Thermochemistry Heat and Chemical Change Agenda Intro Video










































- Slides: 58

Thermochemistry Heat and Chemical Change

Agenda Intro Video Discussions u Notes over Exothermic and Endothermic reactions u Review of Energy Stoichiometry u Notes over Heat Capacity and Specific Heat u

Intro Videos u http: //ed. ted. com/lessons/what-triggersa-chemical-reaction-kareemjarrah#digdeeper

What Triggers a Chemical Reaction Video Questions u Everything you see around you is made of chemicals: the air that you breathe, the water that you drink, and the chair you are sitting on. Entropy is a characteristic of all chemicals, and more ordered chemicals have lower entropy. List two chemicals that you think have low entropy and two that have high entropy. Explain the reason for your choices.

Intro Videos u http: //ed. ted. com/lessons/how-do-coldpacks-get-cold-so-fast-john-pollard

Ice Pack Questions u The breaking of bonds on the molecular level always requires an input of energy. Therefore forming bonds always releases energy. Based on this, build an explanation for why a reaction would release heat (exothermic) and why a reaction would consume heat (endothermic).

Ice Pack Questions u Imagine you were going to design a heat pack that worked in a similar fashion as the cold pack described in the video. Describe how the solid dissolution would occur and how the device would work as a heat pack instead of a cold pack.

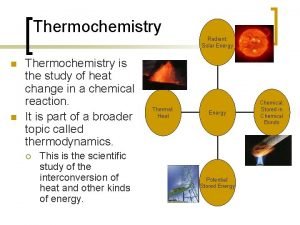
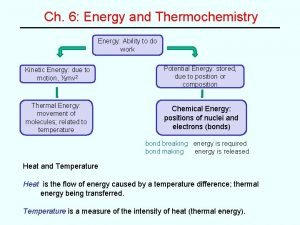
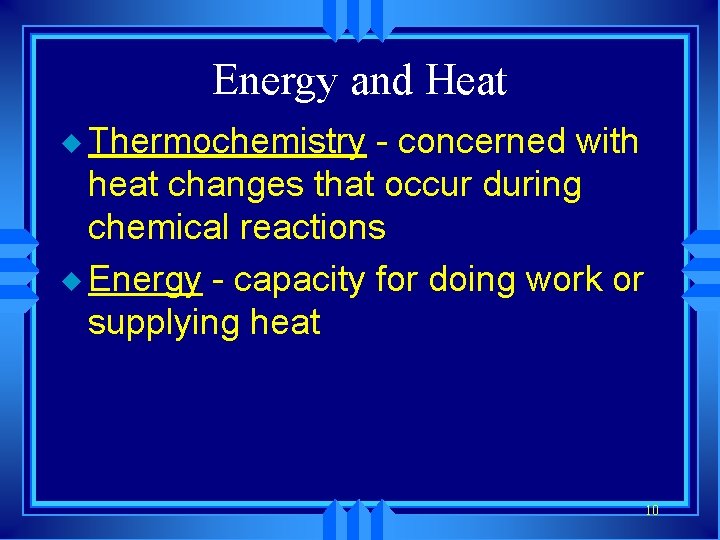
Energy and Heat u Thermochemistry - concerned with heat changes that occur during chemical reactions u Energy - capacity for doing work or supplying heat 10

Energy and Heat u Heat - represented by “q”, is energy that transfers from one object to another, because of a temperature difference between them. 11

Exothermic and Endothermic Processes u In studying heat changes, think of defining these two parts: • the system • the surroundings

Exothermic and Endothermic Processes u The Law of Conservation of Energy states that in any chemical or physical process, energy is neither created nor destroyed. • All the energy is accounted for as work, stored energy, or heat.

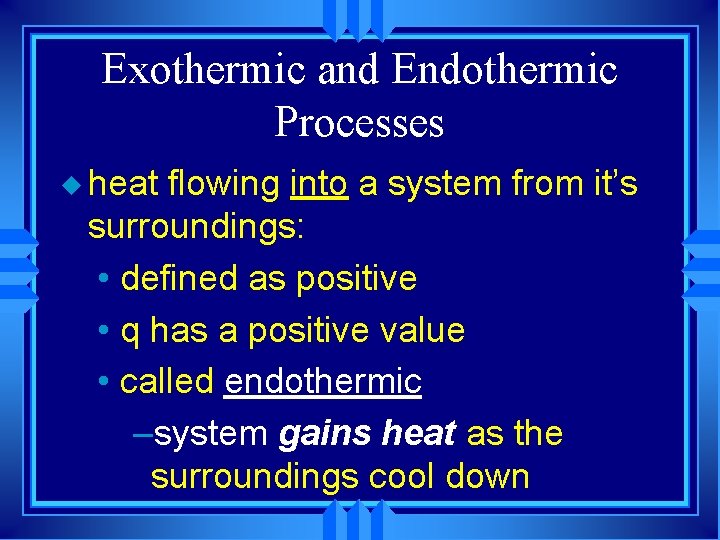
Exothermic and Endothermic Processes u heat flowing into a system from it’s surroundings: • defined as positive • q has a positive value • called endothermic –system gains heat as the surroundings cool down

