Unit 7 Thermochemistry AP CHEMISTRY CHAPTER 6 Energy




























- Slides: 61

Unit 7: Thermochemistry AP CHEMISTRY CHAPTER 6

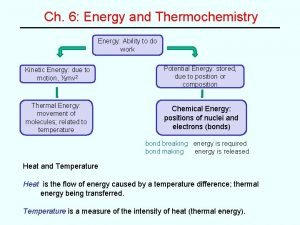
Energy Review v. Energy is capacity to do work or produce heat v. Observable changes to energy is in the form of temperature changes. v. Energy is classified as potential or kinetic energy v. Potential energy is due to a position or composition v. Kinetic energy is due to motion of an object and depends on the object’s mass and velocity. v. On average, warmer objects have more kinetic energy than cooler objects.

Energy cont. v. Collisions between molecules that are in thermal contact (have different average kinetic energies) transfer energy. v. Scientists describe this process as “energy is transferred as heat. ” v. As the molecular collisions continue, thermal equilibrium is eventually reached. v. The average kinetic energies of both substances is the same at thermal equilibrium. v. Heat is not a substance. It is an action that describes the transfer of energy.

Temperature vs. Heat Demonstration Will the volunteers please come to the front? you (and probably everyone else) know who you are Food for thought: Transfer of Thermal Energy between Hot and Cold Bodies Endothermic vs. Exothermic

Change in Energy The energy of a substance is related to both heat and work. ◦ ∆E = q + W ◦ ∆E is change in energy; q is heat and W is work applied by a system. In order to perform energy calculations, we divide the universe into two parts: ◦ The system is the part of the universe that we focus our attention; the surroundings is everything else in the universe.

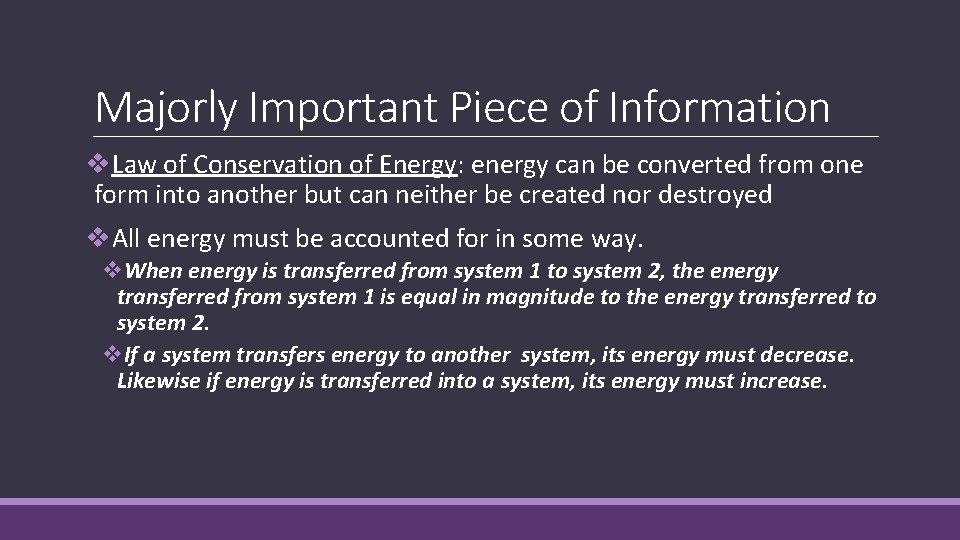
Majorly Important Piece of Information v. Law of Conservation of Energy: energy can be converted from one form into another but can neither be created nor destroyed v. All energy must be accounted for in some way. v. When energy is transferred from system 1 to system 2, the energy transferred from system 1 is equal in magnitude to the energy transferred to system 2. v. If a system transfers energy to another system, its energy must decrease. Likewise if energy is transferred into a system, its energy must increase.

Example Application Imagine a car piston with gas inside the piston. As the gasoline combusts, gas expands and collides with the piston. This creates a force causing the piston to expand. Work is being done on the piston by the gas. There is also gas outside the piston applying pressure to the piston in an effort to cause it to compress. This is the external pressure. ◦ The chemical reaction within the piston is considered “the system” in this example. The piston and everything else, is “the surroundings. ”

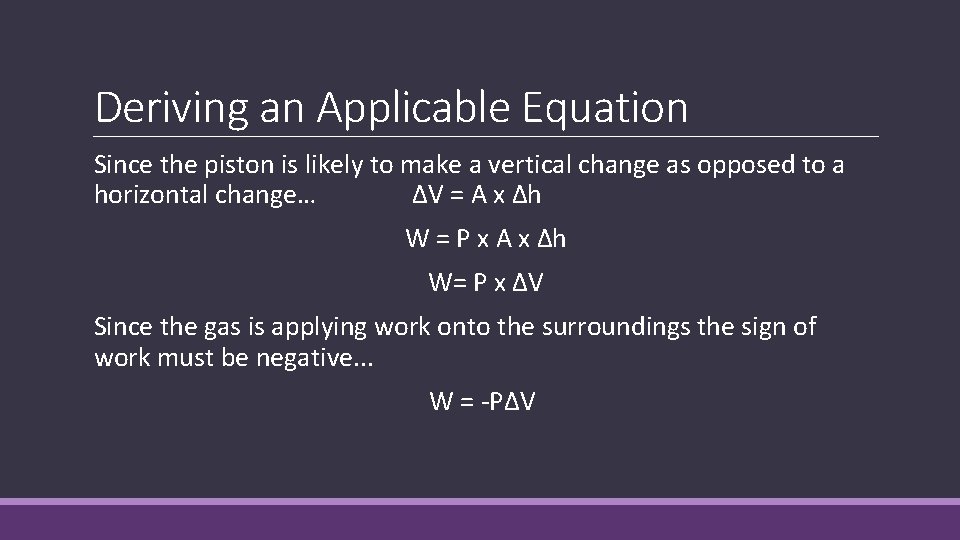
Deriving an Applicable Equation Pressure is defined as a force applied over an area: ◦ P = F/A …thus F = P x A If the piston moves vertically in a cylindrical container, the distance it moves can be expressed as a change in height (∆h). Work is defined as a force applied across a distance: ◦ W = F x ∆h …thus W = P x A x ∆h

Deriving an Applicable Equation Since the piston is likely to make a vertical change as opposed to a horizontal change… ∆V = A x ∆h W = P x A x ∆h W= P x ∆V Since the gas is applying work onto the surroundings the sign of work must be negative. . . W = -P∆V

Example Problem Calculate the work associated with the expansion of a gas from 46 L to 64 L within a piston at a constant external pressure of 15 atm? W= -(15 atm)(64 L-46 L) W= -270 L atm

