Thermochemistry Study of energy and energy transfer All
























- Slides: 44

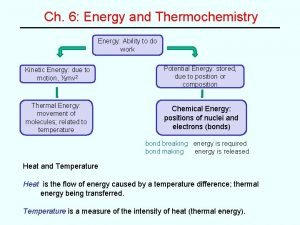
Thermochemistry

�Study of energy and energy transfer ◦ All physical changes and chemical changes are accompanied by a change in energy Thermochemistry

�The total energy of the universe is constant ΔEuniverse = 0 ◦ Ie. Energy cannot be created nor destroyed �Energy CAN, however, be transferred from one form to another! Law of Conservation of Energy

�To interpret energy changes, we need to first define some terms: ◦ The system is the part of the universe being studied/observed (ex. reactants and products) ◦ The surroundings is everything else in the universe. �The relationship between system and surroundings: �ΔEuniverse = ΔEsystem + ΔEsurroundings = 0 System and Surroundings

�Any change in the energy of a system must be accompanied by an equal and opposite change in the energy of the surroundings ΔEsystem = -ΔEsurroundings Surroundings = rest of the universe! System The system is more likely to only significantly influence the immediate surroundings, so this is what we consider. Law of Conservation of Energy

�Open system = open to surroundings- both matter and energy can be exchanged �Closed system= no matter can be exchanged, however energy still can be �Isolated system = completely isolated from surroundings, both in terms of matter and energy Types of Systems

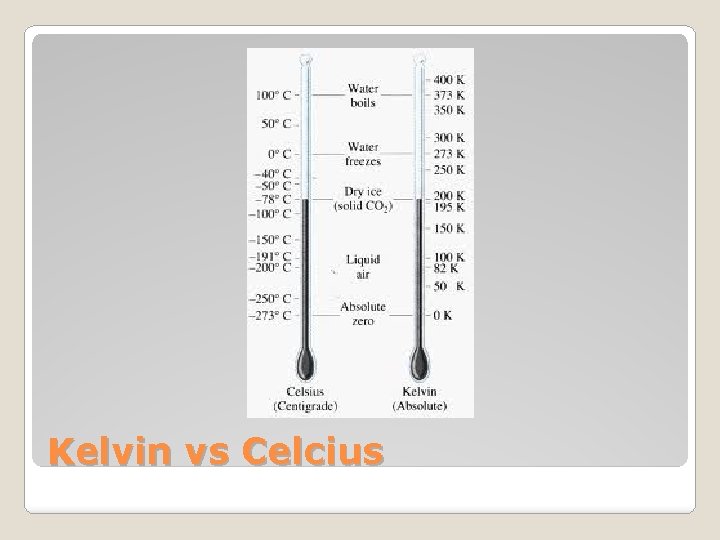
�We know that: ◦ Potential energy = stored energy ◦ Kinetic energy = energy of motion �SI units for both ◦ = 1 kg·m 2/s 2 are joules (J) �Temperature is a measure of the average kinetic energy of the particles that make up a system ◦ Celcius = relative scale (designed relative to H 2 O) ◦ Kelvin = absolute scale (0 = absolute 0, molecules have no kinetic energy!) ◦ 273 K = 0 o. C Types of Energy

Kelvin vs Celcius

�Heat (q) is the transfer of kinetic energy between objects with different temperatures (units = J) ◦ That is why when a substance absorbs heat, it’s particles speed up- they have really absorbed kinetic energy! �According to the first law of thermodynamics: qsystem = -qsurroundings What is Heat?

�What factors are important to consider when measuring changes in energy? ◦ Temperature ◦ Mass ◦ Specific heat capacity (c) �Reflects the nature of a given species to retain heat (aka. store energy) �The energy required to change 1 gram of a substance by 1 degree Celcius �Units = J/g·o. C �For example, the specific heat capacity of water is 4. 184 J/g·o. C ◦ Helps to moderate Earth’s temperature Factors that Affect Change in Energy