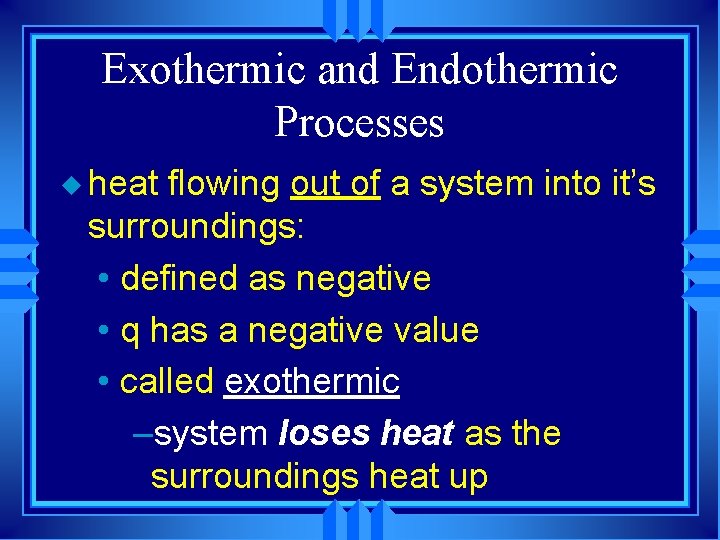
Exothermic and Endothermic Processes u heat flowing out of a system into it’s surroundings: • defined as negative • q has a negative value • called exothermic –system loses heat as the surroundings heat up

Exothemic and Endothermic u Every reaction has an energy change associated with it u Exothermic reactions release energy, usually in the form of heat. u Endothermic reactions absorb energy u Energy is stored in bonds between atoms 16

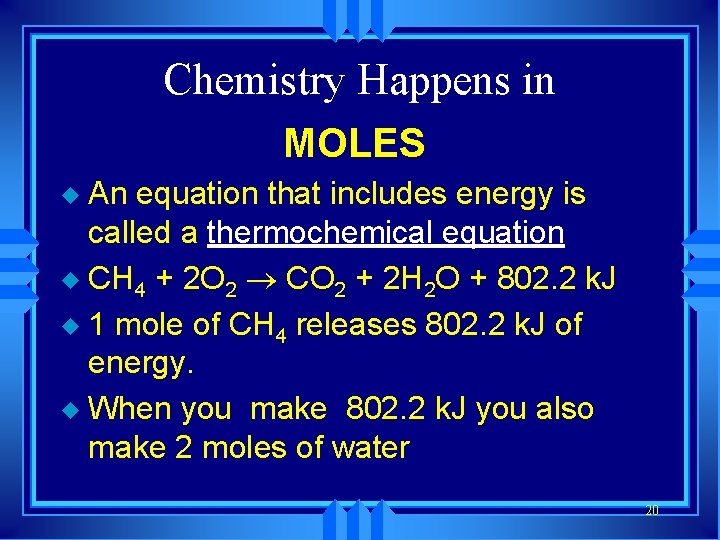
Chemistry Happens in MOLES An equation that includes energy is called a thermochemical equation u CH 4 + 2 O 2 ® CO 2 + 2 H 2 O + 802. 2 k. J u 1 mole of CH 4 releases 802. 2 k. J of energy. u When you make 802. 2 k. J you also make 2 moles of water u 20

Thermochemical Equations u A heat of reaction is the heat change for the equation, exactly as written • The physical state of reactants and products must also be given. • Standard conditions for the reaction is 101. 3 k. Pa (1 atm. ) and 25 o. C 21