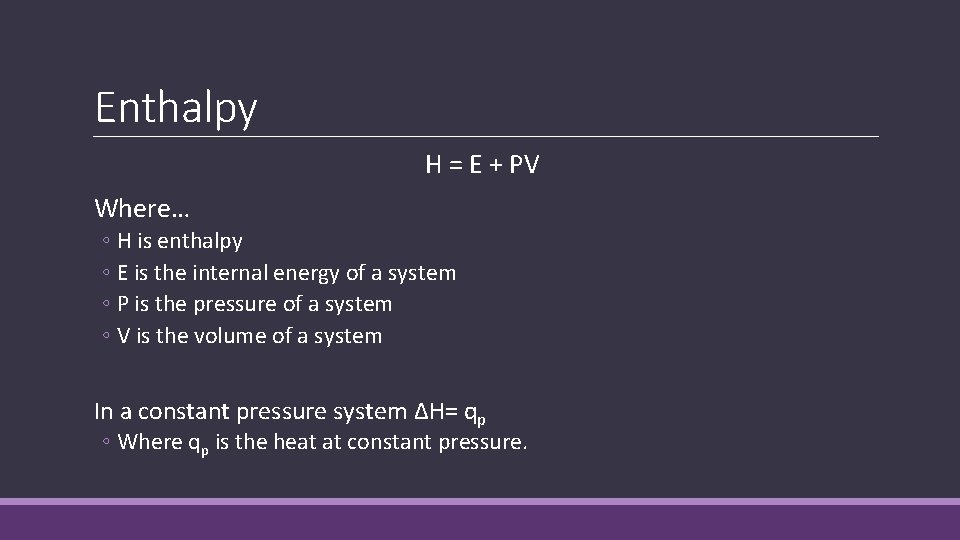
Enthalpy H = E + PV Where… ◦ H is enthalpy ◦ E is the internal energy of a system ◦ P is the pressure of a system ◦ V is the volume of a system In a constant pressure system ∆H= qp ◦ Where qp is the heat at constant pressure.

Calorimetry is the science of measuring heat. ◦ Based on observing the temperature change when a body absorbs or discharges energy as heat. Constant pressure calorimetry vs. constant volume calorimetry Heat Capacity (C) is defined as: ◦ C= (heat absorbed)/(increase in temperature)

Specific Heat Capacity The transfer of a given amount of thermal energy will not produce the same temperature change in equal masses of matter with differing specific heat capacities. ◦ The amount of energy needed to heat one gram of a substance by 1◦C is the specific heat capacity of that substance. ◦ Specific heat of water = 4. 18 J/g °C ◦ The molar heat capacity is the energy needed to heat one mole of a substance by 1 ◦C.

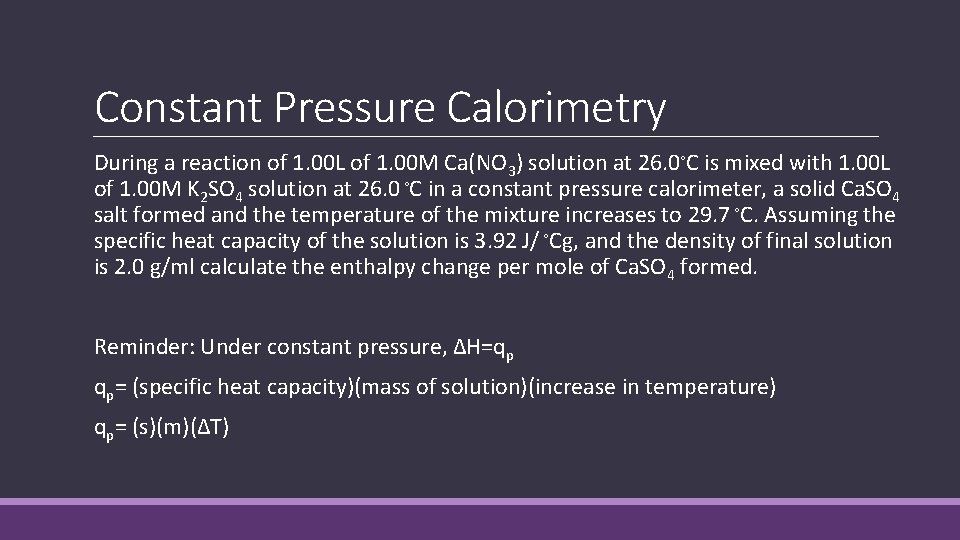
Constant Pressure Calorimetry During a reaction of 1. 00 L of 1. 00 M Ca(NO 3) solution at 26. 0◦C is mixed with 1. 00 L of 1. 00 M K 2 SO 4 solution at 26. 0 ◦C in a constant pressure calorimeter, a solid Ca. SO 4 salt formed and the temperature of the mixture increases to 29. 7 ◦C. Assuming the specific heat capacity of the solution is 3. 92 J/ ◦Cg, and the density of final solution is 2. 0 g/ml calculate the enthalpy change per mole of Ca. SO 4 formed. Reminder: Under constant pressure, ∆H=qp qp= (specific heat capacity)(mass of solution)(increase in temperature) qp= (s)(m)(∆T)

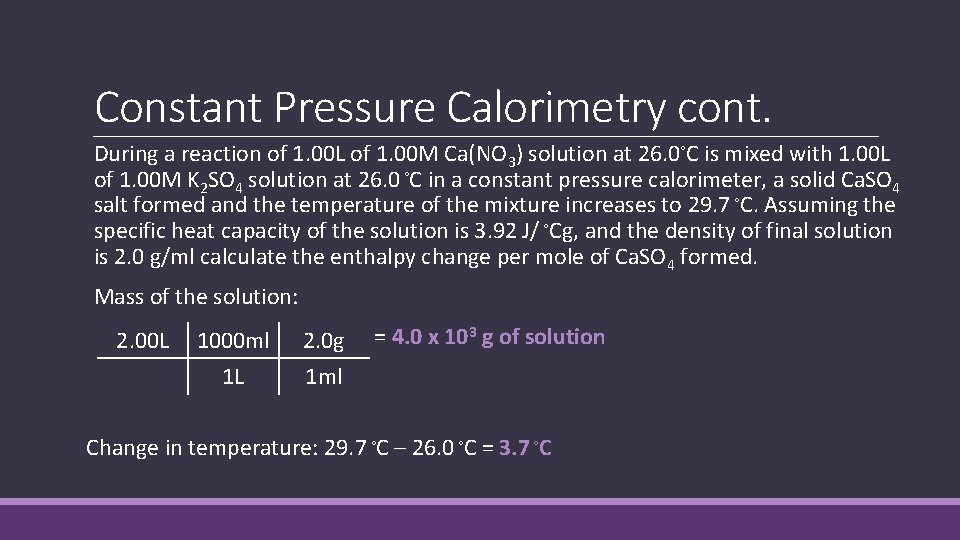
Constant Pressure Calorimetry cont. During a reaction of 1. 00 L of 1. 00 M Ca(NO 3) solution at 26. 0◦C is mixed with 1. 00 L of 1. 00 M K 2 SO 4 solution at 26. 0 ◦C in a constant pressure calorimeter, a solid Ca. SO 4 salt formed and the temperature of the mixture increases to 29. 7 ◦C. Assuming the specific heat capacity of the solution is 3. 92 J/ ◦Cg, and the density of final solution is 2. 0 g/ml calculate the enthalpy change per mole of Ca. SO 4 formed. Mass of the solution: 2. 00 L 1000 ml 1 L 2. 0 g 1 ml = 4. 0 x 103 g of solution Change in temperature: 29. 7 ◦C – 26. 0 ◦C = 3. 7 ◦C

