Chapter 17 Thermochemistry Section 17 1 The Flow


































- Slides: 72

Chapter 17 Thermochemistry

Section 17. 1 The Flow of Energy 2

Energy Transformations Thermochemistry – study of qty of heat absorbed or evolved by chemical rxns n Thermochemistry is concerned with the flow of heat from the system to it’s surroundings, and vice-versa. n 3

Energy Transformations n Energy - capacity for doing work or supplying heat n weightless, odorless, tasteless n if within chemical substances called chemical potential energy Ex: Gasoline contains a significant amt of chemical potential energy 4

Exothermic/Endothermic Processes n n n In chemical reactions, heat is often transferred from the “system” to its “surroundings, ” or vice versa. The system - the substance under study in which a change occurs The surroundings – includes everything in the vicinity of the system

Exothermic/Endothermic Processes n The Law of Conservation of Energy states that during any chemical or physical process, energy is neither created nor destroyed. n All the energy is accounted for as work, stored energy, or heat.

Exothermic/Endothermic Processes n Essentially all chemical reactions and changes in physical state involve either: a) b) evolution or release of heat, or absorption of heat

Direction of Heat Flow n The direction of heat flow is given from the point of view of the system. n Exothermic process is one that releases heat to its surroundings. n Heat flowing out of a system into its surroundings is defined as negative nq has (−) value

Direction of Heat Flow n The direction of heat flow is given from the point of view of the system. n An endothermic process is one that absorbs heat from the surroundings. n Heat flowing into a system into its surroundings is defined as positive nq has (+) value

17. 1 The Flow of Energy > Sample Problem 17. 1 Recognizing Endothermic and Exothermic Processes On a sunny winter day, the snow on a rooftop begins to melt. As the melted water drips from the roof, it refreezes into icicles. Describe the direction of heat flow as the water freezes. Is this process endothermic or exothermic? 10 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 1 The Flow of Energy > Sample Problem 17. 1 1 Analyze Identify the relevant concepts. • Heat flows from a warmer object to a cooler object. • An endothermic process absorbs heat from the surroundings. • An exothermic process releases heat to the surroundings. First identify the system and surroundings. Then determine the direction of the heat flow. 11 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 1 The Flow of Energy > Sample Problem 17. 1 2 Solve Apply concepts to this situation. First identify the system and the surroundings. System: water Surroundings: air 12 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 1 The Flow of Energy > Sample Problem 17. 1 2 Solve Apply concepts to this situation. Determine the direction of heat flow. • In order for water to freeze, its temperature must decrease. • Heat flows out of the water and into the air. 13 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 1 The Flow of Energy > Sample Problem 17. 1 2 Solve Apply concepts to this situation. Determine if the process is endothermic or exothermic. • Heat is released from the system to the surroundings. • The process is exothermic. 14 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Exothermic/Endothermic Processes Every reaction has an energy change associated with it n Exothermic reactions release energy, usually in the form of heat. n Endothermic reactions absorb energy n Energy is stored in bonds between atoms n 15

Gummi Bear Sacrifice 16

Units for Measuring Heat Flow 1) A calorie (cal) is defined as the quantity of heat needed to raise the temperature of 1 g of pure water 1 o. C. n Used except when referring to food n a Calorie, (written with a capital C), always refers to the energy in food n 1 Calorie = 1 kilocalorie = 1000 cal. 17

Units for Measuring Heat Flow 2) calorie is also related to the Joule, Joule – SI unit of heat and energy § One joule of heat raises the temperature of 1 g of pure water 0. 2390°C. § You can convert between calories and joules using the following relationships: 1 J = 0. 2390 cal 4. 184 J = 1 cal 18

Heat Capacity & Specific Heat n Heat Capacity - the amount of heat needed to increase the temperature of an object exactly 1 o. C n Depends on both the object’s mass and its chemical composition 19

17. 1 The Flow of Energy > Heat Capacity and Specific Heat The heat capacity of an object depends on both its mass and its chemical composition. • The greater the mass of the object, the greater its heat capacity. • A massive steel cable requires more heat to raise its temperature by 1 o. C than a steel nail does. 20 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Heat Capacity and Specific Heat n n Specific Heat Capacity (abbreviated “C”) - the amount of heat it takes to raise the temperature of 1 gram of the substance by 1 o. C n often called simply “Specific Heat” n Note Table 17. 1, page 559 Water has a HUGE value, when it is compared to other chemicals 21

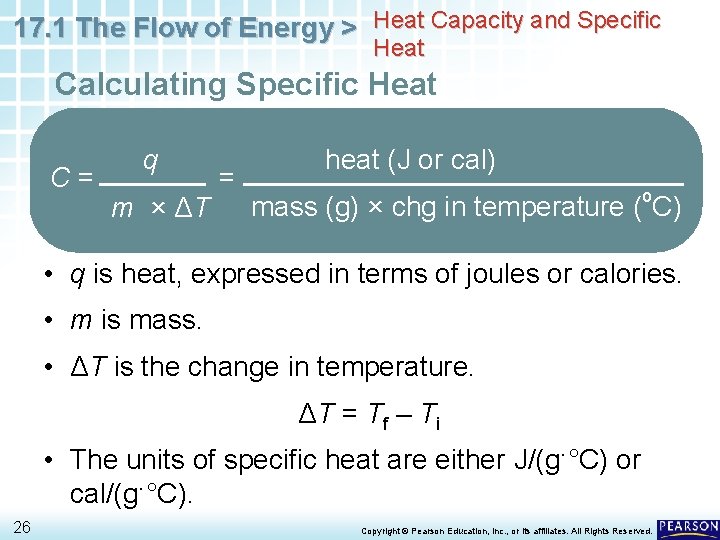
17. 1 The Flow of Energy > Heat Capacity and Specific Heat Calculating Specific Heat To calculate the specific heat (C) of a substance, you divide the heat input by the mass of the substance times the temperature change. C= 25 q m × ΔT = heat (J or cal) mass (g) × chg in temperature (o. C) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 1 The Flow of Energy > Heat Capacity and Specific Heat Calculating Specific Heat C= q m × ΔT = heat (J or cal) mass (g) × chg in temperature (o. C) • q is heat, expressed in terms of joules or calories. • m is mass. • ΔT is the change in temperature. ΔT = Tf – Ti • The units of specific heat are either J/(g·°C) or cal/(g·°C). 26 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