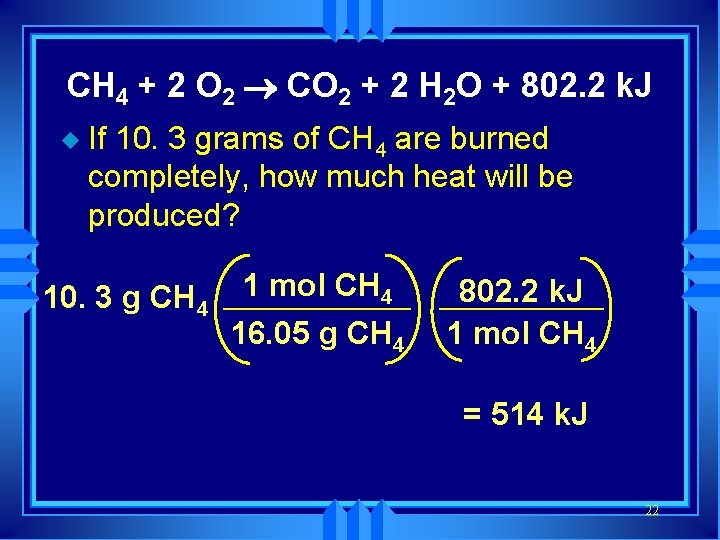
CH 4 + 2 O 2 ® CO 2 + 2 H 2 O + 802. 2 k. J u If 10. 3 grams of CH 4 are burned completely, how much heat will be produced? 10. 3 g CH 4 1 mol CH 4 16. 05 g CH 4 802. 2 k. J 1 mol CH 4 = 514 k. J 22

Heat Capacity and Specific Heat u A calorie is defined as the quantity of heat needed to raise the temperature of 1 g of pure water 1 o. C. • Used except when referring to food • a Calorie, written with a capital C, always refers to the energy in food • 1 Calorie = 1 kilocalorie = 1000 cal. 24

Heat Capacity and Specific Heat u Joule-- the SI unit of heat and energy • 4. 184 J = 1 cal u Specific Heat Capacity - the amount of heat it takes to raise the temperature of 1 gram of the substance by 1 o. C (abbreviated “C”) 25

Heat Capacity and Specific Heat u For water, C = 4. 18 J/(g o. C), and also C = 1. 00 cal/(g o. C) u Thus, for water: • it takes a long time to heat up, and • it takes a long time to cool off! u Water is used as a coolant! • Note Figure 11. 7, page 297 26

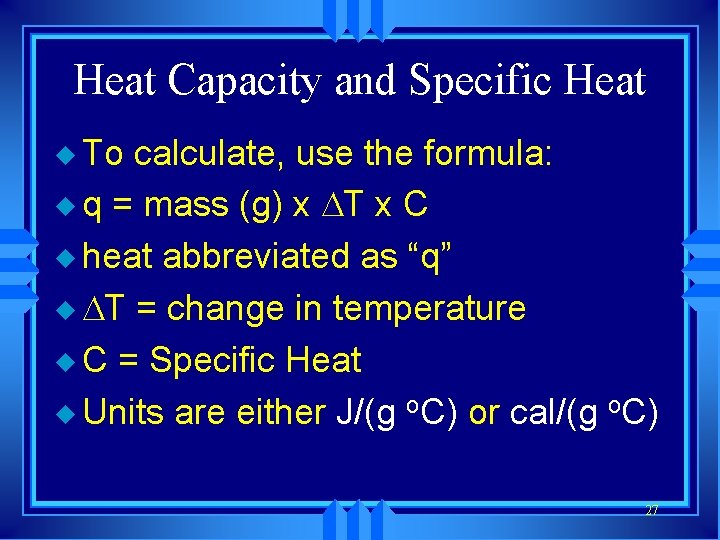
Heat Capacity and Specific Heat u To calculate, use the formula: u q = mass (g) x T x C u heat abbreviated as “q” u T = change in temperature u C = Specific Heat u Units are either J/(g o. C) or cal/(g o. C) 27

Sample Problem # 1 u u The temperature of a a piece of copper with a mass of 95. 4 g increases from 25 °C to 48 °C when the metal absorbs 849 J of heat. What is the specific heat of copper? Solution: Knowns: • Mass= 95. 4 g • Δt =48 -25=23 °C • Q= 849 J Equation: C= q/m* Δt • Plug in numbers: 849/(95. 4*23) = 0. 387 J/g* °C