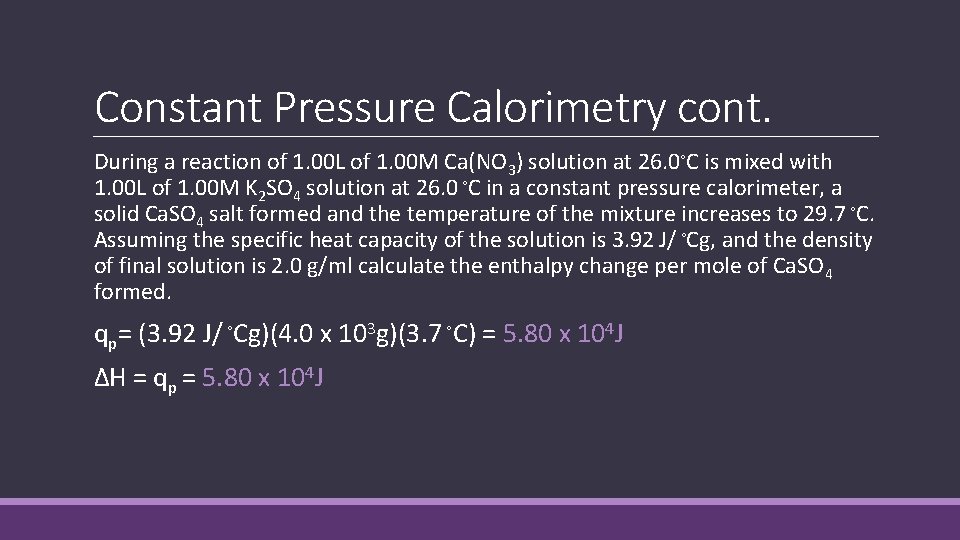
Constant Pressure Calorimetry cont. During a reaction of 1. 00 L of 1. 00 M Ca(NO 3) solution at 26. 0◦C is mixed with 1. 00 L of 1. 00 M K 2 SO 4 solution at 26. 0 ◦C in a constant pressure calorimeter, a solid Ca. SO 4 salt formed and the temperature of the mixture increases to 29. 7 ◦C. Assuming the specific heat capacity of the solution is 3. 92 J/ ◦Cg, and the density of final solution is 2. 0 g/ml calculate the enthalpy change per mole of Ca. SO 4 formed. qp= (3. 92 J/ ◦Cg)(4. 0 x 103 g)(3. 7 ◦C) = 5. 80 x 104 J ΔH = qp = 5. 80 x 104 J

Homework Pg. 266 # 21, 26, 29, 44, 48, 51

Enthalpy Changes The enthalpy change of a reaction gives the amount of energy released (for negative values) or absorbed (for positive values) by a chemical reaction at constant pressure. Hess’ Law: in going from a particular set of reactants to a particular set of products, the change in enthalpy is the same whether the reaction takes place in one step or in many.

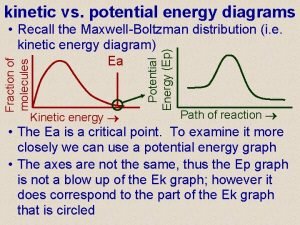
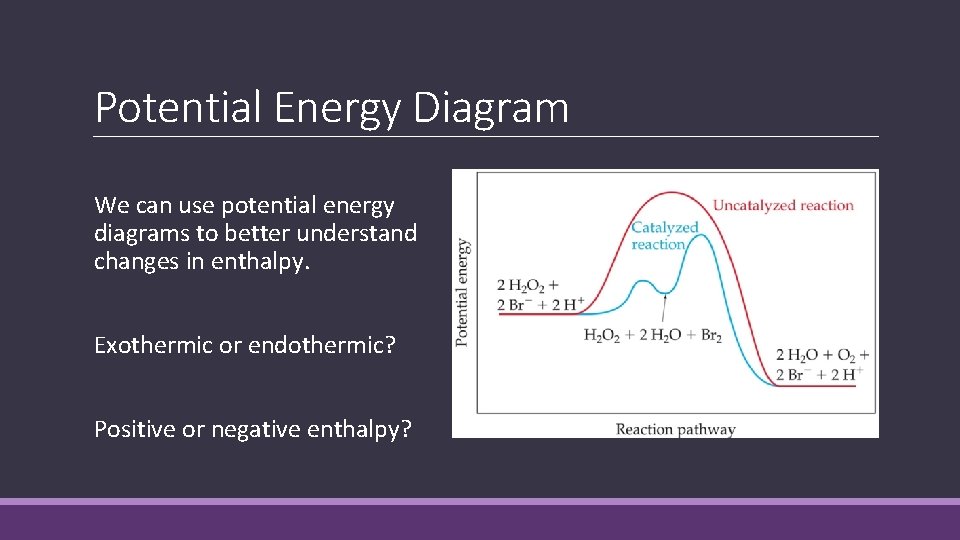
Potential Energy Diagram We can use potential energy diagrams to better understand changes in enthalpy. Exothermic or endothermic? Positive or negative enthalpy?

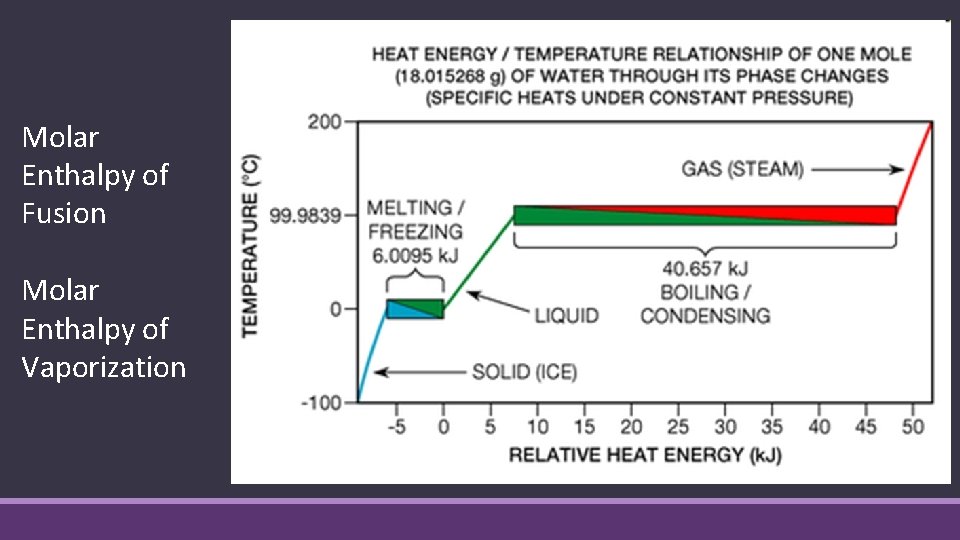
Molar Enthalpy of Fusion Molar Enthalpy of Vaporization

Characteristics of Enthalpy Changes 1. If a reaction is reversed, the sign of ΔH is also reversed. 2. The magnitude of ΔH is directly proportional to the quantities of reactants and products in a reaction. If the coefficients in a balanced reaction are multiplied by an integer, the value of ΔH is multiplied by the same integer.

