Thermochemistry Unit 17 Chapter 17 Fun fact Thermochemistry







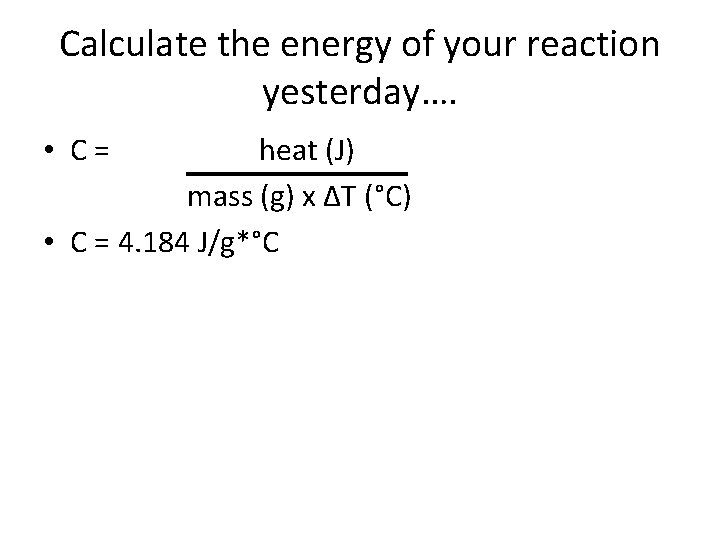











- Slides: 27

Thermochemistry Unit 17 Chapter 17

Fun fact: • Thermochemistry is technically physics, and not chemistry…

First, some terminology • “The system” in chemistry is defined as the chemicals involved with reaction or heat change • “The surroundings” or “the environment” is where the heat goes or comes from – Ex) In a propane stove the system is the flame, and the air around it is the surroundings

• https: //www. youtube. com/watch? v=Gqt. UWy DR 1 fg&list=PL 1 R 4 f. AABv. X_P_QWo. VK 3 g 02 Mev z. Msy. Ri. Tt

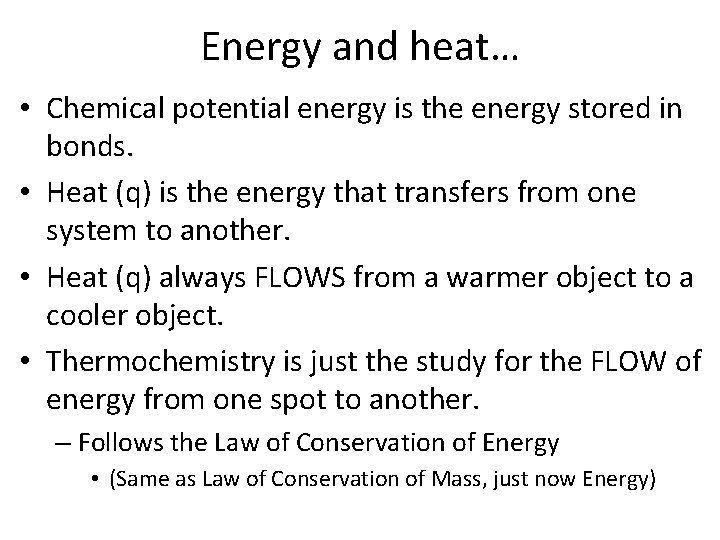
Energy and heat… • Chemical potential energy is the energy stored in bonds. • Heat (q) is the energy that transfers from one system to another. • Heat (q) always FLOWS from a warmer object to a cooler object. • Thermochemistry is just the study for the FLOW of energy from one spot to another. – Follows the Law of Conservation of Energy • (Same as Law of Conservation of Mass, just now Energy)

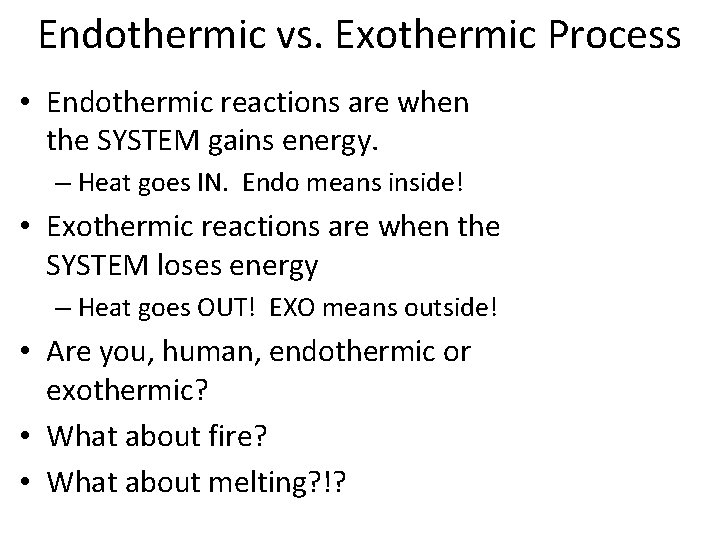
Endothermic vs. Exothermic Process • Endothermic reactions are when the SYSTEM gains energy. – Heat goes IN. Endo means inside! • Exothermic reactions are when the SYSTEM loses energy – Heat goes OUT! EXO means outside! • Are you, human, endothermic or exothermic? • What about fire? • What about melting? !?