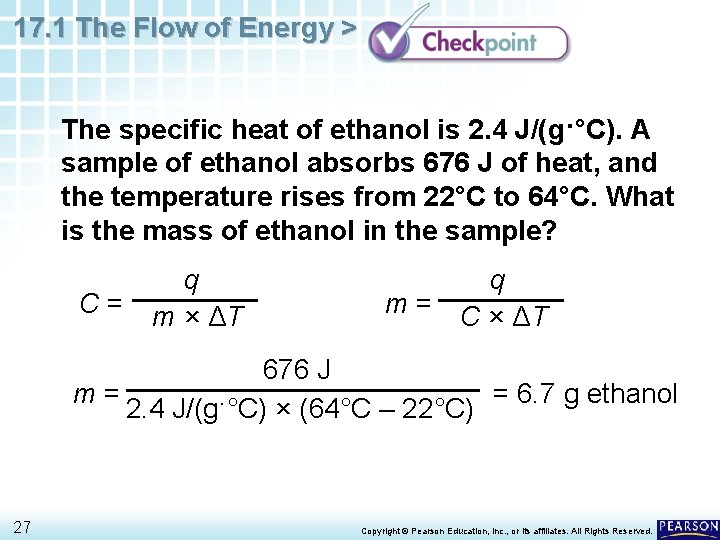
17. 1 The Flow of Energy > The specific heat of ethanol is 2. 4 J/(g·°C). A sample of ethanol absorbs 676 J of heat, and the temperature rises from 22°C to 64°C. What is the mass of ethanol in the sample? C= q m × ΔT q m = C × ΔT 676 J m= = 6. 7 g ethanol 2. 4 J/(g·°C) × (64°C – 22°C) 27 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Section 17. 2 Measuring and Expressing Enthalpy Changes 28

Calorimetry - the measurement of the heat into or out of a system for chemical and physical processes. n Based on the fact that the heat released = the heat absorbed n The device used to measure the absorption or release of heat in chemical or physical processes is called a “Calorimeter” n

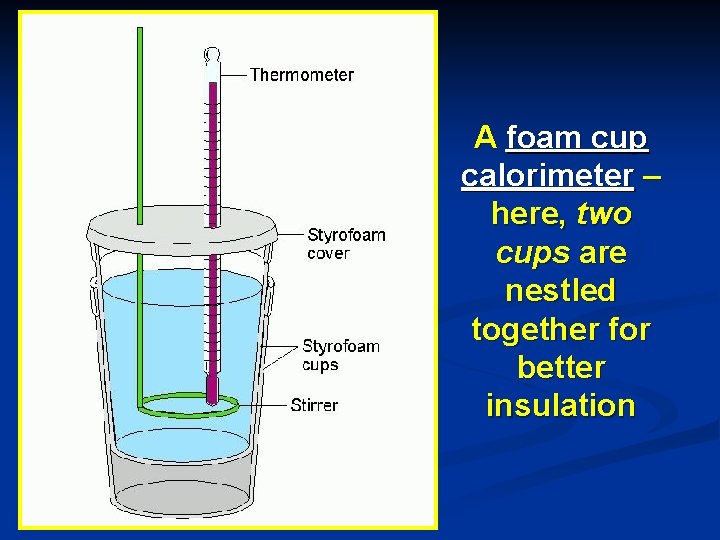
Calorimetry Foam cups are excellent heat insulators, and are commonly used as simple calorimeters under constant pressure. (They are good because they are well-insulated. ) n Fig. 17. 5, page 511 n What about a Dewar’s flask? n For systems at constant pressure, the “heat content” is the same as a property called Enthalpy (H) of the system n

A foam cup calorimeter – here, two cups are nestled together for better insulation

Calorimetry Changes in enthalpy = ΔH n q = ΔH These terms will be used interchangeably in this textbook n Thus, q = H = m x C x ΔT 4 H is negative for an exothermic reaction 4 H is positive for an endothermic reaction n

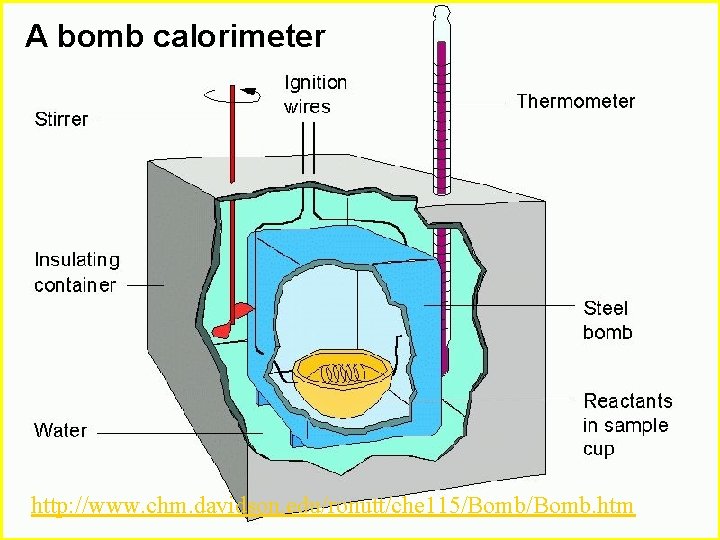
Calorimetry n Calorimetry experiments can be performed at a constant volume using a device called a “bomb calorimeter” - a closed system n Used by nutritionists to measure energy content of food

A bomb calorimeter A Bomb Calorimeter http: //www. chm. davidson. edu/ronutt/che 115/Bomb. htm