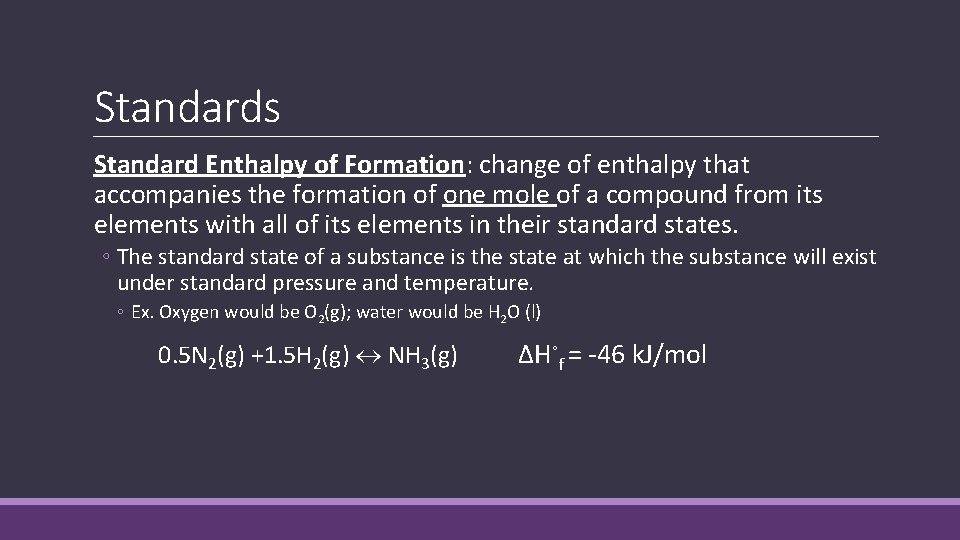
Standards Standard Enthalpy of Formation: change of enthalpy that accompanies the formation of one mole of a compound from its elements with all of its elements in their standard states. ◦ The standard state of a substance is the state at which the substance will exist under standard pressure and temperature. ◦ Ex. Oxygen would be O 2(g); water would be H 2 O (l) 0. 5 N 2(g) +1. 5 H 2(g) NH 3(g) ΔH◦f = -46 k. J/mol

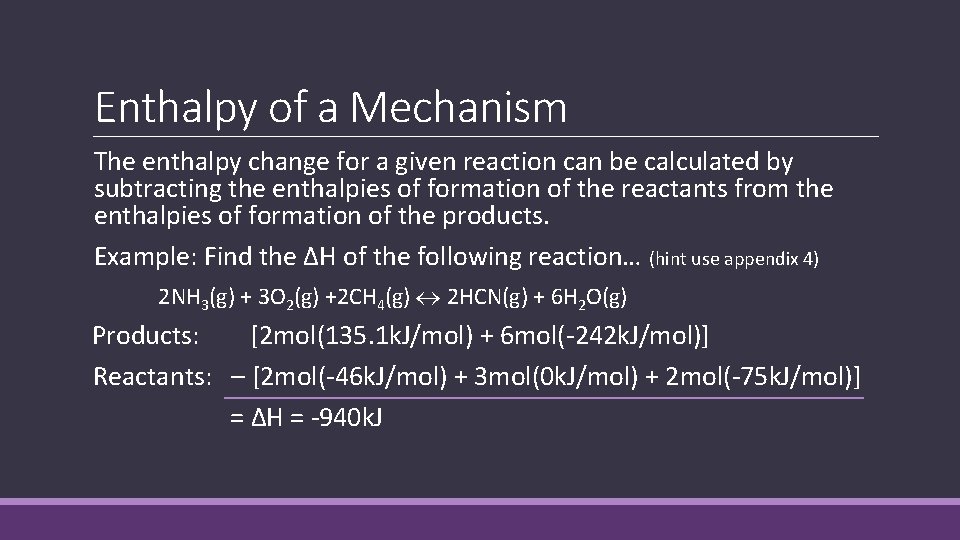
Enthalpy of a Mechanism The enthalpy change for a given reaction can be calculated by subtracting the enthalpies of formation of the reactants from the enthalpies of formation of the products. Example: Find the ΔH of the following reaction… (hint use appendix 4) 2 NH 3(g) + 3 O 2(g) +2 CH 4(g) 2 HCN(g) + 6 H 2 O(g) Products: [2 mol(135. 1 k. J/mol) + 6 mol(-242 k. J/mol)] Reactants: – [2 mol(-46 k. J/mol) + 3 mol(0 k. J/mol) + 2 mol(-75 k. J/mol)] = ΔH = -940 k. J

Homework Pg. 267 -270 ◦ # 33, 35, 57, 61, 65, 71 Hint: for 71 you will need to look at Appendix Four in the back of the book.

Unit 7 part dos…Entropy AP CHEMISTRY CHAPTER 16

What is Entropy? Entropy is a measure of the dispersal of matter and energy. ΔSuniv = ΔSsys + ΔSsurr Entropy suggests the spontaneity of a reaction to occur. ◦ Entropy does not suggest anything about the rate a reaction will occur, but merely if it will occur without assistance of outside factors.

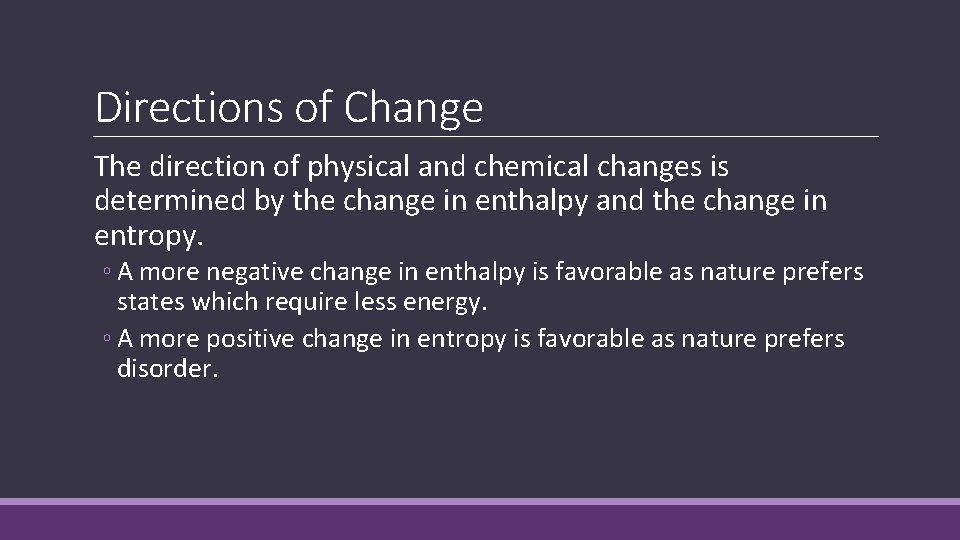
Directions of Change The direction of physical and chemical changes is determined by the change in enthalpy and the change in entropy. ◦ A more negative change in enthalpy is favorable as nature prefers states which require less energy. ◦ A more positive change in entropy is favorable as nature prefers disorder.