Two units to measure energy • A joule is the SI measure that is the measure of actual potential and kinetic energy – It’s actually a very small amount of energy, and its unit is J • A calorie is the non-SI measure that is the amount of energy needed to increase the temperature of 1. 00 g of water exactly 1. 00°C. – It’s still a very small number (1. 00 cal) so we use Cal (or 1000 cal. or 1 kcal) more. • 1 cal = 4. 184 J

Who cares? • Well, this is how we can actually measure the heat gained or lost by a system! – The more the temperature goes up, the more heat it released. (Or, the more the temperature goes down, the more heat it gained…) • This amount of energy it takes for the substance to increase energy is called SPECIFIC HEAT CAPACITY (C). C= heat (J) mass (g) x ΔT (°C)

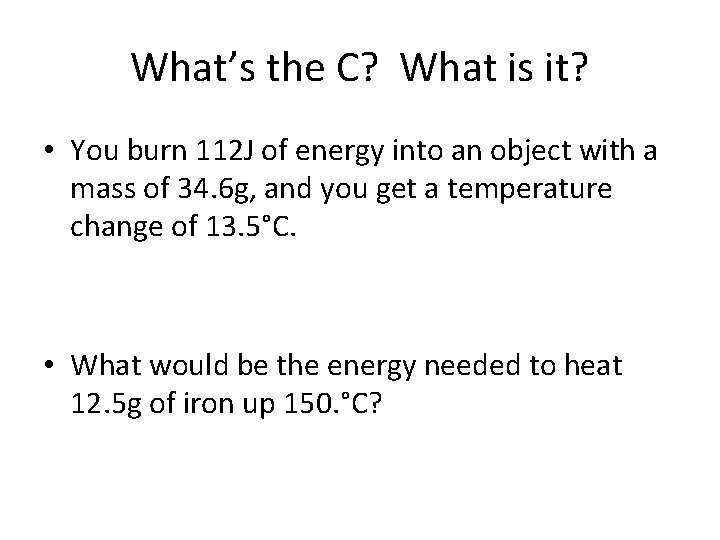
What’s the C? What is it? • You burn 112 J of energy into an object with a mass of 34. 6 g, and you get a temperature change of 13. 5°C. • What would be the energy needed to heat 12. 5 g of iron up 150. °C?

So? • That means if we know energy, and mass, we can know how much the temperature goes up. • Or if we know how much mass, and how much temperature goes up, we can calculate energy!
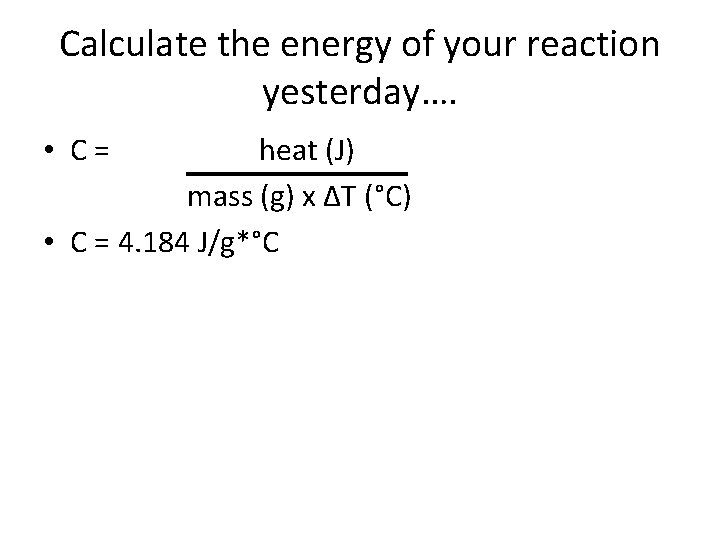
Calculate the energy of your reaction yesterday…. • C= heat (J) mass (g) x ΔT (°C) • C = 4. 184 J/g*°C

• https: //www. youtube. com/watch? v=Ju. Wt. BRr. DQk • Or • https: //www. youtube. com/watch? v=EAgbkn. I DKNo

Calorimetry • Because the system (in this case the chemicals) releases (or gains) the energy into (or from) the system, we can measure the reaction by measuring the environment – A Calorimeter is an environment where the system can be measured by measuring the change in temperature of the environment • Ideally loses no energy to the environment outside • This allows us to measure the enthalpy (H) of the system, which is the heat content of a system at constant pressure. (Still, no work done. ) – q = ΔH (so, change in enthalpy is equal to heat)

The one equation to rule them all… • Is actually just a rearranged version of the one we did in 17. 1 • qsys = ΔH = -qsurr = - m x C x ΔT • If the system loses heat, the surrounding gains the opposite amount of heat. – Hence the negative sign…

There actually TWO types of calorimeters… • Constant pressure (reaction takes place in water, and we measure the water). – Like we did in class! • Constant volume (reaction occurs in gas) – The gas then reacts, and then heats the water around it. – Used to be called a “bomb calorimeter”, which sounds super cool, until you realize it’s not. • No work is done, so all the energy is moved as temperature changes (heat)!