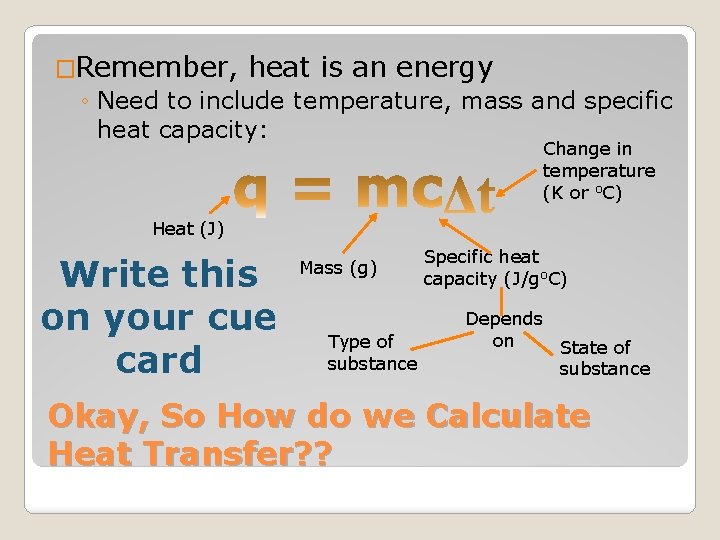
�Remember, heat is an energy ◦ Need to include temperature, mass and specific heat capacity: Change in temperature (K or o. C) Heat (J) Write this on your cue card Mass (g) Type of substance Specific heat capacity (J/go. C) Depends on State of substance Okay, So How do we Calculate Heat Transfer? ?

� 10. 0 g of ice is added to 60. 0 g of water that has an initial temperature of 26. 5 o. C. The final temperature of the mixture is 9. 7 o. C. How much heat is lost by the water? ◦ m = 60. 0 g (we are only concerned about the water!) ◦ Ti = 26. 5 o. C ◦ Tf = 9. 7 o. C ◦ c = 4. 184 J/g o. C �q= mcΔt = (60. 0 g)(4. 184 J/g o. C) (26. 5 o. C – 9. 7 o. C) = - 4. 22 x 103 J (or -4. 22 k. J) Therefore, the water lost 4. 22 k. J of heat Okay, let’s do an example

�Which has a greater heat capacity? �Water has the same specific heat capacity in both cases- but different masses have different heat capacities Heat Capacity

�Relates the heat of a sample to it’s change in temperature C= c x m ◦ C = heat capacity (k. J/o. C) ◦ c = specific heat capacity (k. J/kg o. C) ◦ m = mass (kg) �And, therefore q = CΔT Heat Capacity Write this on your cue card

�A bathtub contains 100 kg of water. What is the heat capacity of the water in the tub? We need to use units of k. J and kg for the specific heat capacity C = 4. 184 k. J/kgo. C x 100 kg = 418 k. J/o. C �How much heat is transferred to the surroundings as the water cools from 60 o. C to 20 o. C? q = 418 k. J/o. C x (20 o. C – 60 o. C) = -1. 67 x 104 k. J qsystem = - qsurroundings Therefore the surroundings gained 1. 67 x 103 k. J of heat For Example

�What about the teacup? �The teacup contains 0. 100 kg of water. What is the heat capacity of the water in the teacup? What is the energy transferred to the surroundings if the water cools from 60 o. C to 20 o. C? For Example

�What is heat? �Aluminum has a specific heat capacity of 0. 902 J/go. C. Copper has a specific heat capacity of 0. 389 J/go. C. If an equal amount of heat is transferred to similar masses of each metal, which will increase more in temperature? Quick Review

�okay, so we can see a change in kinetic energy through a change in temperature �What happens during a ◦ Ie. solid liquid gas �What phase change? happens during a chemical reaction? �These changes are due to changes in the potential energy of the system Enthalpy Change