Field Trip!! … well sort of Everyone will participate in the activity, but one person will have a specific role. I need someone who is either absolutely terrible at card games or is very messy

Laws of Thermodynamics 1 st Law of Thermodynamics: the energy of the universe is constant. 2 nd Law of Thermodynamics: the entropy of the universe is increasing. ◦ For all spontaneous reactions

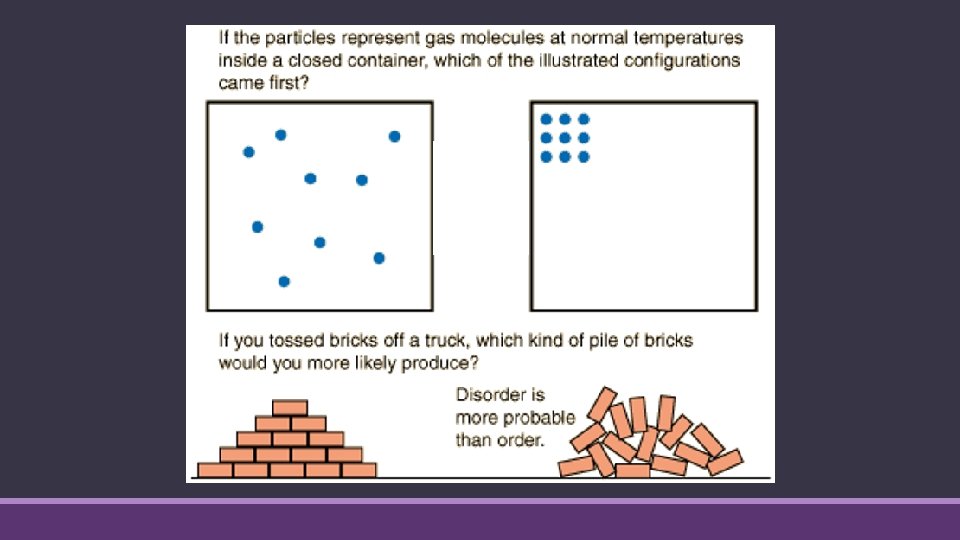
Increasing Entropy increases when: ◦ Matter is dispersed (solid to liquid, etc. ) ◦ Particles become free to move ◦ Number of individual particles increases during a chemical reaction ◦ With respect to gases… an increase in volume. ◦ Gas particles can move in a larger space ◦ Energy is dispersed ◦ Temperature increases

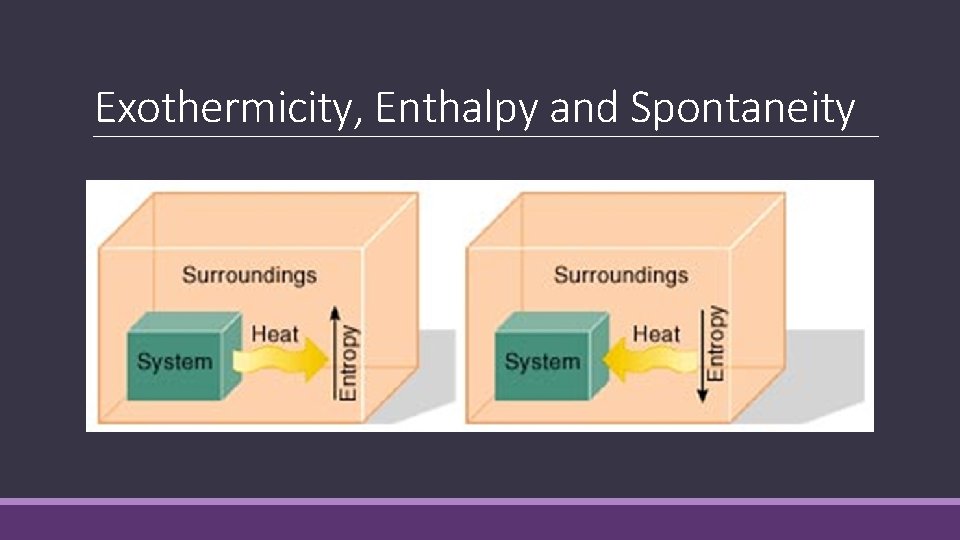
Exothermicity, Enthalpy and Spontaneity

Entropy of Surroundings The sign of ΔSsurr depends on the direction of heat flow. ◦ If the heat flows into the surroundings, the change in enthalpy will be negative with respect to the reacting system. Adding heat to surroundings increases energy of surroundings and the entropy. ◦ - ΔH = + ΔSsurr The magnitude of ΔSsurr depends on the temperature of the surroundings. ◦ If the surrounding temperature is lower, energy exchange will have a greater impact thus greater change in entropy.

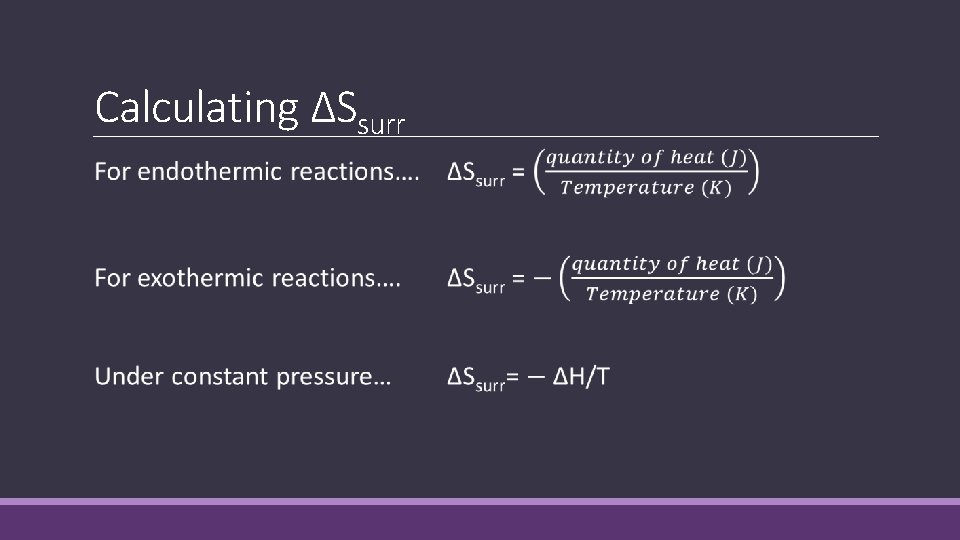
Calculating ΔSsurr

Homework Pg. 783 #19 -23, 26

Favorable Reactions “Thermodynamically Favored” means the products are favored at equilibrium. ◦ In regards to chemistry, this phrase has the same meaning as “spontaneous. ” In some reactions, it is necessary to consider both enthalpy and entropy to determine if a reaction will be thermodynamically favorable. ◦ The freezing of water and the dissolution of sodium nitrate in water provide good examples of such situations.

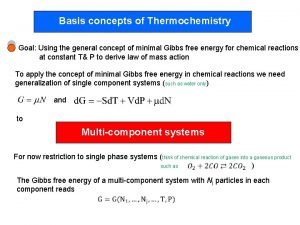
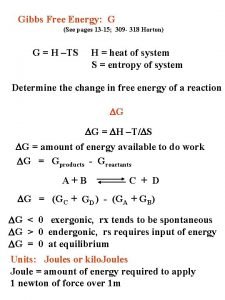
Gibbs Free Energy Free energy is a function that describes spontaneity/thermodynamic favor. ◦ Free energy is especially helpful with temperature dependence. ◦ Symbolized by “G” ◦ G = H-TSsys ◦ Thus… ΔG = ΔH – TΔS …at constant temperature and pressure.

