Chapter 7 Chemical Formulas and Chemical Compounds Taken























- Slides: 51

Chapter 7 – Chemical Formulas and Chemical Compounds Taken from Modern Chemistry written by Davis, Metcalfe, Williams & Castka

Section 7. 1 – Chemical Names and Formulas HW – Notes on section 7. 1 pgs 203 -215 Objectives Students will be able to : • Explain the significance of a chemical formula • Determine the formula of an ionic compound formed between two given ions • Name an ionic compound given its formula • Using prefixes, name a binary molecular compound from its formula. • Write the formula of a binary molecular compound given its name.

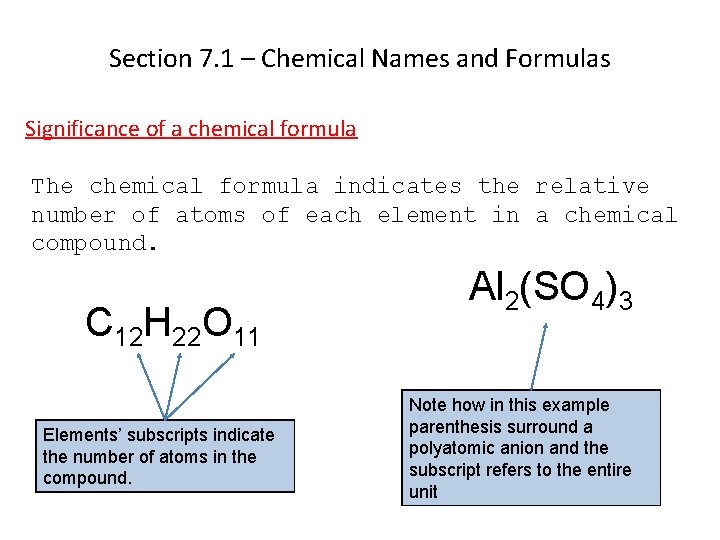
Section 7. 1 – Chemical Names and Formulas Significance of a chemical formula The chemical formula indicates the relative number of atoms of each element in a chemical compound. C 12 H 22 O 11 Elements’ subscripts indicate the number of atoms in the compound. Al 2(SO 4)3 Note how in this example parenthesis surround a polyatomic anion and the subscript refers to the entire unit

Section 7. 1 – Chemical Names and Formulas Monatomic Ions Group 1 metals lose one e- to give 1+ cations. Group 2 metals lose two e- to give 2+ cations. By gaining or losing electrons many main-group elements form ions with stable configurations. Ions formed from a single atom are known as monatomic ions

Section 7. 1 – Chemical Names and Formulas Monatomic Ions (continued) The nonmetals in groups 15, 16 & 17 gain e- to form anions. Not all main-group elements readily form ions, C and Si form covalent bonds where they share electrons.

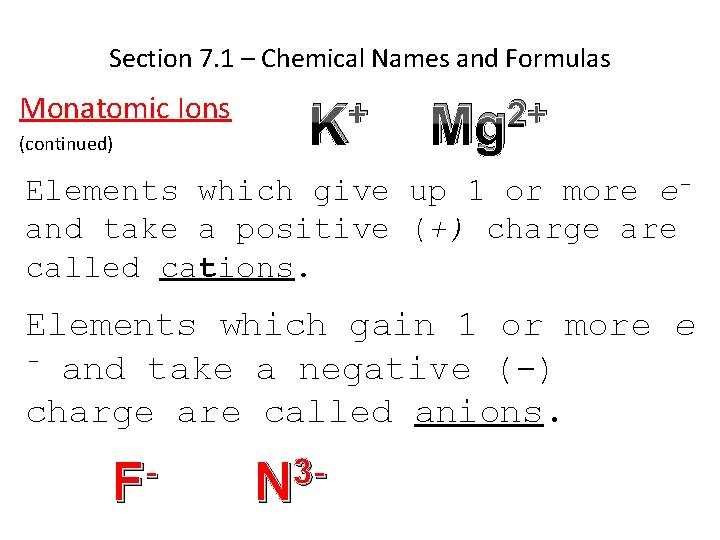
Section 7. 1 – Chemical Names and Formulas Monatomic Ions (continued) + K 2+ Mg Elements which give up 1 or more eand take a positive (+) charge are called cations. Elements which gain 1 or more e - and take a negative (-) charge are called anions. F 3 N