- Page 513

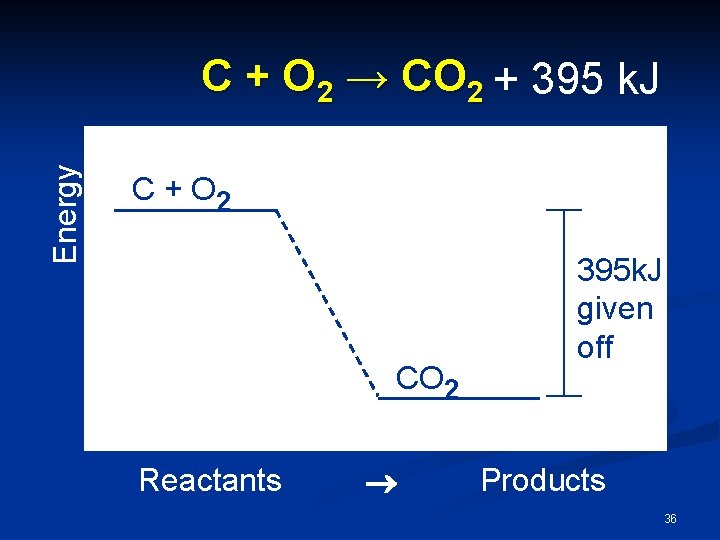
Energy C + O 2 → CO 2 + 395 k. J C + O 2 CO 2 Reactants ® 395 k. J given off Products 36

Exothermic n The products are lower in energy than the reactants n Thus, energy is released. n ΔH = -395 k. J n. The negative sign does not mean negative energy, but instead that energy is lost. 37

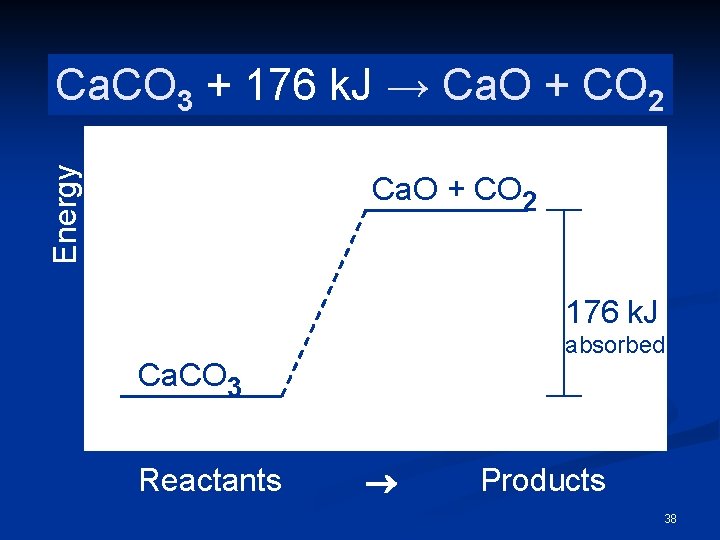
Energy Ca. CO Ca. O + CO+2 CO 2 Ca. CO → Ca. O 3 →k. J 3 + 176 Ca. O + CO 2 176 k. J absorbed Ca. CO 3 Reactants ® Products 38

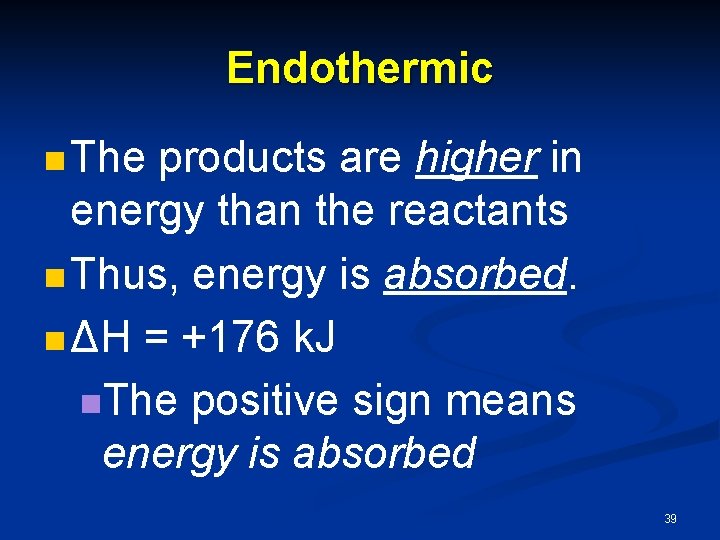
Endothermic n The products are higher in energy than the reactants n Thus, energy is absorbed. n ΔH = +176 k. J n. The positive sign means energy is absorbed 39

Chemistry Happens in MOLES n n An equation that includes energy is called a thermochemical equation CH 4 + 2 O 2 CO 2 + 2 H 2 O + 802. 2 k. J n 1 mole of CH 4 releases 802. 2 k. J of energy. n When you make 802. 2 k. J you also make 2 moles of water 40

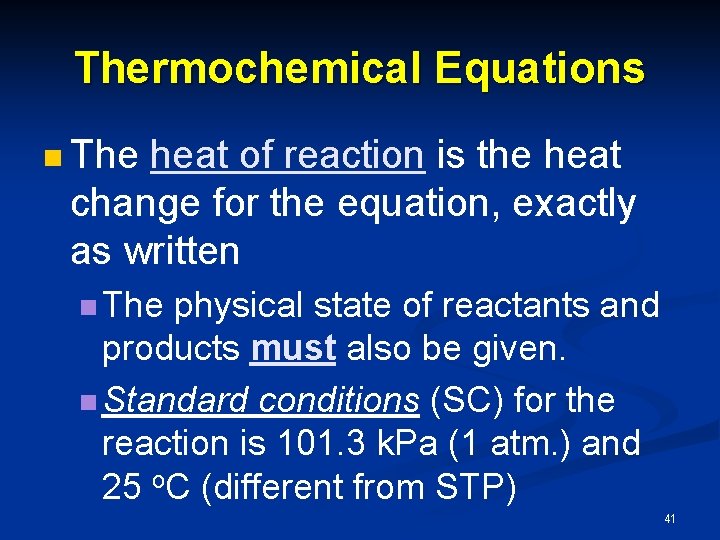
Thermochemical Equations n The heat of reaction is the heat change for the equation, exactly as written n The physical state of reactants and products must also be given. n Standard conditions (SC) for the reaction is 101. 3 k. Pa (1 atm. ) and 25 o. C (different from STP) 41