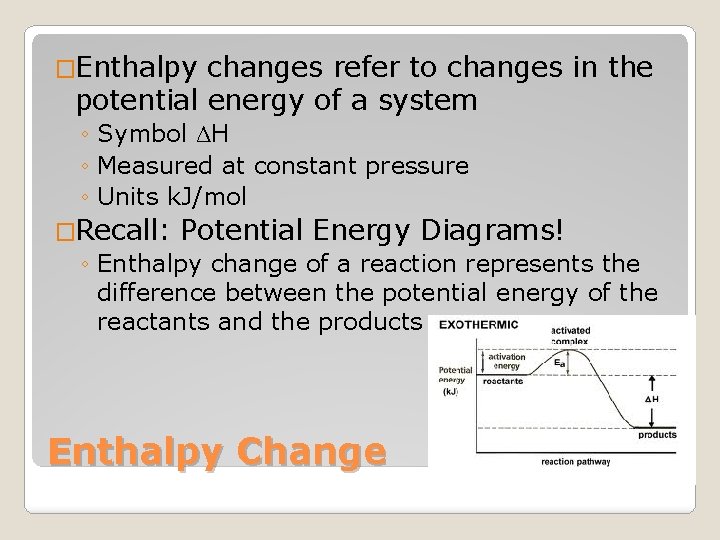
�Enthalpy changes refer to changes in the potential energy of a system ◦ Symbol ΔH ◦ Measured at constant pressure ◦ Units k. J/mol �Recall: Potential Energy Diagrams! ◦ Enthalpy change of a reaction represents the difference between the potential energy of the reactants and the products Enthalpy Change

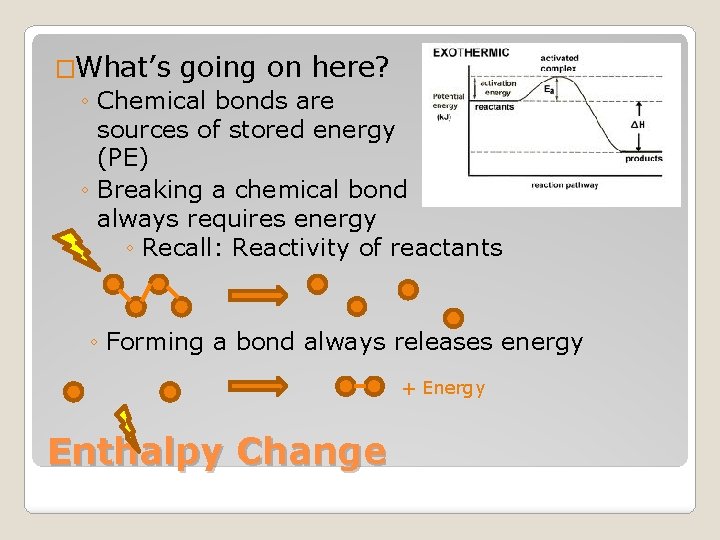
�What’s going on here? ◦ Chemical bonds are sources of stored energy (PE) ◦ Breaking a chemical bond always requires energy ◦ Recall: Reactivity of reactants ◦ Forming a bond always releases energy + Energy Enthalpy Change

�How much energy is required to break a bond depends on the strength of the bond ◦ Consider a synthesis reaction: N 2 + O 2 2 NO ◦ If more energy is required to break the triple bonds of N 2 and double bonds of O 2 than is given off in forming N-O bonds, the reaction has a net absorption of energy �ENDOTHERMIC ◦ If more energy was given off during the formation of N-O bonds, the reaction would have a net release of energy �EXOTHERMIC Enthalpy Change

� The internal energy of a substance (at constant pressure) = enthalpy ◦ Chemists never care about the absolute enthalpy ◦ We study the change in enthalpy that accompanies a process (relative) � Enthalpy of reaction ΔHrxn ◦ Depends on temperature and pressure ◦ Aka. heat of reaction � Standard enthalpy of reaction ΔH 0 rxn ◦ Occurs at SATP (25 o. C and 100 k. Pa) � Standard molar enthalpy of formation ΔH 0 f ◦ Quantity of energy absorbed or released when one mole of a compound is formed directly from it’s elements in their standard states. Enthalpy Change