Section 7. 1 – Chemical Names and Formulas Naming Monatomic Ions (continued) Potassium + K cation 2+ Mg Magnesium cation Cation naming is simple, element name and the word cation. For anions you drop the end of the element name and add –ide to the root. 3 N Nitrogen Nitride F Fluorine fluoride

Section 7. 1 – Chemical Names and Formulas Binary Ionic Compounds composed of two different elements are known as binary compounds Cation goes first : Mg 2+, Br-, Br. Balance to become electrically neutral And you get Mg. Br 2

Section 7. 1 – Chemical Names and Formulas Naming Binary Ionic Compounds The naming system involves combining the names of the compound’s positive and negative ions Aluminum cation & oxide And you get Aluminum Oxide Al 2 O 3

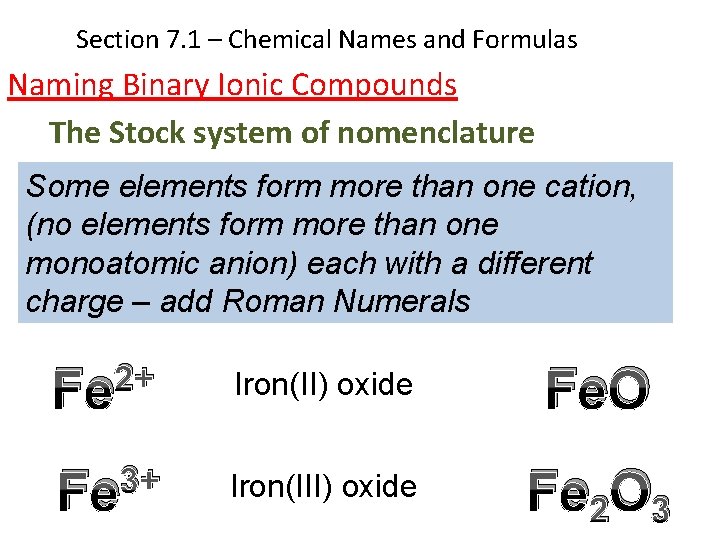
Section 7. 1 – Chemical Names and Formulas Naming Binary Ionic Compounds The Stock system of nomenclature Some elements form more than one cation, (no elements form more than one monoatomic anion) each with a different charge – add Roman Numerals 2+ Fe Iron(II) oxide Fe. O 3+ Fe Iron(III) oxide Fe 2 O 3

Section 7. 1 – Chemical Names and Formulas Naming Binary Ionic Compounds Containing Polyatomic Ions Oxyanions each is a polyatomic ion that contains oxygen. Most Common (-ate) ending One Less O (-ite) ending Nitrate Nitrite NO 3 NO 2 - -

Section 7. 1 – Chemical Names and Formulas Naming Binary Molecular Compounds 1. Less electronegative element given first, prefix only for multiples 2. Second element named with prefix indicating # of atoms, with few exceptions ends with –ide (only 2 elements) 3. The o or a at the end of the prefix is dropped when the word following begins with another vowel. 1 mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca-

Section 7. 1 – Chemical Names and Formulas Naming Binary Molecular Compounds Example pg 212 P 4 O 10 Prefix need if more than one Prefix indicating number Less-electronegative element Root name +ide tetraphosphorus decoxide

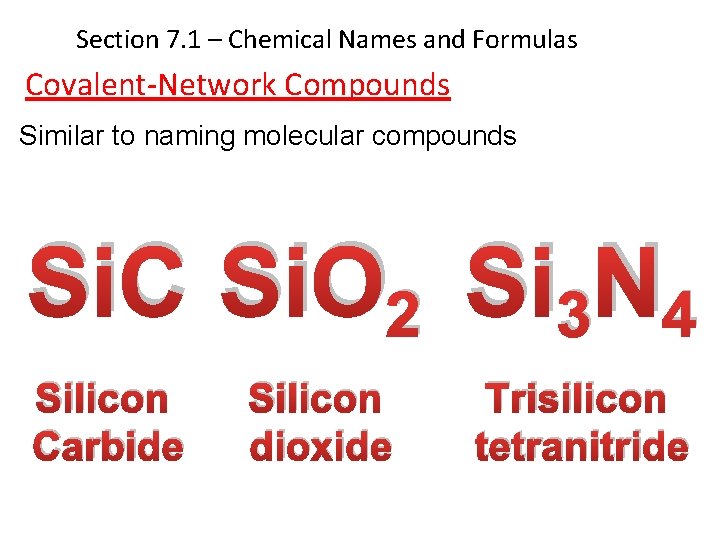
Section 7. 1 – Chemical Names and Formulas Covalent-Network Compounds Similar to naming molecular compounds Si. C Si. O 2 Si 3 N 4 Silicon Carbide Silicon dioxide Trisilicon tetranitride