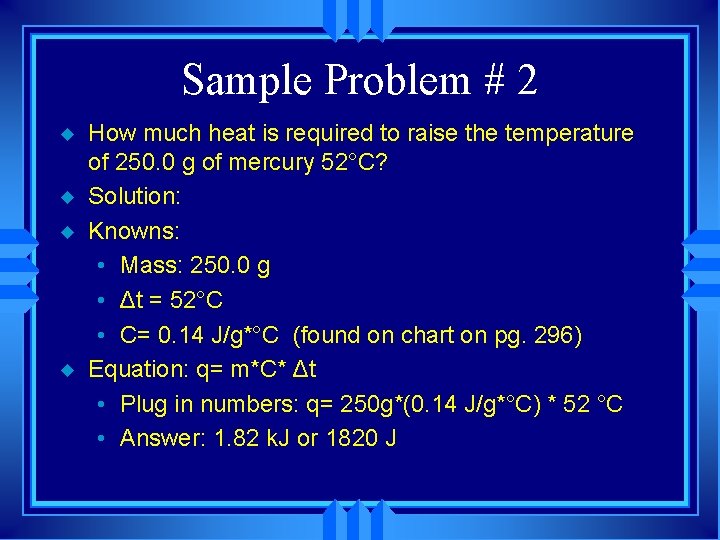
Sample Problem # 2 u u How much heat is required to raise the temperature of 250. 0 g of mercury 52°C? Solution: Knowns: • Mass: 250. 0 g • Δt = 52°C • C= 0. 14 J/g*°C (found on chart on pg. 296) Equation: q= m*C* Δt • Plug in numbers: q= 250 g*(0. 14 J/g*°C) * 52 °C • Answer: 1. 82 k. J or 1820 J

Practice Problem 1 (Yay!!!!) u What is the specific heat capacity of Mercury if 1400. 0 J of heat are used to raise the temp of 500. 0 g of Mercury from 14. 0 degrees to 34. 0 degrees?

Practice Problem 2 (Woo. Hoo) u How much heat energy is used to raise the temp of 56. 0 g of Al from 0. 0 degrees to 95. 0 degrees?

Practice Problem 3 (Awesome) u A 3. 0 kg piece of iron is cooled from 300 o. C to 20 o. C. How much heat energy was lost from the iron?

Homework Finish any and all missing assignments u Do specific heat practice u

Warm-Up Problem #1 14 KMn. O 4 + 4 C 3 H 5(OH)3 ------> 7 K 2 CO 3 + 7 Mn 2 O 3 +5 CO 2 + 16 H 2 O u ΔH = -566 k. J u If I used 24. 0 g of Potassium Permangante with an excess of glycerin, then how much heat was released from this reaction? u

Warm-Up Problem #2 Ba(OH)2. 8 H 2 O + 2 NH 4 SCN -----> Ba(SCN)2 + 2 NH 3 + 10 H 2 O u ΔH = 330 k. J u If I used 50. 0 g of Ammonium Thiocyanate in excess Barium Hydroxide how much heat would the reaction absorb? u

Warm-Up Problem # 3 u Calculate the specific heat capacity of copper given that 204. 75 J of energy raises the temperature of 15 g of copper from 25 o to 60 o.

Warm-Up Problem # 4 u How much heat energy is required to raise the temperature of 55. 0 g of water from 25. 0°C to 28. 6°C? The specific heat of water is 4. 18 J/g°C.

Section 11. 2 Measuring and Expressing Heat Changes u OBJECTIVES: • Construct equations that show the heat changes for chemical and physical processes. 40

Section 11. 2 Measuring and Expressing Heat Changes u OBJECTIVES: • Calculate heat changes in chemical and physical processes. 41

Calorimetry u Calorimetry - the accurate and precise measurement of heat change for chemical and physical processes.

Calorimetry u For systems at constant pressure, the heat content is the same as a property called Enthalpy (H) of the system

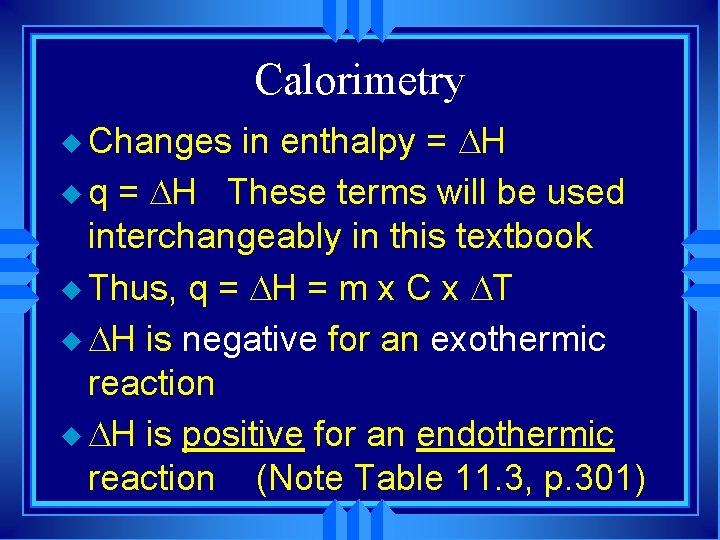
Calorimetry u Changes in enthalpy = H u q = H These terms will be used interchangeably in this textbook u Thus, q = H = m x C x T u H is negative for an exothermic reaction u H is positive for an endothermic reaction (Note Table 11. 3, p. 301)