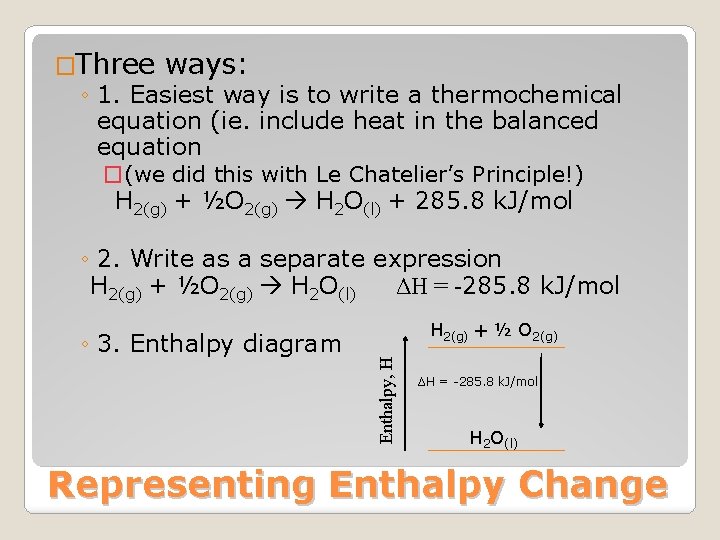
�Three ways: ◦ 1. Easiest way is to write a thermochemical equation (ie. include heat in the balanced equation �(we did this with Le Chatelier’s Principle!) H 2(g) + ½O 2(g) H 2 O(l) + 285. 8 k. J/mol ◦ 2. Write as a separate expression H 2(g) + ½O 2(g) H 2 O(l) ΔH = -285. 8 k. J/mol H 2(g) + ½ O 2(g) Enthalpy, H ◦ 3. Enthalpy diagram ΔH = -285. 8 k. J/mol H 2 O(l) Representing Enthalpy Change

�Show the standard molar enthalpies of formation for the following substances using a balanced thermochemical equation and by writing it as a separate expression. ◦ H 2 O ◦ Ca. Cl 2 ◦ CH 4 ◦ C 6 H 6 �Draw an enthalpy diagram for the standard molar enthalpy of formation of sodium chloride TRY IT

�Enthalpy changes also depend on the amount of reactants you have moles �So: q = n ΔH Heat and Enthalpy Write this on your cue card

�How much heat is released when 50. 00 g of methane (natural gas) is formed from it’s elements? ◦ Remember that to find moles of an element we have to divide the mass of the substance by the molar mass (Y diagram) n = 50. 00 g CH 4 / (12. 01 g/mol + 4(1. 01 g/mol) = 3. 13 mols CH 4 q = nΔH 0 f = 3. 13 mols x -74. 6 k. J/mol = -232 k. J Example

�Hydrogen gas and oxygen gas react to form 0. 534 g of liquid water. How much heat is released to the surroundings? �How my heat is released if 0. 543 g of gaseous water is produced? Try This Try some enthalpy calculations now

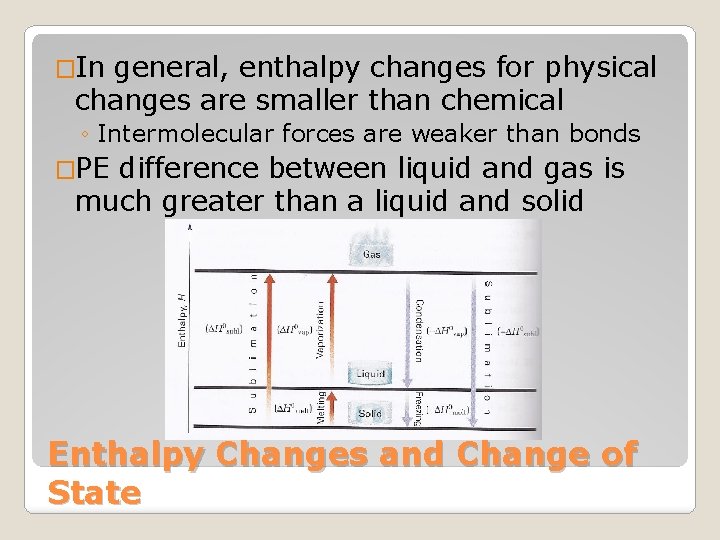
�In general, enthalpy changes for physical changes are smaller than chemical ◦ Intermolecular forces are weaker than bonds �PE difference between liquid and gas is much greater than a liquid and solid Enthalpy Changes and Change of State

�ΔHvap : enthalpy change for to change one mole from liquid to gas �ΔHcond : enthalpy change for to change one mole from gas to liquid �ΔHmelt : enthalpy change for to change one mole from solid to liquid (aka. fusion, ΔHfus) �ΔHfre : enthalpy change for to change one mole from liquid to solid ΔHvap = -ΔHcond ΔHmelt = -ΔHfre Molar Enthalpy

: Heat transfer when 1 mole of solute dissolves in solvent �ΔHsoln ◦ Can be endothermic or exothermic �Ex. hot and cold packs �Calculate heat changes for state changes the same way as chemical changes ◦ Ex: An ice cube with a mass of 8. 2 g is placed in some lemonade. The ice cube melts completely. How much heat does the ice cube absorb from the lemonade as it melts? Enthalpy of Solution