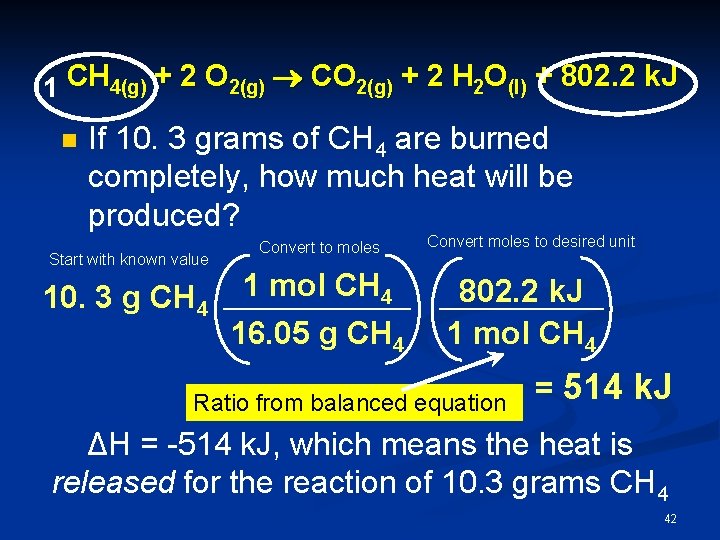
1 CH 4(g) + 2 O 2(g) ® CO 2(g) + 2 H 2 O(l) + 802. 2 k. J n If 10. 3 grams of CH 4 are burned completely, how much heat will be produced? Start with known value 10. 3 g CH 4 Convert to moles 1 mol CH 4 16. 05 g CH 4 Convert moles to desired unit 802. 2 k. J 1 mol CH 4 Ratio from balanced equation = 514 k. J ΔH = -514 k. J, which means the heat is released for the reaction of 10. 3 grams CH 4 42

- Page 516

Summary, so far. . .

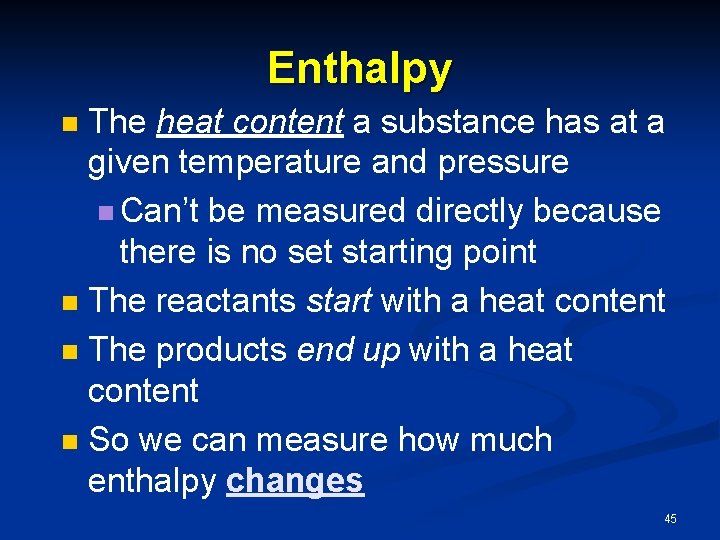
Enthalpy The heat content a substance has at a given temperature and pressure n Can’t be measured directly because there is no set starting point n The reactants start with a heat content n The products end up with a heat content n So we can measure how much enthalpy changes n 45

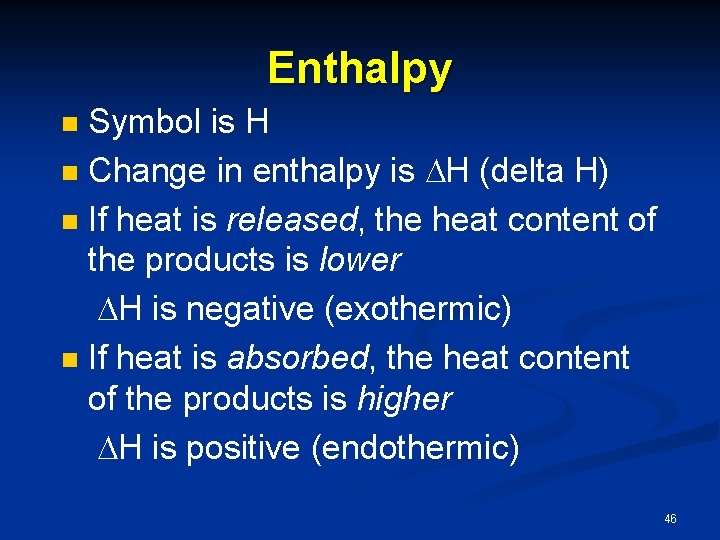
Enthalpy Symbol is H n Change in enthalpy is H (delta H) n If heat is released, the heat content of the products is lower H is negative (exothermic) n If heat is absorbed, the heat content of the products is higher H is positive (endothermic) n 46

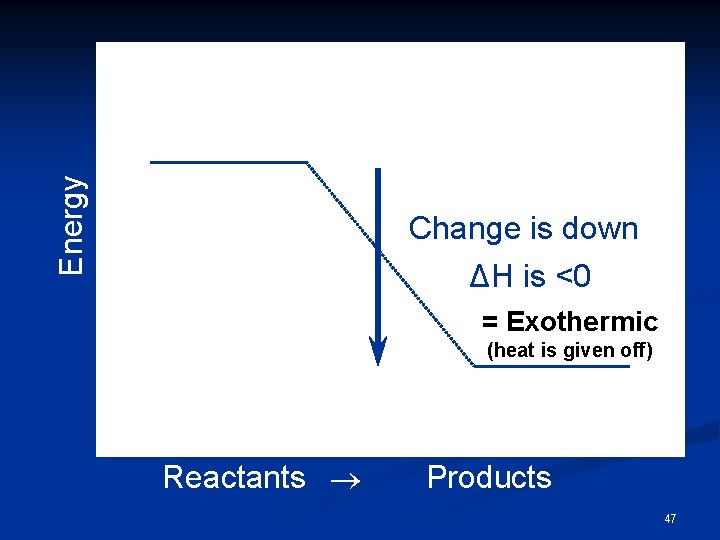
Energy Change is down ΔH is <0 = Exothermic (heat is given off) Reactants Products 47