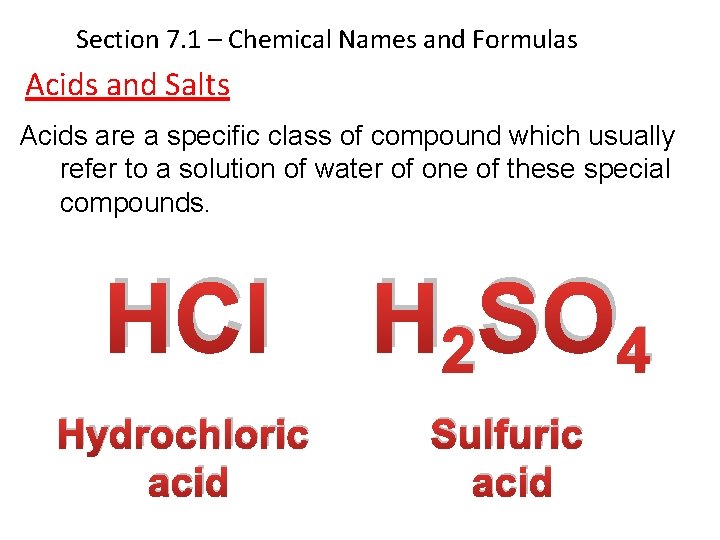
Section 7. 1 – Chemical Names and Formulas Acids and Salts Acids are a specific class of compound which usually refer to a solution of water of one of these special compounds. HCl H 2 SO 4 Hydrochloric acid Sulfuric acid

Section 7. 1 – Chemical Names and Formulas Acids and Salts - (continued) An ionic compound composed of a cation and the anion from an acid is often referred to as a salt. Na. Cl Common Table Salt

Section 7. 1 – Chemical Names and Formulas Practices PRACTICE – pg 207 Q 1 & 2 all Practice Naming PRACTICE – pg 209 Q 1 & 2 all PRACTICE – pg 211 Q 1 & 2 all PRACTICE – pg 213 Q 1 & 2 all SECTION REVIEW – pg 215 Q 2, 3 & 4 all

Section 7. 1 – Chemical Names and Formulas Quiz Break Quiz Key

Section 7. 1 – Chemical Names and Formulas - POGIL Ions How are ions made from neutral atoms? KEY

Section 7. 1 – Chemical Names and Formulas - POGIL Naming Ionic Compounds What are the structural units that make up Ionic compounds and how are they named? KEY

Section 7. 1 – Chemical Names and Formulas - POGIL Polyatomic Ions Can a group of atoms have a charge? KEY

Section 7. 1 – Chemical Names and Formulas - POGIL Naming Molecular Compounds How are the chemical formula and name of a molecular compound related? KEY

Section 7. 1 – V 2 – Ions and Compounds Quiz Break Quiz Key

Section 7. 1 – Chemical Names and Formulas - POGIL Naming Acids KEY

Section 7. 2 - Oxidation Numbers Objectives HW – Notes on section 7. 2 pgs 216 -219 Students will be able to : • List the rules for assigning oxidation numbers. • Give the oxidation number for each element in the formula of a chemical compound. • Name the binary molecular compounds using oxidation numbers and the Stock sytem.

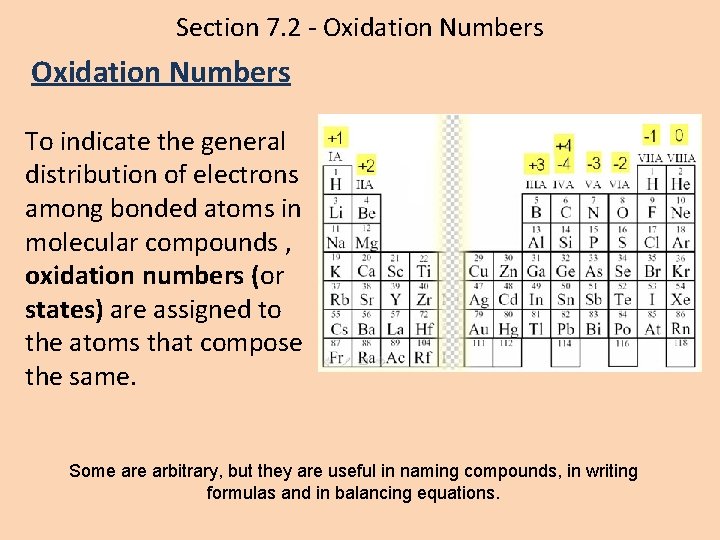
Section 7. 2 - Oxidation Numbers To indicate the general distribution of electrons among bonded atoms in molecular compounds , oxidation numbers (or states) are assigned to the atoms that compose the same. Some arbitrary, but they are useful in naming compounds, in writing formulas and in balancing equations.