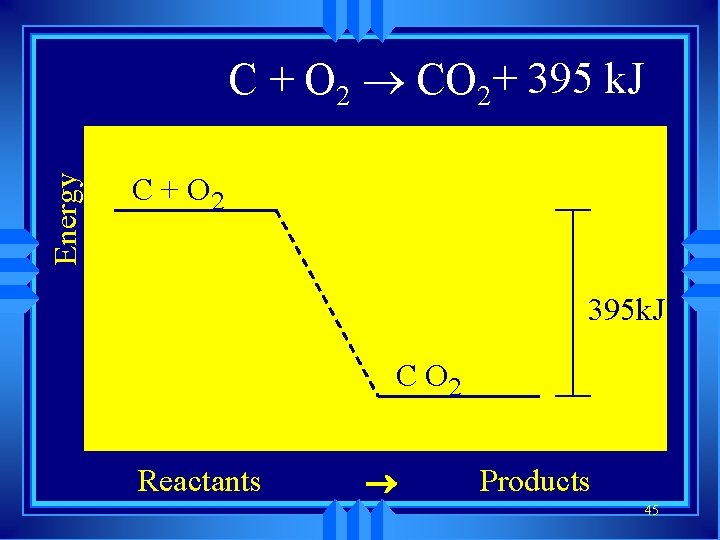
Energy C + O 2 ® CO 2+ 395 k. J C + O 2 395 k. J C O 2 Reactants ® Products 45

In terms of bonds C O O Breaking this bond will require energy. O C O O Making these bonds gives you energy. In this case making the bonds gives you more energy than breaking them. 46

Exothermic u The products are lower in energy than the reactants u Releases energy 47

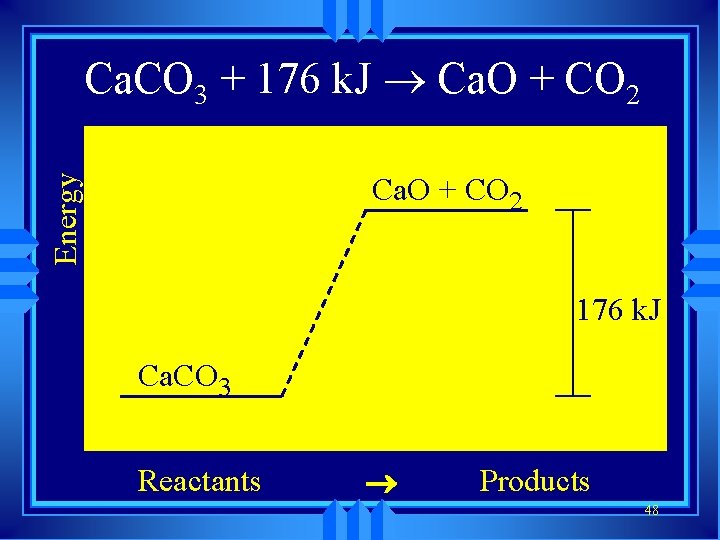
Ca. CO ® Ca. O Ca. CO Ca. O + CO+2 CO 2 3 + 176 3 ®k. J Energy Ca. O + CO 2 176 k. J Ca. CO 3 Reactants ® Products 48

Summary, so far. . .

Enthalpy u The heat content a substance has at a given temperature and pressure u Can’t be measured directly because there is no set starting point u The reactants start with a heat content u The products end up with a heat content u So we can measure how much enthalpy changes 50

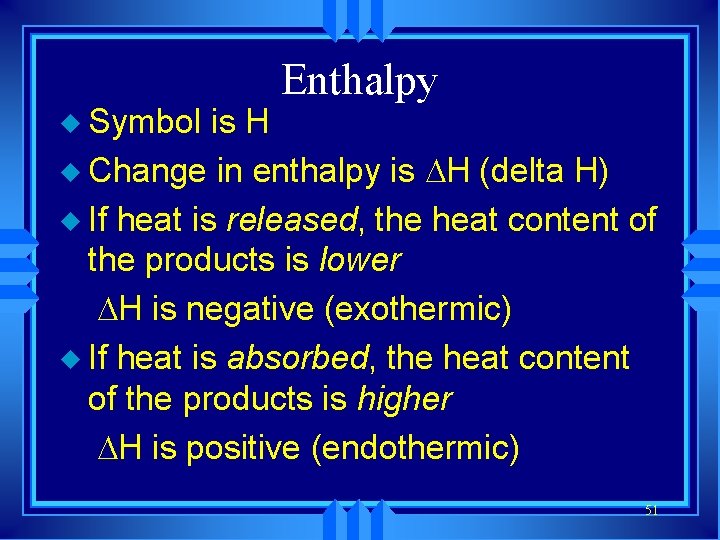
u Symbol is H Enthalpy u Change in enthalpy is H (delta H) u If heat is released, the heat content of the products is lower H is negative (exothermic) u If heat is absorbed, the heat content of the products is higher H is positive (endothermic) 51