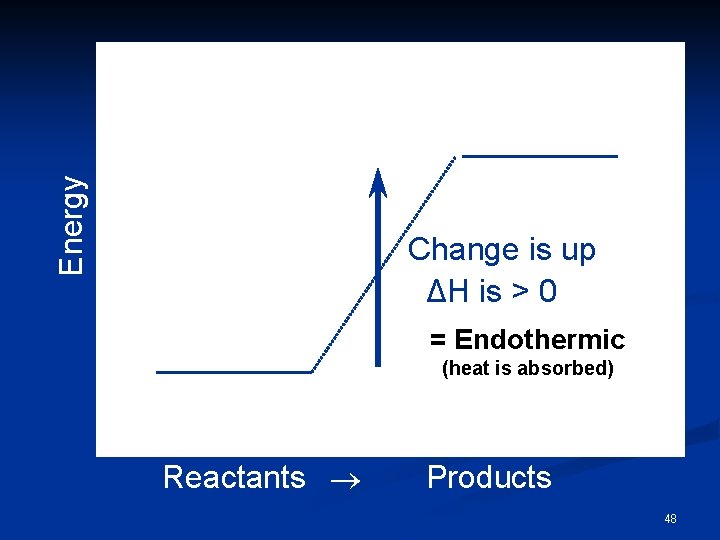
Energy Change is up ΔH is > 0 = Endothermic (heat is absorbed) Reactants Products 48

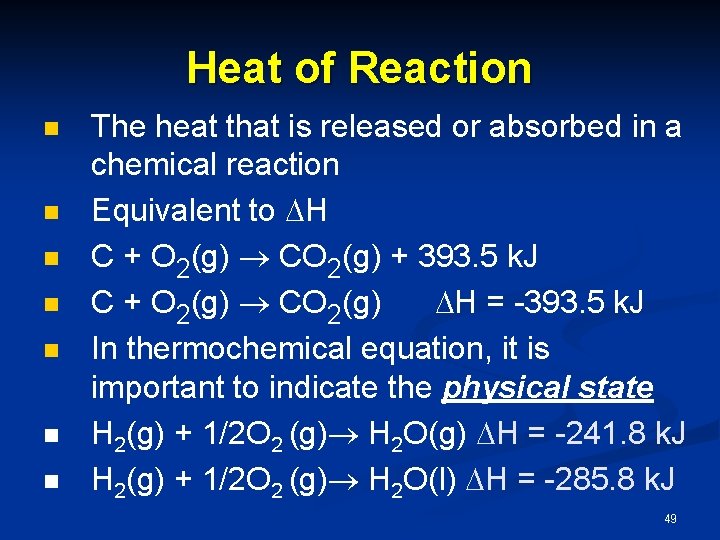
Heat of Reaction n n n The heat that is released or absorbed in a chemical reaction Equivalent to H C + O 2(g) CO 2(g) + 393. 5 k. J C + O 2(g) CO 2(g) H = -393. 5 k. J In thermochemical equation, it is important to indicate the physical state H 2(g) + 1/2 O 2 (g) H 2 O(g) H = -241. 8 k. J H 2(g) + 1/2 O 2 (g) H 2 O(l) H = -285. 8 k. J 49

Heat of Combustion n The heat from the reaction that completely burns 1 mole of a substance: n n C + O 2(g) CO 2(g) + 393. 5 k. J C + O 2(g) CO 2(g) H = -393. 5 k. J n Note Table 17. 2, page 517 n DVD: Thermite Reaction 50

Section 17. 3 Heat in Changes of State n OBJECTIVES: n. Classify the enthalpy change that occurs when a substance: a) melts, b) freezes, c) boils, d) condenses, or e) dissolves. 51

Section 17. 3 Heat in Changes of State n OBJECTIVES: n. Solve for the enthalpy change that occurs when a substance: a) melts, b) freezes, c) boils, d) condenses, or e) dissolves. 52

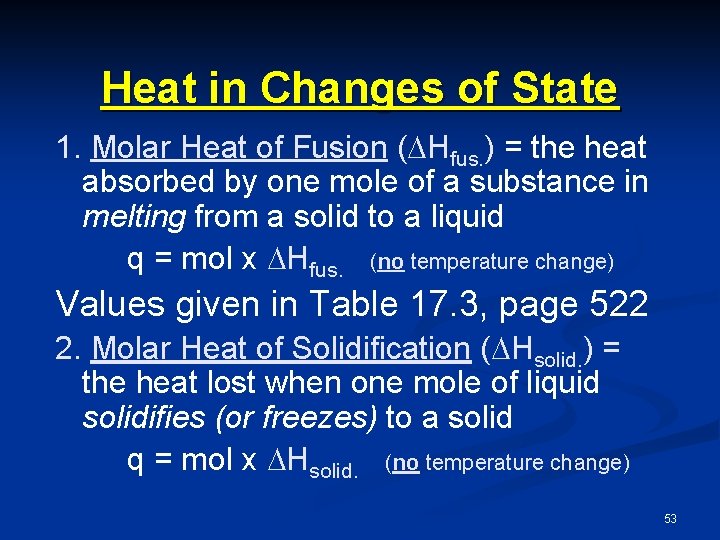
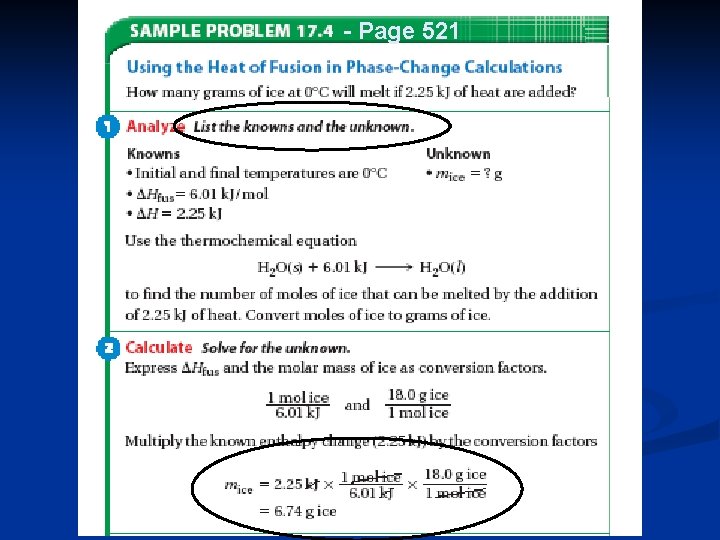
Heat in Changes of State 1. Molar Heat of Fusion ( Hfus. ) = the heat absorbed by one mole of a substance in melting from a solid to a liquid q = mol x Hfus. (no temperature change) Values given in Table 17. 3, page 522 2. Molar Heat of Solidification ( Hsolid. ) = the heat lost when one mole of liquid solidifies (or freezes) to a solid q = mol x Hsolid. (no temperature change) 53