So how does this help? If ΔG<0, the products are favored at equilibrium and the forward process is said to be “thermodynamically favored. ” If ΔG>0, the reactants are favored at equilibrium and the reverse reaction is said to be “thermodynamically favored. ” If ΔG=0, the reaction is at equilibrium and there is no driving force in either direction.

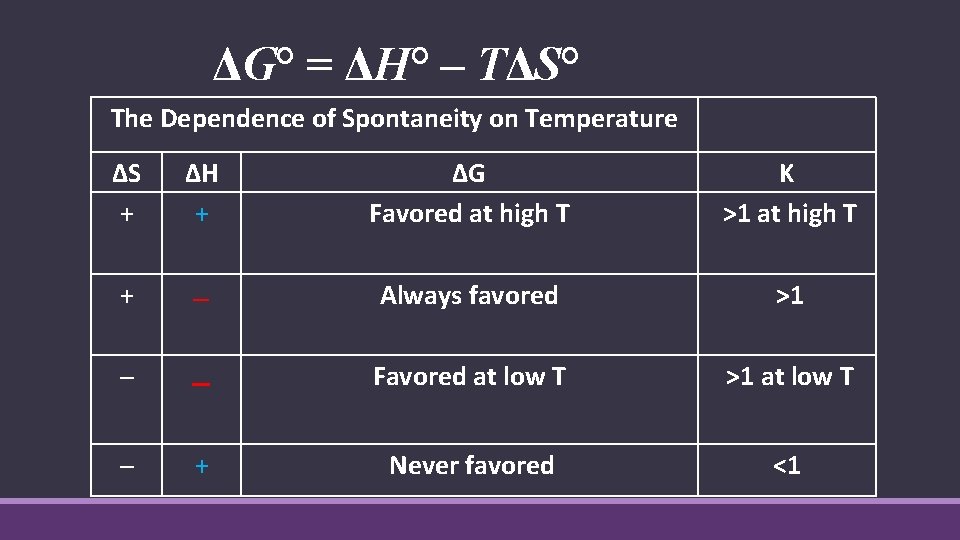
ΔG° = ΔH° – TΔS° The Dependence of Spontaneity on Temperature ΔS + ΔH + ΔG Favored at high T K >1 at high T + ─ Always favored >1 ─ ─ Favored at low T >1 at low T ─ + Never favored <1

Rate of Favoritism Do thermodynamically favored reactions occur quickly or slowly? ◦ Yes “Thermodynamically favored” doesn’t imply anything about the rate and so some thermodynamically favored reactions will occur quickly while other will go slowly. ◦ Many processes that are thermodynamically favored do not occur to any measurable extent, or they are at extremely slow rates.

“Kinetics be hating” Processes that are thermodynamically favored, but do not proceed at a measureable rate, are said to be under “Kinetic Control. ” ◦ High activation energy is a common reason for a process to be under kinetic control. ◦ This does not mean that the reaction is at equilibrium.

Homework Pg. 784 ◦ # 27, 28, 33, 35,

Bellwork Identify the sign or value of ΔG for the following situations: ◦ Q < 1 ◦ Q > 1 ◦ Q = 1


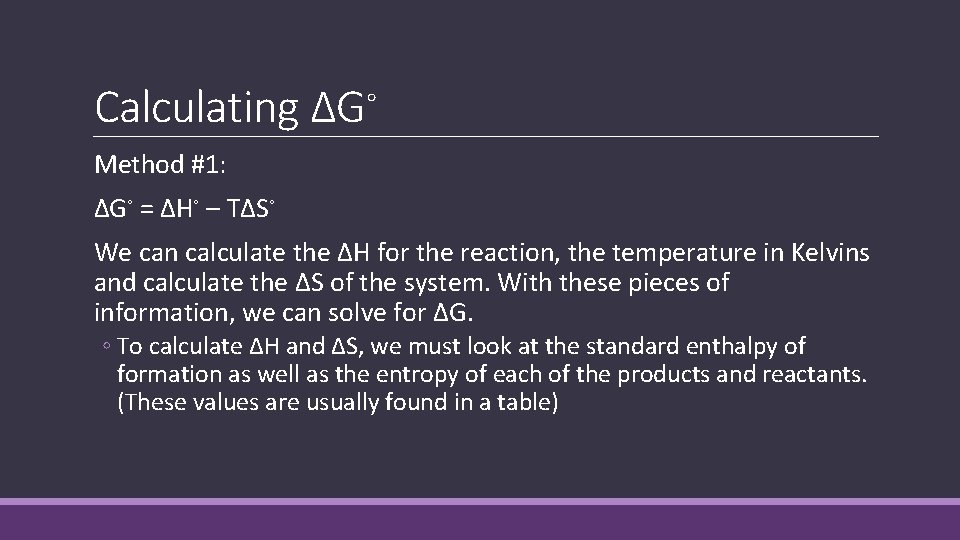
Calculating ΔG◦ Method #1: ΔG◦ = ΔH◦ – TΔS◦ We can calculate the ΔH for the reaction, the temperature in Kelvins and calculate the ΔS of the system. With these pieces of information, we can solve for ΔG. ◦ To calculate ΔH and ΔS, we must look at the standard enthalpy of formation as well as the entropy of each of the products and reactants. (These values are usually found in a table)

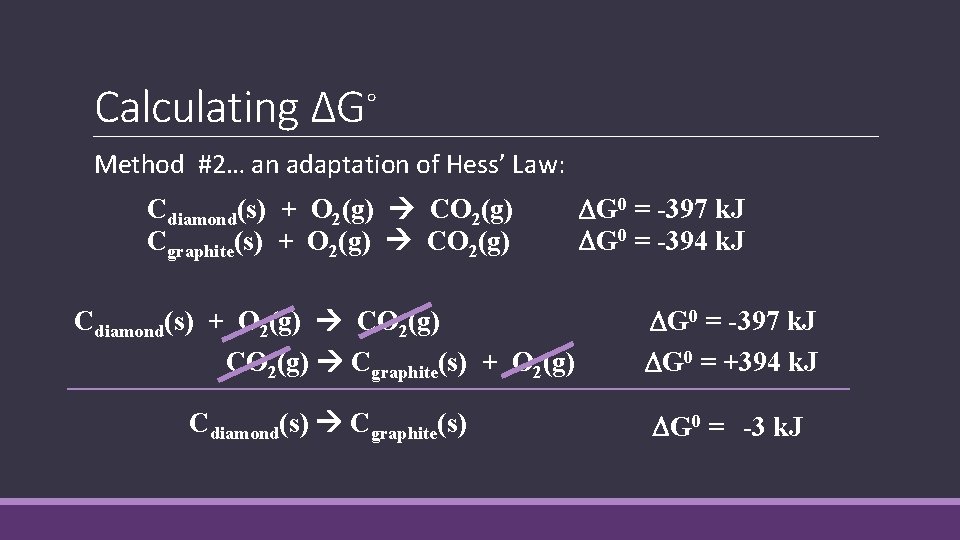
Calculating ΔG◦ Method #2… an adaptation of Hess’ Law: Cdiamond(s) + O 2(g) CO 2(g) Cgraphite(s) + O 2(g) CO 2(g) G 0 = -397 k. J G 0 = -394 k. J Cdiamond(s) + O 2(g) CO 2(g) Cgraphite(s) + O 2(g) G 0 = -397 k. J G 0 = +394 k. J Cdiamond(s) Cgraphite(s) G 0 = -3 k. J