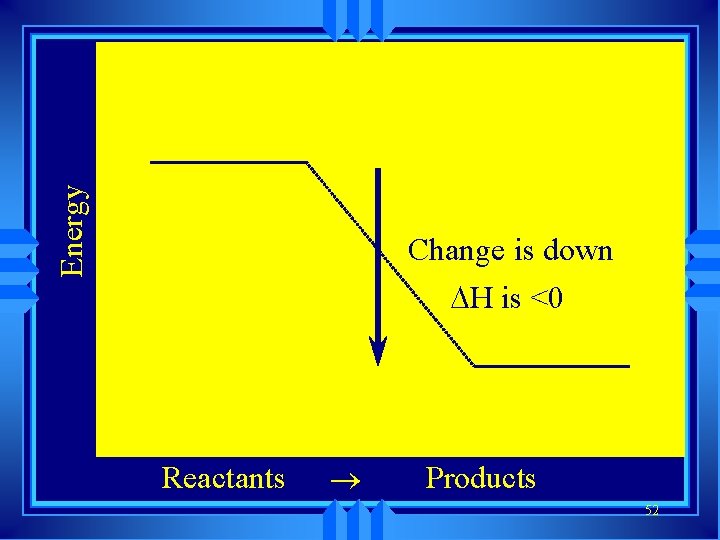
Energy Change is down H is <0 Reactants ® Products 52

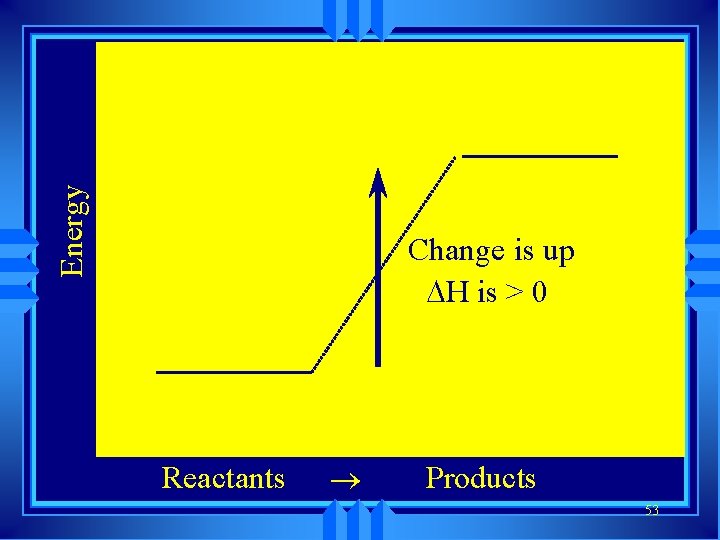
Energy Change is up H is > 0 Reactants ® Products 53

More Fun with Thermo Practice Problem 5 u 25. 0 m. L of water containing 0. 025 moles HCl is added to 25. 0 m. L of water containing 0. 025 mols Na. OH in a foam cup calorimeter. At the start, the solutions and the calorimeter are all at 25. 0 o. C. During the reaction, the highest temperature observed is 32. 0 o. C. Calculate the heat (in k. J) released during this reaction. Assume the densities of the solutions are 1. 00 g/m. L.

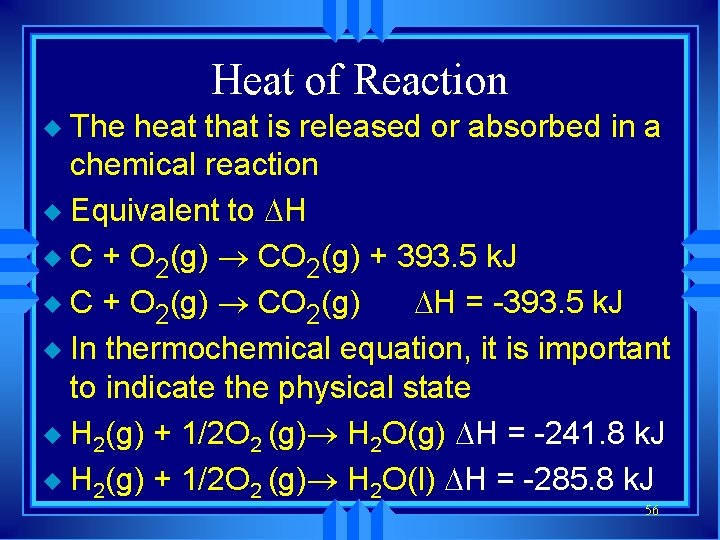
Heat of Reaction The heat that is released or absorbed in a chemical reaction u Equivalent to H u C + O 2(g) ® CO 2(g) + 393. 5 k. J u C + O 2(g) ® CO 2(g) H = -393. 5 k. J u In thermochemical equation, it is important to indicate the physical state u H 2(g) + 1/2 O 2 (g)® H 2 O(g) H = -241. 8 k. J u H 2(g) + 1/2 O 2 (g)® H 2 O(l) H = -285. 8 k. J u 56