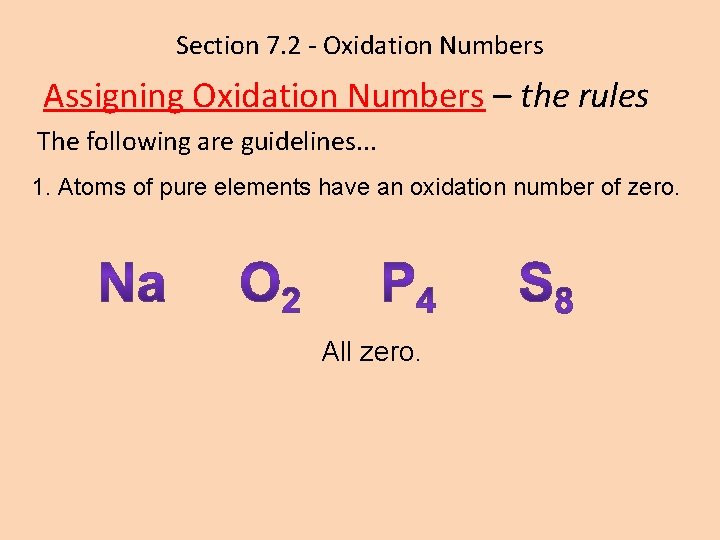
Section 7. 2 - Oxidation Numbers Assigning Oxidation Numbers – the rules The following are guidelines. . . 1. Atoms of pure elements have an oxidation number of zero. All zero.

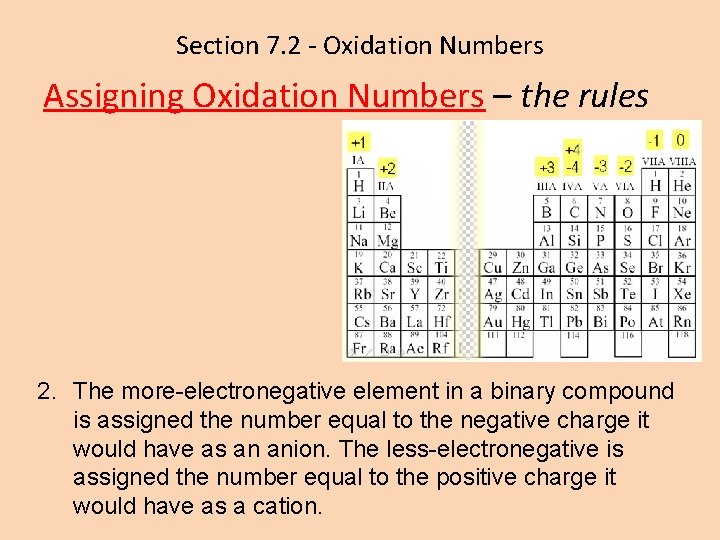
Section 7. 2 - Oxidation Numbers Assigning Oxidation Numbers – the rules 2. The more-electronegative element in a binary compound is assigned the number equal to the negative charge it would have as an anion. The less-electronegative is assigned the number equal to the positive charge it would have as a cation.

