of Reaction from Standard Enthalpies of Formation Standard States and Standard Enthalpy Changes • Standard Enthalpy of Formation (DHf°) • For a pure compound – the change in enthalpy when 1 mole of the compound forms from its constituent elements in their standard states. • For a pure element in its standard state DHf°= 0 123

Section 6. 9 Determining Enthalpies of Reaction from Standard Enthalpies of Formation 124

Section 6. 9 Determining Enthalpies of Reaction from Standard Enthalpies of Formation Standard Enthalpy of Formation (DHf°) • Add up all the DHf° for the products – Because the are being formed • Subtract all the DHf° of the reactants – Because they are being broken down • Pay attention to stoichiometry of equation • DHrxn° = Snp. DHf(products) – Snp. DHf° (reactants) • Heats of Formation Practice 125

Section 6. 9 Determining Enthalpies of Reaction from Standard Enthalpies of Formation Example Calculate DH° for the following reaction: 2 Na(s) + 2 H 2 O(l) → 2 Na. OH(aq) + H 2(g) Given the following information: DHf° (k. J/mol) Na(s) 0 H 2 O(l) – 286 Na. OH(aq) – 470 H 2(g) 0 • [2(– 470) + 0] – [0 + 2(– 286)] = – 368 k. J DH = – 368 k. J 126

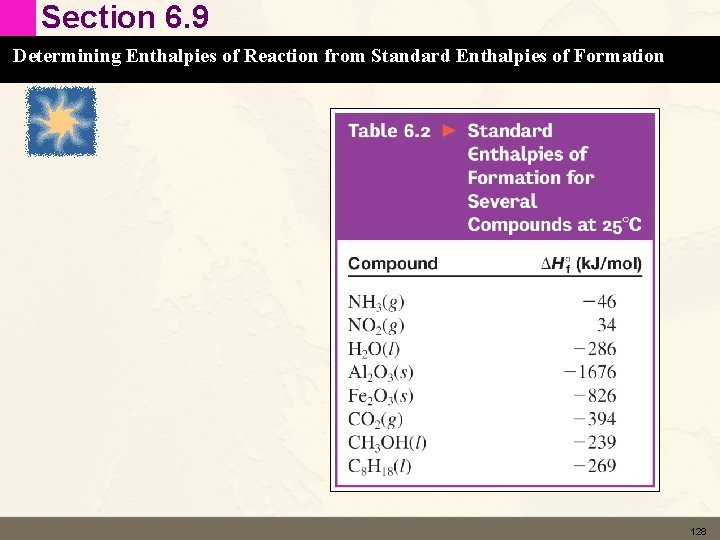
Section 6. 9 Determining Enthalpies of Reaction from Standard Enthalpies of Formation Learning Check Calculate DH° for the following reaction: 4 NH 3 (g) + 7 O 2(g) → 4 NO 2(g) + 6 H 2 O(l) Use the Table on the next slide. 127

Section 6. 9 Determining Enthalpies of Reaction from Standard Enthalpies of Formation 128

Section 6. 9 Determining Enthalpies of Reaction from Standard Enthalpies of Formation Solution Calculate DH° for the following reaction: 4 NH 3 (g) + 7 O 2(g) → 4 NO 2(g) + 6 H 2 O(l) [4(34) + 6(– 286)] – [4(– 46) + 0] = – 1396 k. J DH = – 1396 k. J = 1. 40 x 103 k. J 129

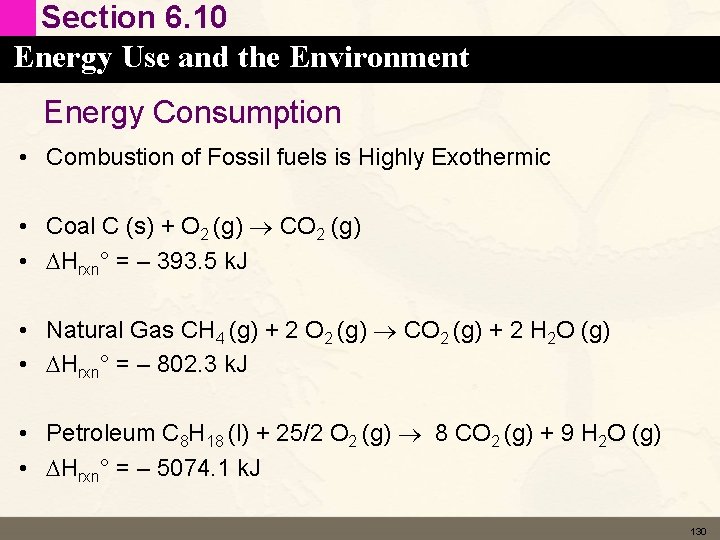
Section 6. 10 Energy Use and the Environment Energy Consumption • Combustion of Fossil fuels is Highly Exothermic • Coal C (s) + O 2 (g) CO 2 (g) • DHrxn° = – 393. 5 k. J • Natural Gas CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) • DHrxn° = – 802. 3 k. J • Petroleum C 8 H 18 (l) + 25/2 O 2 (g) 8 CO 2 (g) + 9 H 2 O (g) • DHrxn° = – 5074. 1 k. J 130

Section 6. 10 Energy Use and the Environmental Problems Associated with Fossil Fuel Use • Non renewable • Produce greenhouse gases (CO 2, H 2 O) • Produce other gases from side reactions due to impurities – Sulfur oxides – contribute to acid rain – Nitrogen oxides – contribute to SMOG – Ozone – ground level ozone is a dangerous pollutant 131

Section 6. 10 Review • Difference between heat (q) and work (w) • Difference between constant volume and constant pressure calorimetry – Know what each one measures • Difference between DE and DH – Know how to calculate DE – Know three ways to calculate DH • • Calorimetry Hess’s Law Stoichiometry DH formation 132

Section 6. 10 Energy Use and the Environment Suggested Homework • • • Chapter 6 Review Questions Odd numbered problems Cumulative and Challenge Questions Finally – do the Sapling HW – try to do this without your notes to determine what topics you still need to review. 133

Section 6. 10 Suggested Homework • • 3 rd Edition (original – orange cover) Review Questions Odd numbered problems 33 – 139 3 rd Edition (updated – green cover) Self Assessment Quiz Odd numbered problems 1 – 88 134