Heat of Combustion u The heat from the reaction that completely burns 1 mole of a substance u Note Table 11. 4, page 305 57

Heats of Fusion and Solidification u Molar Heat of Fusion ( Hfus) - the heat absorbed by one mole of a substance in melting from a solid to a liquid u Molar Heat of Solidification ( Hsolid) - heat lost when one mole of liquid solidifies 60

Heats of Vaporization and Condensation u Molar Heat of Vaporization ( Hvap) - the amount of heat necessary to vaporize one mole of a given liquid. 61

Heats of Vaporization and Condensation u Molar Heat of Condensation ( Hcond) - amount of heat released when one mole of vapor condenses u Hvap = - Hcond 62

Heats of Vaporization and Condensation u The large values for Hvap and Hcond are the reason hot vapors such as steam is very dangerous • You can receive a scalding burn from steam when the heat of condensation is released! 63

Heat of Solution u Heat changes can also occur when a solute dissolves in a solvent. u Molar Heat of Solution ( Hsoln) - heat change caused by dissolution of one mole of substance 65

Hess’s Law u If you add two or more thermochemical equations to give a final equation, then you can also add the heats of reaction to give the final heat of reaction. Called Hess’s law of heat summation u Example shown on page 314 for graphite and diamonds 68

Why Does It Work? If you turn an equation around, you change the sign: u If H 2(g) + 1/2 O 2(g)® H 2 O(g) H=-285. 5 k. J u then, H 2 O(g) ® H 2(g) + 1/2 O 2(g) H =+285. 5 k. J u also, u If you multiply the equation by a number, you multiply the heat by that number: u 2 H 2 O(g) ® 2 H 2(g) + O 2(g) H =+571. 0 k. J u 69

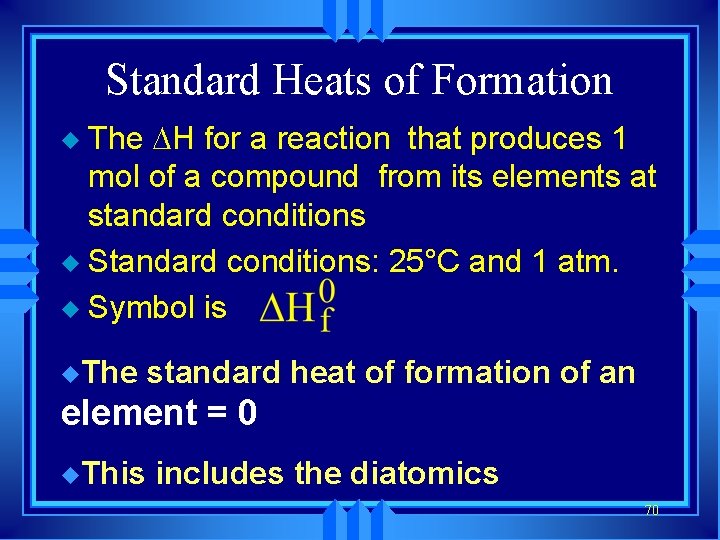
Standard Heats of Formation The H for a reaction that produces 1 mol of a compound from its elements at standard conditions u Standard conditions: 25°C and 1 atm. u Symbol is u u. The standard heat of formation of an element = 0 u. This includes the diatomics 70

What good are they? Table 11. 6, page 316 has standard heats of formation u The heat of a reaction can be calculated by: • subtracting the heats of formation of the reactants from the products u Ho = ( Products) - ( Reactants) 71