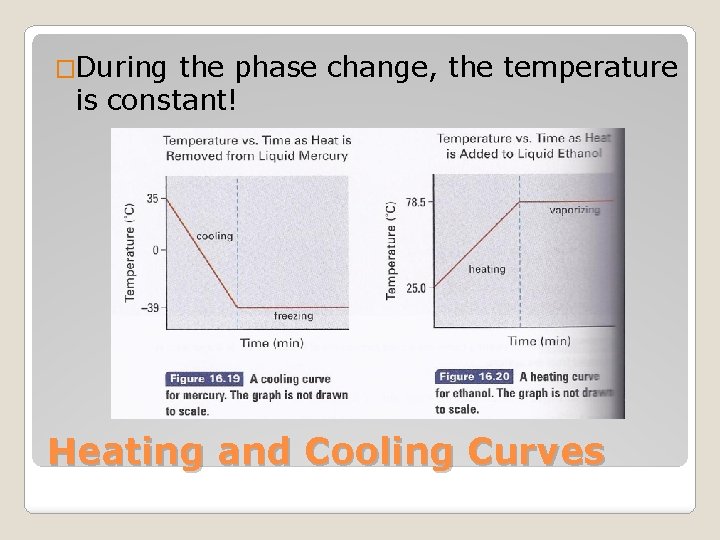
�Show temperature changes as heat is removed or added to a substance Heating and Cooling Curves

�During the phase change, the temperature is constant! Heating and Cooling Curves

�Okay, lets say you want to make some spaghetti. You put water on the stove to boil. �Meanwhile, you get distracted by an episode of Vampire Diaries and water boils off completely. ◦ What would the heating curve for this look like? ◦ How much heat was required to completely vaporize all of the water?

�Heat Curve: �To find the heat required, we have 2 Use this for a change in kinetic energy equations: (ie. temperature is changing) q = n ΔH Use this for a change in potential energy (ie. phase is changing)

�How much heat is absorbed by 5. 00 kg of water at 10 o. C if it is heated until all of the water has evaporated? ◦ Step 1: Heating the water to it’s boiling point q = mcΔT = (5000 g)(4. 184 J/go. C)(100 o. C-10 o. C) =1. 88 x 106 J or 1880 k. J ◦ Step 2: Change liquid water into water vapour q = nΔHvap = (5000 g/18 gmol-1)(40. 7 k. J/mol) = 1. 13 x 104 k. J ◦ Finally, 1880 k. J + 11300 k. J = 13180 k. J �So, the total heat absorbed by the water was 1. 32 x 104 k. J Example Now practice calculating heat over phase changes

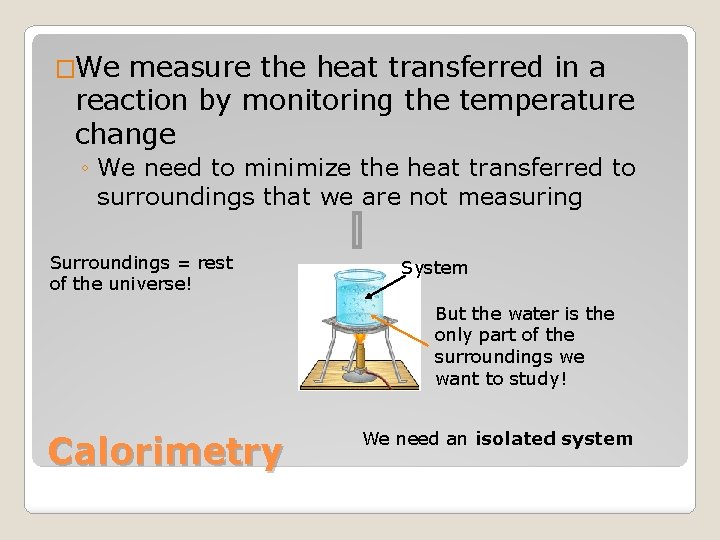
�We measure the heat transferred in a reaction by monitoring the temperature change ◦ We need to minimize the heat transferred to surroundings that we are not measuring Surroundings = rest of the universe! System But the water is the only part of the surroundings we want to study! Calorimetry We need an isolated system