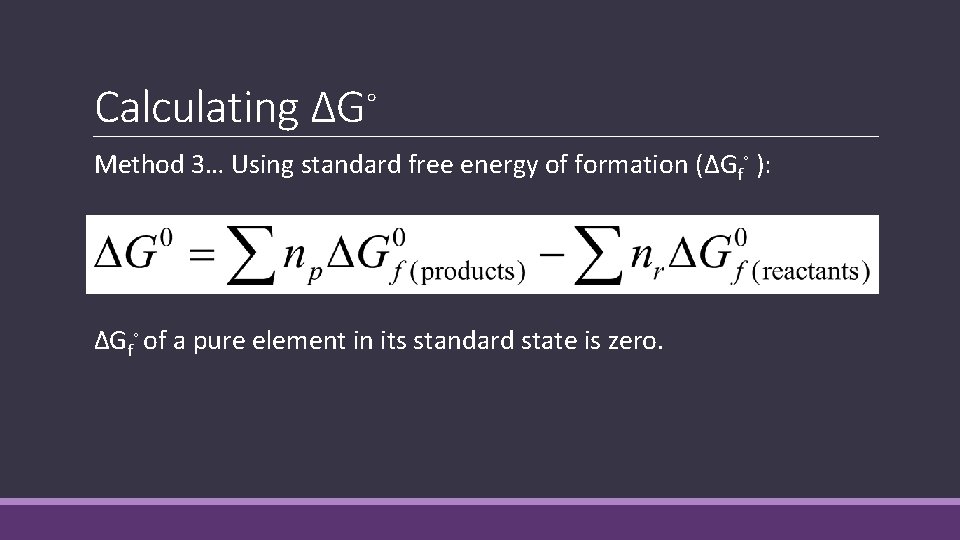
Calculating ΔG◦ Method 3… Using standard free energy of formation (ΔGf◦ ): ΔGf◦ of a pure element in its standard state is zero.

When do we use each method? Method 1: ◦ If the standard enthalpy of formations and the entropies of each species is provided. ◦ If the ΔH and the ΔS are provided for the reaction. Method 2: ◦ If there are different steps to a mechanism and the change in Gibb’s free energy of each of the steps is provided. Method 3: ◦ If the Gibb’s free energy is provided for each of the species present.

Homework Pg. 784 -785 Method 1: ◦ # 45, 46, 47 Method 2: ◦ # 51, Method 3: ◦ # 53

ΔG◦ and pressure


New Terms yay! (even if they are bio-related)

Homework Pg. 785 ◦ # 57, 59, 61

Energy and Work ΔG for a spontaneous reaction represents the amount of energy to perform useful work. All real pathways waste energy. ◦ Only hypothetical pathways can achieve the maximum work available from a spontaneous reaction. ◦ All real process are irreversible since work is changed to heat in the surroundings and the entropy increases. Thermodynamicist, Henry Bent: ◦ 1 st law: You can’t win, you only break even. ◦ 2 nd law: You can’t break even.

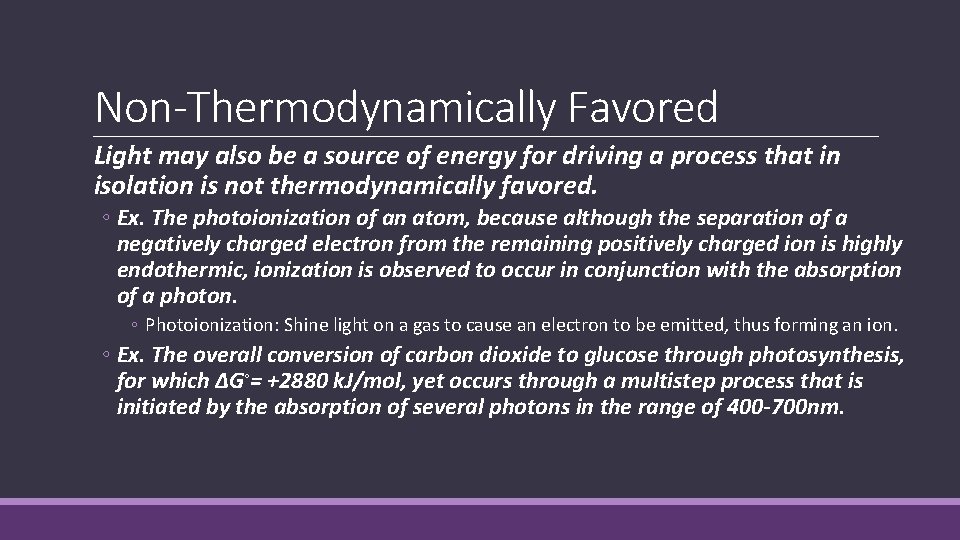
Non-Thermodynamically Favored External sources can be used to drive change in cases where the change in Gibbs free energy is positive: ◦ Electricity may be used to cause a process to occur that is not thermodynamically favored. ◦ Ex. Charging a battery, electrolysis.

Non-Thermodynamically Favored Light may also be a source of energy for driving a process that in isolation is not thermodynamically favored. ◦ Ex. The photoionization of an atom, because although the separation of a negatively charged electron from the remaining positively charged ion is highly endothermic, ionization is observed to occur in conjunction with the absorption of a photon. ◦ Photoionization: Shine light on a gas to cause an electron to be emitted, thus forming an ion. ◦ Ex. The overall conversion of carbon dioxide to glucose through photosynthesis, for which ΔG◦= +2880 k. J/mol, yet occurs through a multistep process that is initiated by the absorption of several photons in the range of 400 -700 nm.

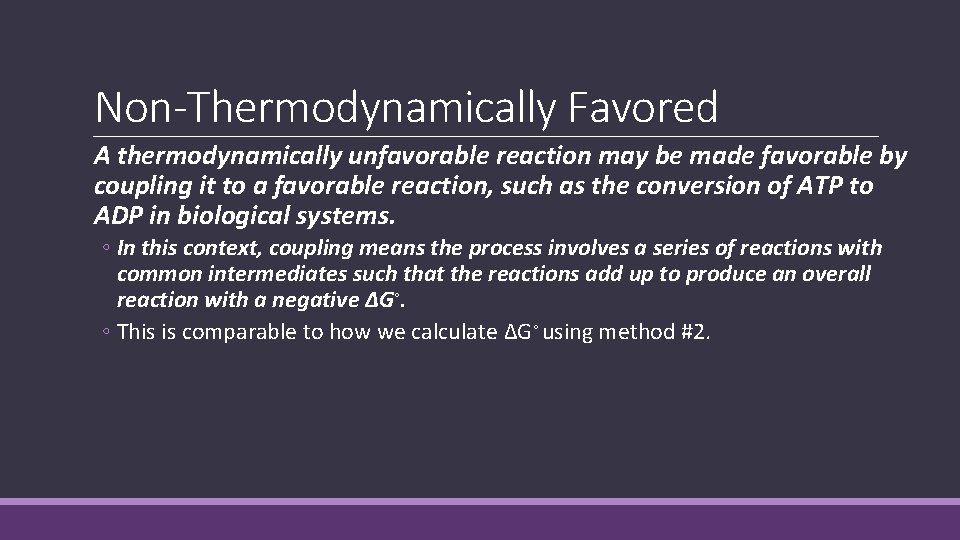
Non-Thermodynamically Favored A thermodynamically unfavorable reaction may be made favorable by coupling it to a favorable reaction, such as the conversion of ATP to ADP in biological systems. ◦ In this context, coupling means the process involves a series of reactions with common intermediates such that the reactions add up to produce an overall reaction with a negative ΔG◦. ◦ This is comparable to how we calculate ΔG◦ using method #2.