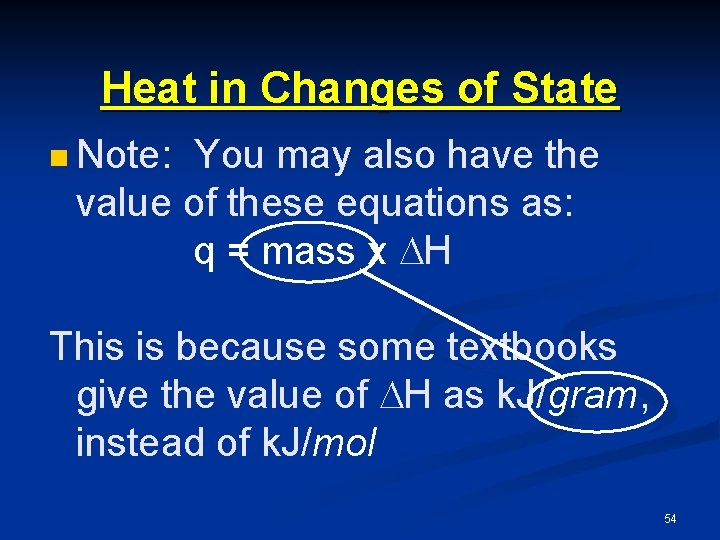
Heat in Changes of State n Note: You may also have the value of these equations as: q = mass x H This is because some textbooks give the value of H as k. J/gram, instead of k. J/mol 54

Heat in Changes of State n Heat absorbed by a melting solid is equal to heat lost when a liquid solidifies n Thus, Hfus. = - Hsolid. n Note Table 17. 3, page 522 for the number values. Why is there no value listed for the molar heat of solidification? 55

- Page 521

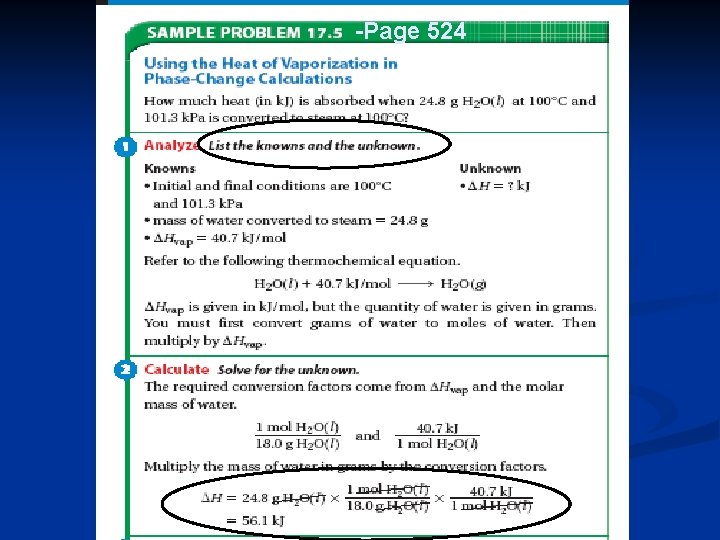
Heats of Vaporization and Condensation When liquids absorb heat at their boiling points, they become vapors. 3. Molar Heat of Vaporization ( Hvap. ) = the amount of heat necessary to vaporize one mole of a given liquid. q = mol x Hvap. (no temperature change) n Table 17. 3, page 522 n 57

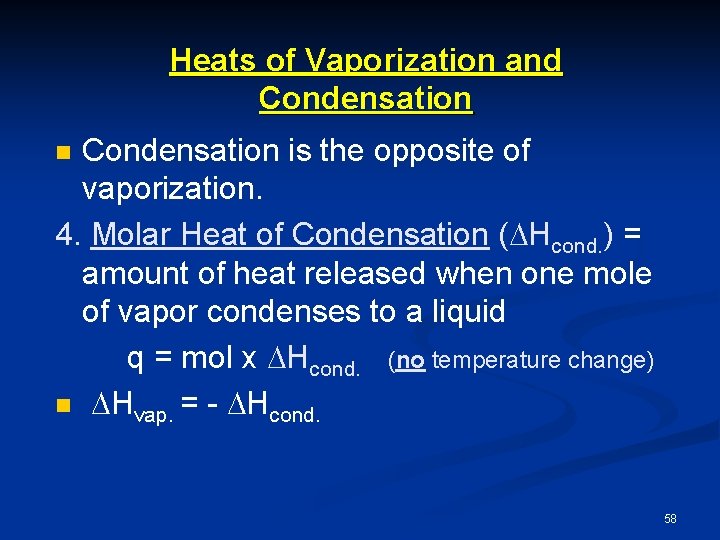
Heats of Vaporization and Condensation is the opposite of vaporization. 4. Molar Heat of Condensation ( Hcond. ) = amount of heat released when one mole of vapor condenses to a liquid q = mol x Hcond. (no temperature change) n Hvap. = - Hcond. n 58

Heats of Vaporization and Condensation n Lets look at Table 17. 3, page 522… n The large values for water Hvap. and Hcond. is the reason hot vapors such as steam are very dangerous! n You can receive a scalding burn from steam when the heat of condensation is released! H 20(g) H 20(l) Hcond. = - 40. 7 k. J/mol 59