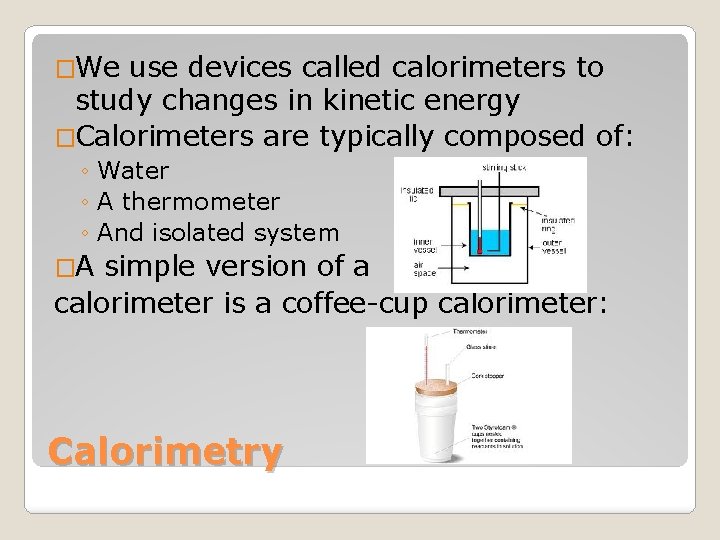
�We use devices called calorimeters to study changes in kinetic energy �Calorimeters are typically composed of: ◦ Water ◦ A thermometer ◦ And isolated system �A simple version of a calorimeter is a coffee-cup calorimeter: Calorimetry

�Recall the law of conservation of energy: qsystem = -qsurroundings �Assumes: ◦ The system is isolated ◦ The amount of heat exchanged with the calorimeter itself is negligible ◦ The properties of the water remains the same �The system is at thermal equilibrium when all components have the same temperature Calorimetry

�A 70. 0 g piece of metal was heated to 95 o. C in a hot water bath. This was quickly transferred to a coffee cup calorimeter. The calorimeter contained 100 g of water at 19. 8 o. C. The final temperature of the contents was 22. 6 o. C. What is the specific heat capacity of the metal? ◦ Recall: I already gave you a question similar to this when we started this unit! Example

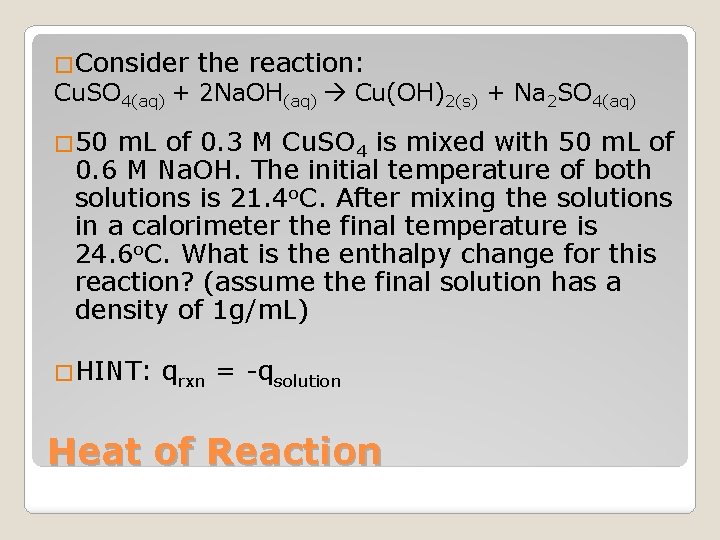
�Consider the reaction: Cu. SO 4(aq) + 2 Na. OH(aq) Cu(OH)2(s) + Na 2 SO 4(aq) � 50 m. L of 0. 3 M Cu. SO 4 is mixed with 50 m. L of 0. 6 M Na. OH. The initial temperature of both solutions is 21. 4 o. C. After mixing the solutions in a calorimeter the final temperature is 24. 6 o. C. What is the enthalpy change for this reaction? (assume the final solution has a density of 1 g/m. L) �HINT: qrxn = -qsolution Heat of Reaction

�What we know: ◦ Volume Cu. SO 4 solution = 50 m. L, [Cu. SO 4] = 0. 3 M ◦ Volume Na. OH = 50 m. L, [Na. OH] = 0. 6 M ◦ Ti = 21. 4 o. C ◦ Tf = 24. 6 o. C