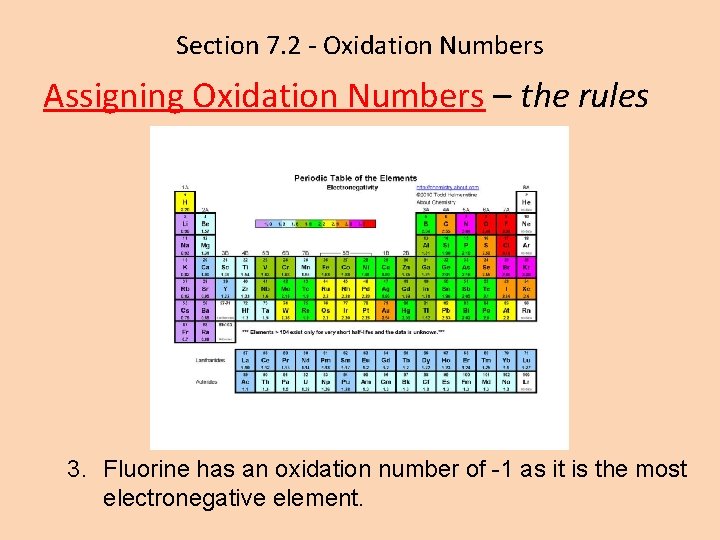
Section 7. 2 - Oxidation Numbers Assigning Oxidation Numbers – the rules 3. Fluorine has an oxidation number of -1 as it is the most electronegative element.

Section 7. 2 - Oxidation Numbers Assigning Oxidation Numbers – the rules 4. Oxygen has an oxidation number of -2 in almost all compounds. Exceptions peroxides is -1, compounds with halogens +2 5. Hydrogen has an oxidation number of +1 in all compounds containing elements that are more- electronegative; it has an oxidation number of -1 in compounds with metals.

Section 7. 2 - Oxidation Numbers Assigning Oxidation Numbers – the rules 6. The algebraic sum of the oxidation numbers of all atoms in a neutral compound = zero. 7. The algebraic sum of the oxidation numbers of all atoms in a polyatomic ion = the charge of the ion. 8. Rules 1 -7 apply to covalently bonded atoms, oxidation numbers can also be assigned to atoms in ionic compounds.

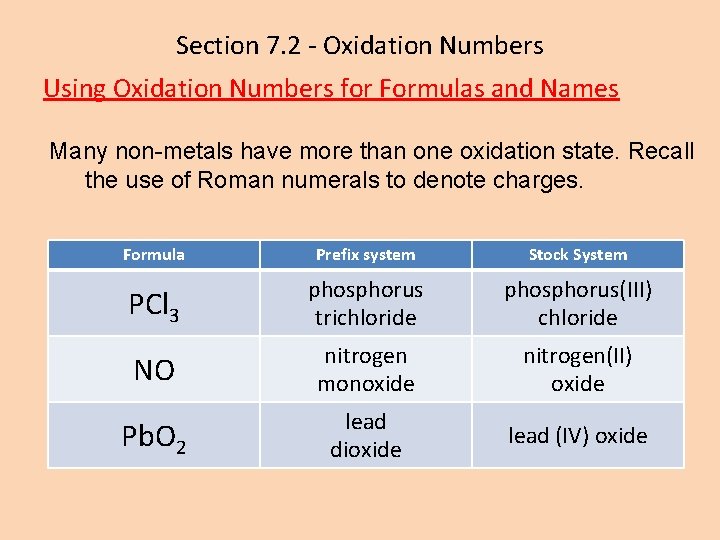
Section 7. 2 - Oxidation Numbers Using Oxidation Numbers for Formulas and Names Many non-metals have more than one oxidation state. Recall the use of Roman numerals to denote charges. Formula Prefix system Stock System PCl 3 phosphorus trichloride phosphorus(III) chloride NO nitrogen monoxide nitrogen(II) oxide Pb. O 2 lead dioxide lead (IV) oxide

Section 7. 2 Practices Ba(NO 3)2 Is the substance elemental? No, three elements are present. Is the substance ionic? Yes, metal + non-metal. Are there any monoatomic ions? Yes, barium ion is monoatomic. Barium ion = Ba 2+ Oxidation # for Ba = +2 Which elements have specific rules? Oxygen has a rule. . -2 in most compounds Oxidation # for O = -2 Which element does not have a specific rule? N does not have a specific rule. Use rule 8 to find the oxidation # of N Let N = Oxidation # for nitrogen (# Ba) (Oxid. # of Ba) + (# N) (Oxid. # N) + (# of O) (Oxid. # of O) = 0 1(+2) + 2(N) + 6(-2) = 0 N = +5