-Page 524

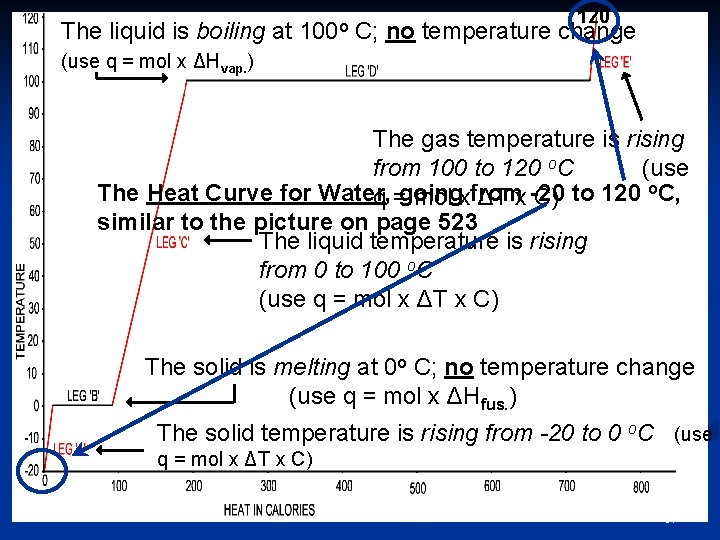
The liquid is boiling at 100 o 120 C; no temperature change (use q = mol x ΔHvap. ) The gas temperature is rising from 100 to 120 o. C (use The Heat Curve for Water, q =going mol xfrom ΔT x -20 C) to 120 o. C, similar to the picture on page 523 The liquid temperature is rising from 0 to 100 o. C (use q = mol x ΔT x C) The solid is melting at 0 o C; no temperature change (use q = mol x ΔHfus. ) The solid temperature is rising from -20 to 0 o. C (use q = mol x ΔT x C) 61

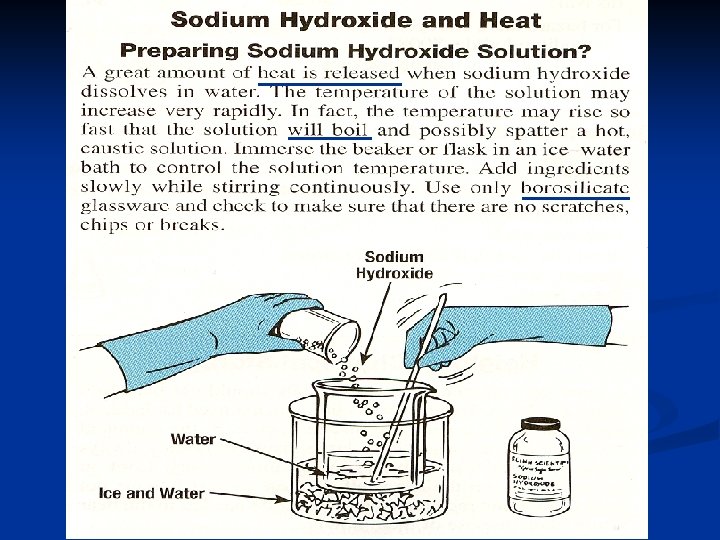
Heat of Solution Heat changes can also occur when a solute dissolves in a solvent. 5. Molar Heat of Solution ( Hsoln. ) = heat change caused by dissolution of one mole of substance q = mol x Hsoln. (no temperature change) n Sodium hydroxide provides a good example of an exothermic molar heat of solution (next slide) n 62

Heat of Solution Na. OH(s) H 2 O(l) Na 1+(aq) + OH 1 -(aq) Hsoln. = - 445. 1 k. J/mol n The heat is released as the ions separate (by dissolving) and interact with water, releasing 445. 1 k. J of heat as Hsoln. n thus becoming so hot it steams! 63


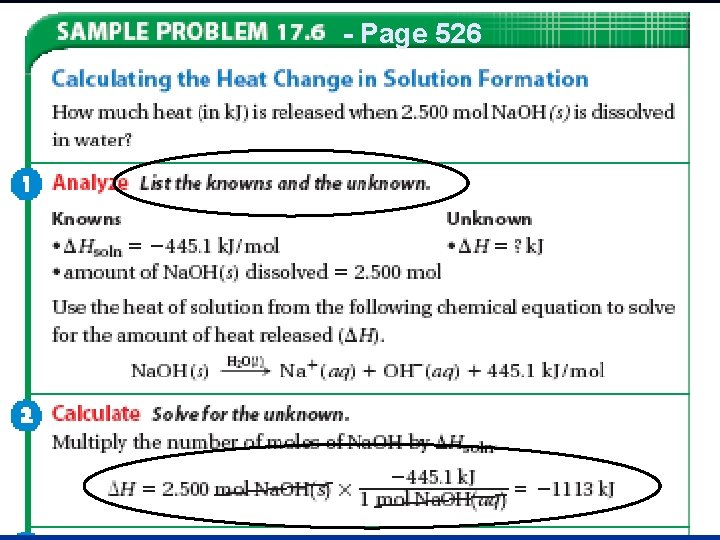
- Page 526

Section 17. 4 Calculating Heats of Reaction n OBJECTIVES: n. State Hess’s Law of Heat Summation, and describe how (or why) it is used in chemistry. 66

Section 17. 4 Calculating Heats of Reaction n OBJECTIVES: n. Solve for enthalpy changes by using Hess’s law or standard heats of formation. 67

Hess’s Law (developed in 1840) Germain Henri Hess (1802 -1850) n If you add two or more thermochemical equations to give a final equation, then you can also add the heats of reaction to give the final heat of reaction. Called Hess’s Law of Heat Summation 68

How Does It Work? 1) n n If you turn an equation around, you change the sign: If H 2(g) + 1/2 O 2(g) H 2 O(g) H=-285. 5 k. J then the reverse is: H 2 O(g) H 2(g) + 1/2 O 2(g) H =+285. 5 k. J If you multiply the equation by a number, you multiply the heat by that number: 2 H 2 O(g) 2 H 2(g) + O 2(g) H =+571. 0 k. J Or, you can just leave the equation “as is” 69

Hess’s Law - Procedure Options: 1. Use the equation as written 2. Reverse the equation (and change heat sign + to -, etc. ) 3. Increase the coefficients in the equation (and increase heat by same amount) n Note samples from pages 528 and 529 70