A new type of balanced chemical equation results… • A thermochemical equation! – Has all the information of a balanced equation, but also shows the amount of energy that is released, or that changes. • The “Heat of reaction” – Are assumed to be at 1 atm and 25°C, and 1 mol. • Exothermic reactions will have negative enthalpy values, while endothermic will be positive. – The state will be important, because different states actually produce different energy

Heat of Combustion is the same as heat of reaction… • It is just for a combustion reaction ( + O 2) • Basically always negative, and is measured for 1 mole of the chemical that combines with O 2 at 25°C at 1 atm. • Other than that, it’s the same.

So, let’s burn some ethanol… • If I burn 25 g of ethanol, how much heat is lost by the system? • How much would that increase the temperature of a liter of water?

Heat of Change in State • In addition to taking energy to increase temperature, it also takes (or releases) energy to change states. • It takes energy to change to the more disorganized state. (Solid is the most organized, gas is the least organized. ) • The heat to melt is called the molar heat of fusion. (in k. J/mol. ) • The heat to freeze is called the molar heat of solidification – It is always the opposite of the heat of fusion

The same rules apply for vaporization and condensation. • Vaporization takes energy from the surroundings, condensation gives off energy, and they are always equally opposite.

Why? • By turning a chemical from a solid to a liquid (or a liquid to a gas) you are breaking attractions, and that takes energy away from the system to do it. • Have you ever noticed how the snow stays on the grass much longer than it stays on the pavement? – The grass doesn’t have as much heat transfer as the pavement.

Alright, fine, some problems! • How much energy would it take to melt 360. 5 g of water? – Heat of fusion is on page 522 • How much energy would be released from condensing 25. 2 kg of ethanol?

There is also energy in forming solutions. • Attractions are broken, and new attractions are made, so energy is released or absorbed by the system. • This is called the “Heat of Solution”, and can be positive or negative. – Just 36. 5 g (less than 1 oz. ) of pure HCl put in water can release 75, 000 J of energy. Which is why you always SLOWLY pour acids into water. • Let’s see how much energy is produced by putting solid Na. OH into water…

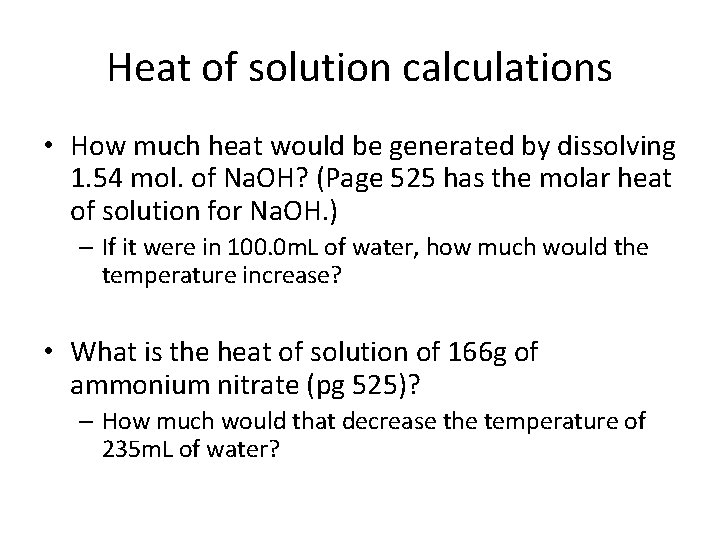
Heat of solution calculations • How much heat would be generated by dissolving 1. 54 mol. of Na. OH? (Page 525 has the molar heat of solution for Na. OH. ) – If it were in 100. 0 m. L of water, how much would the temperature increase? • What is the heat of solution of 166 g of ammonium nitrate (pg 525)? – How much would that decrease the temperature of 235 m. L of water?

Hess’s Law of Heat Summation • Why is enthalpy H? WHY? Seriously, man, what the heck? • Answer: Hess! • Hess said that it didn’t matter how the reaction occurred, all that mattered was the starting and ending states. • So: Heat of formation ΔHf 0 = ΔHf 0 (products) - ΔHf 0 (reactants)

Pure elements, yo… • Have a ΔHf 0 = 0. 0 k. J/mol in their room temperature state. • Compounds will have a ΔHf 0 that is not zero. • Basically, if a pure element in it’s periodic table state, it’s going to be zero, everything else is a number • Same 2 equations apply, yo.

Some reactions… • John has unwisely decided to make 10, 000. g of water. How much energy will be released? • Thermite is 760. 0 g aluminum powder plus iron oxide in a single replacement reaction. How much energy is produced?