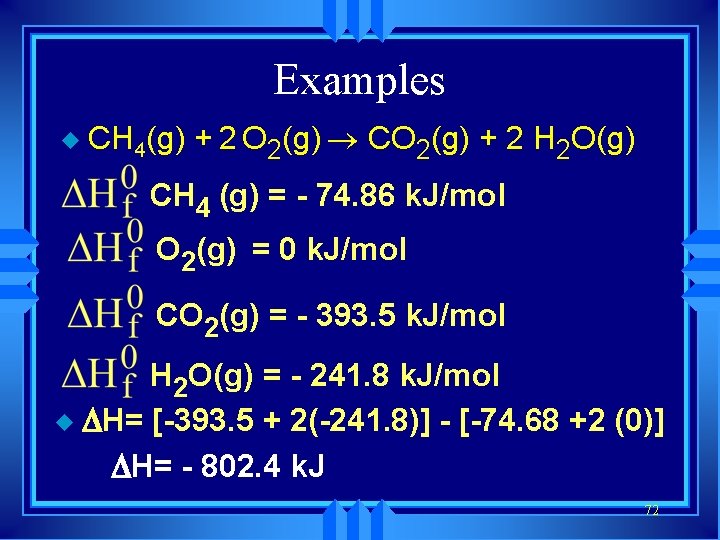
Examples u CH 4(g) + 2 O 2(g) ® CO 2(g) + 2 H 2 O(g) CH 4 (g) = - 74. 86 k. J/mol O 2(g) = 0 k. J/mol CO 2(g) = - 393. 5 k. J/mol H 2 O(g) = - 241. 8 k. J/mol u DH= [-393. 5 + 2(-241. 8)] - [-74. 68 +2 (0)] DH= - 802. 4 k. J 72
 Thermochemistry
Thermochemistry Intro to thermochemistry
Intro to thermochemistry Thermochemistry video
Thermochemistry video Physical changes examples
Physical changes examples Whats the difference between a chemical and physical change
Whats the difference between a chemical and physical change What is example of physical change
What is example of physical change Whats the difference between physical and chemical change
Whats the difference between physical and chemical change What is a physical change
What is a physical change Spare change physical versus chemical change
Spare change physical versus chemical change Physical change
Physical change Baking is a chemical change
Baking is a chemical change Chopping wood chemical or physical change
Chopping wood chemical or physical change Agenda sistemica y agenda institucional
Agenda sistemica y agenda institucional Intro to change management
Intro to change management Video yandex ru
Video yandex ru Gravity yahoo
Gravity yahoo Httptw
Httptw The frame size of a video refers to the video’s
The frame size of a video refers to the video’s Define specific latent heat
Define specific latent heat Knowledge and skills framework band 6
Knowledge and skills framework band 6 Agenda for change ksf
Agenda for change ksf Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test 7-3 practice problems chemistry answers
7-3 practice problems chemistry answers Chapter 7 heat transfer and change of phase
Chapter 7 heat transfer and change of phase Meta means change and morph means heat
Meta means change and morph means heat Physical and chemical change grade 10
Physical and chemical change grade 10 Whats the difference between chemical and physical change
Whats the difference between chemical and physical change Mixing red and green marbles physical or chemical change
Mixing red and green marbles physical or chemical change Chapter 15 energy and chemical change
Chapter 15 energy and chemical change Differentiate between physical and chemical change
Differentiate between physical and chemical change Styrofoam and acetone chemical or physical change
Styrofoam and acetone chemical or physical change Class 7 chemical and physical change
Class 7 chemical and physical change Specific heat capacity
Specific heat capacity Dry heat method
Dry heat method Absolute change and relative change formula
Absolute change and relative change formula Change in supply and change in quantity supplied
Change in supply and change in quantity supplied Rocks change due to temperature and pressure change
Rocks change due to temperature and pressure change First order change examples
First order change examples Thermochemistry
Thermochemistry Chapter 17 thermochemistry practice problems answers
Chapter 17 thermochemistry practice problems answers Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is the study of: *
Thermochemistry is the study of: * Balanced thermochemical equation
Balanced thermochemical equation Thermochemistry
Thermochemistry Gaussian thermochemistry
Gaussian thermochemistry Thermochemistry equations
Thermochemistry equations Cartoon thermochemistry
Cartoon thermochemistry Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Thermochemistry is study of
Thermochemistry is study of Thermochemistry equations
Thermochemistry equations Thermochemistry one pager
Thermochemistry one pager Thermochemistry equations
Thermochemistry equations Kirchhoff's law enthalpy example
Kirchhoff's law enthalpy example Chapter 17 thermochemistry
Chapter 17 thermochemistry Thermochemistry equations
Thermochemistry equations Thermochemistry cartoon
Thermochemistry cartoon Ap chemistry thermochemistry
Ap chemistry thermochemistry Ap chemistry unit 7
Ap chemistry unit 7 Thermochemistry is study of
Thermochemistry is study of