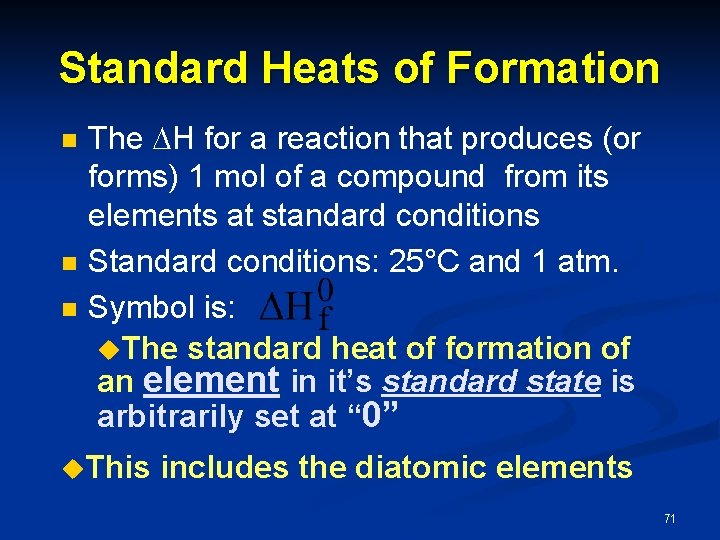
Standard Heats of Formation n The H for a reaction that produces (or forms) 1 mol of a compound from its elements at standard conditions Standard conditions: 25°C and 1 atm. Symbol is: u. The standard heat of formation of an element in it’s standard state is arbitrarily set at “ 0” u. This includes the diatomic elements 71

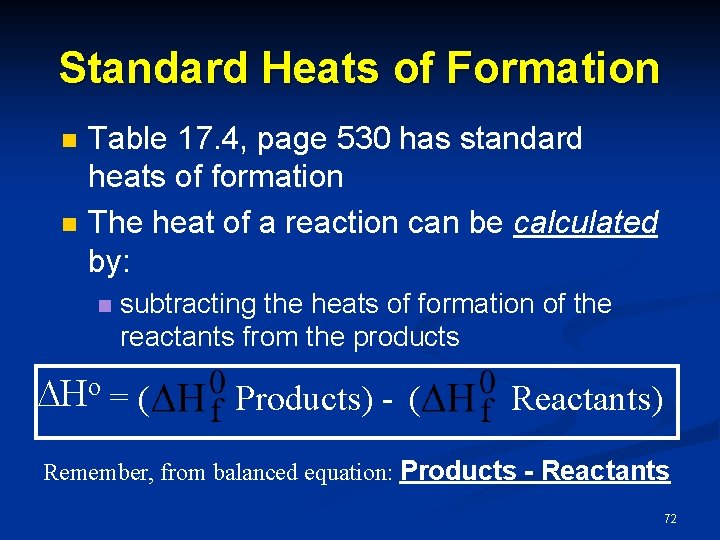
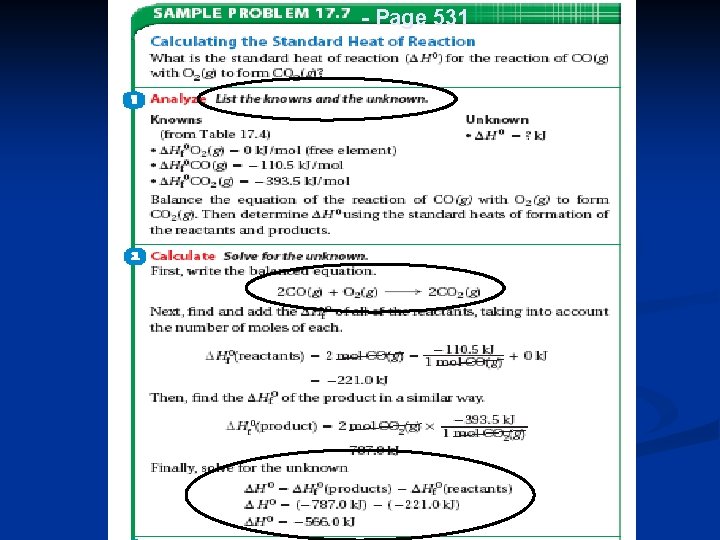
Standard Heats of Formation n n Table 17. 4, page 530 has standard heats of formation The heat of a reaction can be calculated by: n subtracting the heats of formation of the reactants from the products Ho = ( Products) - ( Reactants) Remember, from balanced equation: Products - Reactants 72

- Page 531

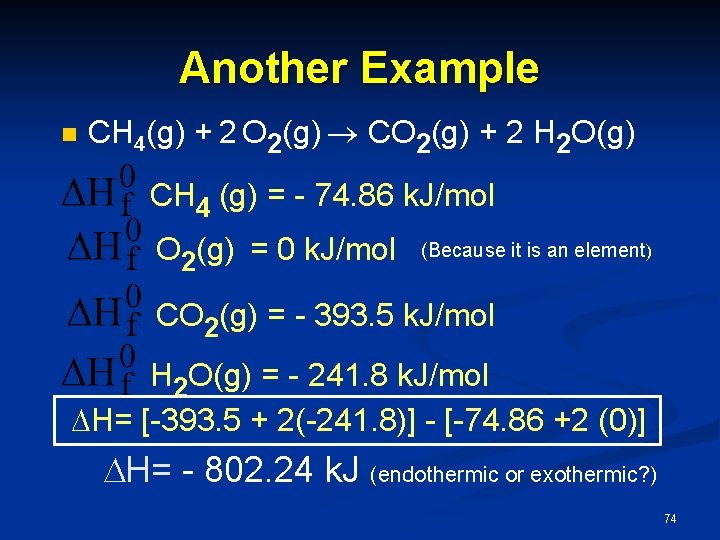
Another Example n CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) CH 4 (g) = - 74. 86 k. J/mol O 2(g) = 0 k. J/mol (Because it is an element) CO 2(g) = - 393. 5 k. J/mol H 2 O(g) = - 241. 8 k. J/mol H= [-393. 5 + 2(-241. 8)] - [-74. 86 +2 (0)] H= - 802. 24 k. J (endothermic or exothermic? ) 74

75