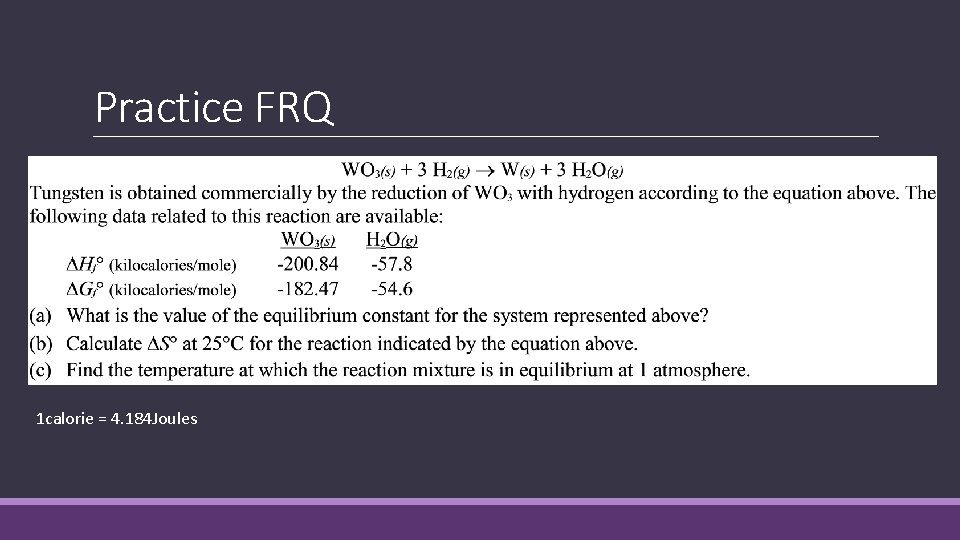
Practice FRQ 1 calorie = 4. 184 Joules

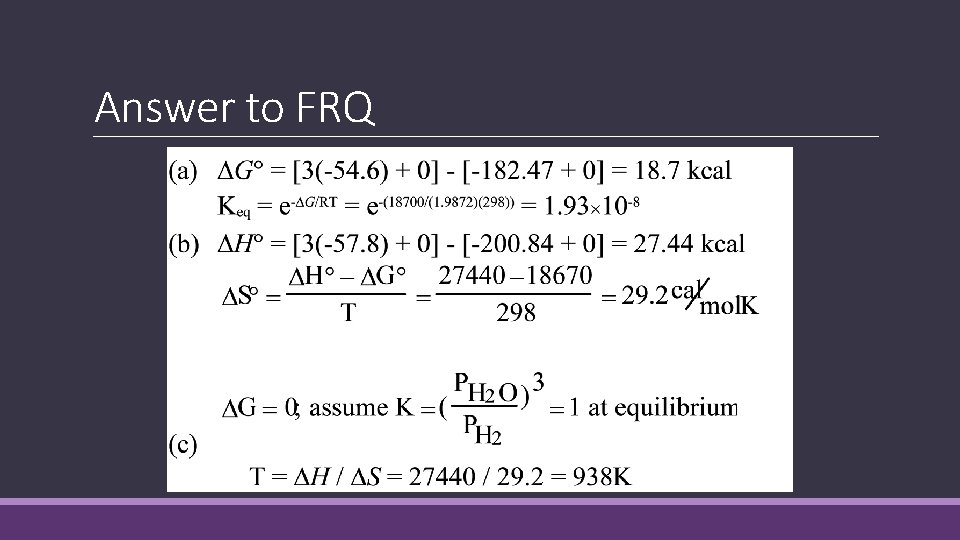
Answer to FRQ

Practice FRQ

Answer to FRQ
 Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Ap chemistry thermochemistry
Ap chemistry thermochemistry Ap chemistry thermochemistry frq
Ap chemistry thermochemistry frq General chemistry thermochemistry
General chemistry thermochemistry Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry Ap chemistry unit 9
Ap chemistry unit 9 Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Chapter 17 thermochemistry
Chapter 17 thermochemistry Chapter 17 thermochemistry answer key
Chapter 17 thermochemistry answer key Thermochemistry form 3 exercise
Thermochemistry form 3 exercise Quiz 2: heat flow and technology
Quiz 2: heat flow and technology Chapter 6 thermochemistry
Chapter 6 thermochemistry Chapter 17 thermochemistry
Chapter 17 thermochemistry Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Functional groups ib chemistry
Functional groups ib chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Unit 10, unit 10 review tests, unit 10 general test
Unit 10, unit 10 review tests, unit 10 general test Thermochemistry
Thermochemistry Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is study of: *
Thermochemistry is study of: * Thermochemistry equations
Thermochemistry equations Thermochemistry
Thermochemistry Thermochemistry in gaussian
Thermochemistry in gaussian Thermochemistry equations
Thermochemistry equations Thermochemistry cartoon
Thermochemistry cartoon Thermochemistry is study of
Thermochemistry is study of Thermochemistry equations
Thermochemistry equations Thermochemistry one pager
Thermochemistry one pager Thermochemical equation
Thermochemical equation Kirchhoff's law enthalpy example
Kirchhoff's law enthalpy example Thermochemistry equations
Thermochemistry equations Thermochemistry cartoon
Thermochemistry cartoon Thermochemistry is the study of
Thermochemistry is the study of Study of energy transformations
Study of energy transformations Termokimia mempelajari hubungan antara reaksi kimia dengan
Termokimia mempelajari hubungan antara reaksi kimia dengan What is thermochemistry
What is thermochemistry What is thermochemistry
What is thermochemistry Thermochemistry concepts
Thermochemistry concepts Thermochemistry is study of
Thermochemistry is study of Thermochemistry is concerned with the
Thermochemistry is concerned with the Thermochemistry
Thermochemistry A piece of metal is heated then submerged in cool water
A piece of metal is heated then submerged in cool water Intro to thermochemistry
Intro to thermochemistry Thermochemistry video
Thermochemistry video Thermochemistry conversions
Thermochemistry conversions Thermochemistry
Thermochemistry Thermochemistry exam review
Thermochemistry exam review State and explain hess's law of constant heat summation
State and explain hess's law of constant heat summation Thermochemistry notes
Thermochemistry notes Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry problems
Thermochemistry problems Why are chemists interested in studying thermochemistry?
Why are chemists interested in studying thermochemistry? Thermochemistry vocabulary
Thermochemistry vocabulary Chapter 7 energy conservation of energy
Chapter 7 energy conservation of energy Binding energy chemistry
Binding energy chemistry Epgraph
Epgraph Ap chemistry spontaneity entropy and free energy
Ap chemistry spontaneity entropy and free energy What is lattice energy
What is lattice energy Binding energy chemistry
Binding energy chemistry Free energy in chemistry
Free energy in chemistry