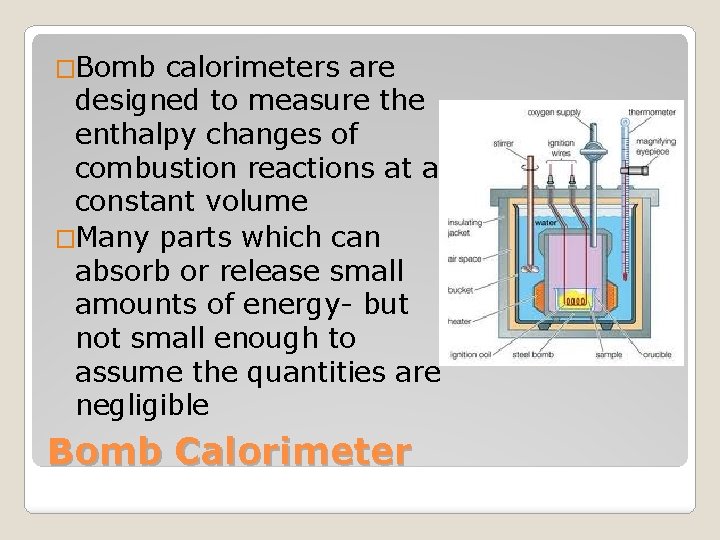
�Bomb calorimeters are designed to measure the enthalpy changes of combustion reactions at a constant volume �Many parts which can absorb or release small amounts of energy- but not small enough to assume the quantities are negligible Bomb Calorimeter

�To get precise measurements we need to know the heat capacity of the bomb calorimeter Ctotal = Cwater + Cthermometer + Cstirrer + Ccontainer �Bomb calorimeters are calibrated using constant masses, so the heat capacities do not include mass units qcal = CΔT Bomb Calorimeter

�A laboratory wants to test the energy content of peanut butter. A 16 g sample is placed in a steel bomb calorimeter (calibrated to C = 8. 28 k. J/o. C). During the experiment the temperature increased by 50. 5 o. C. What is the heat released by the sample? qcal = (8. 28 k. J/o. C)(50. 5 o. C) =418 k. J qcal = -qsample �Therefore the sample released 418 k. J of energy. Example- Bomb Calorimeter
 Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is study of
Thermochemistry is study of Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of
Thermochemistry is the study of Entalpi pengatoman standar
Entalpi pengatoman standar Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of
Thermochemistry is the study of Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry Name all the lines name all the segments name all the rays
Name all the lines name all the segments name all the rays Thermochemistry intro and joule conversions
Thermochemistry intro and joule conversions What is a disturbance that transfers energy
What is a disturbance that transfers energy Thermochemistry
Thermochemistry Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Thermochemical equation
Thermochemical equation Introduction to thermochemistry
Introduction to thermochemistry Zero point energy correction gaussian
Zero point energy correction gaussian Thermochemistry equations
Thermochemistry equations Cartoon thermochemistry
Cartoon thermochemistry Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Thermochemistry equations
Thermochemistry equations Symbolic border one pager
Symbolic border one pager Thermochemical equation examples
Thermochemical equation examples Kirchhoff's law of thermochemistry
Kirchhoff's law of thermochemistry Chapter 17 thermochemistry
Chapter 17 thermochemistry Thermochemistry equations
Thermochemistry equations Thermochemistry cartoon
Thermochemistry cartoon Thermodynamics ap chemistry
Thermodynamics ap chemistry Unit 7 ap chemistry
Unit 7 ap chemistry General chemistry thermochemistry
General chemistry thermochemistry Chapter 5 science form 3
Chapter 5 science form 3 Chapter 17 thermochemistry answer key
Chapter 17 thermochemistry answer key Energy changes
Energy changes What is thermochemistry
What is thermochemistry Quiz 2: heat flow and technology
Quiz 2: heat flow and technology Thermochemistry concepts
Thermochemistry concepts Ap chemistry unit 9
Ap chemistry unit 9 Thermochemistry is concerned with the
Thermochemistry is concerned with the Thermochemistry
Thermochemistry Thermochemistry review
Thermochemistry review Bomb calorimetry equation
Bomb calorimetry equation Thermochemistry video
Thermochemistry video