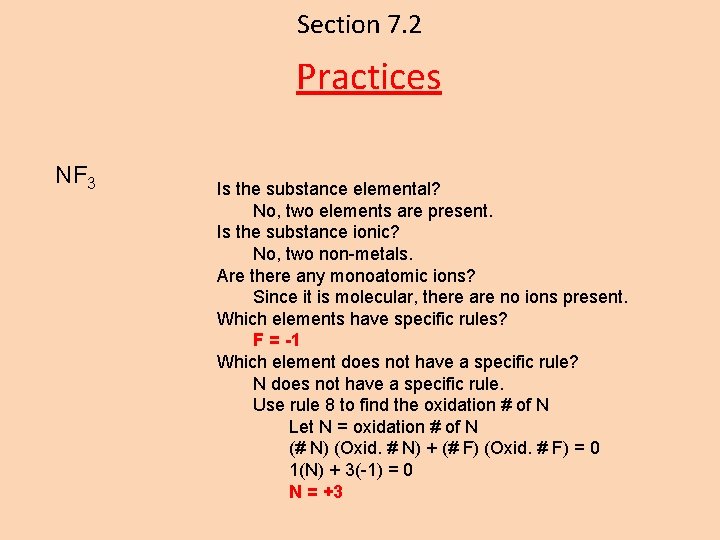
Section 7. 2 Practices NF 3 Is the substance elemental? No, two elements are present. Is the substance ionic? No, two non-metals. Are there any monoatomic ions? Since it is molecular, there are no ions present. Which elements have specific rules? F = -1 Which element does not have a specific rule? N does not have a specific rule. Use rule 8 to find the oxidation # of N Let N = oxidation # of N (# N) (Oxid. # N) + (# F) (Oxid. # F) = 0 1(N) + 3(-1) = 0 N = +3

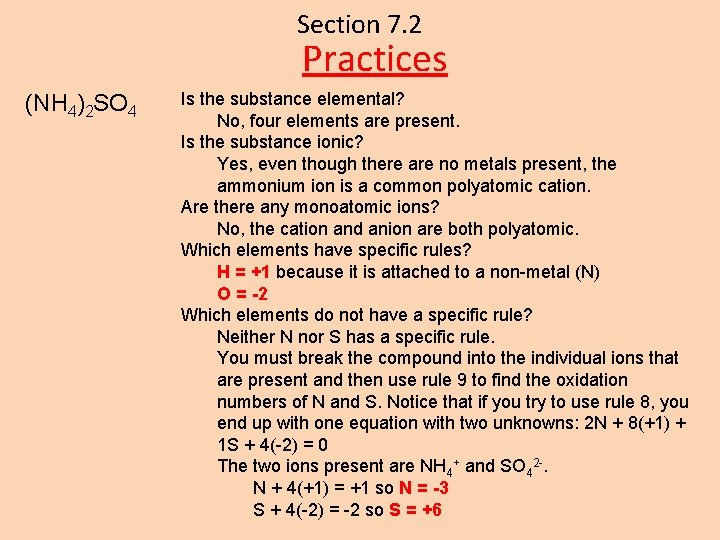
Section 7. 2 Practices (NH 4)2 SO 4 Is the substance elemental? No, four elements are present. Is the substance ionic? Yes, even though there are no metals present, the ammonium ion is a common polyatomic cation. Are there any monoatomic ions? No, the cation and anion are both polyatomic. Which elements have specific rules? H = +1 because it is attached to a non-metal (N) O = -2 Which elements do not have a specific rule? Neither N nor S has a specific rule. You must break the compound into the individual ions that are present and then use rule 9 to find the oxidation numbers of N and S. Notice that if you try to use rule 8, you end up with one equation with two unknowns: 2 N + 8(+1) + 1 S + 4(-2) = 0 The two ions present are NH 4+ and SO 42 -. N + 4(+1) = +1 so N = -3 S + 4(-2) = -2 so S = +6

Section 7. 2 Practices PRACTICE – pg 218 Q 1 all SECTION REVIEW – pg 219 Q 1 & 2 all Practice Sheet Key

Section 7. 2 Quiz Key

Section 7. 3 - Using Chemical Formulas Objectives HW – Notes on section 7. 3 pgs 221 -228 Students will be able to : • Calculate the formula mass or molar mass of any given compound. • Use molar mass to convert between mass in grams and amount in moles of a chemical compound. • Calculate the # of molecules, formula units, or ions in a given molar amount of a chemical compound. • Calculate the % composition of a given chemical compound.

Section 7. 3 - Using Chemical Formulas Formula Masses The formula mass of any molecule, formula unit, or ion is the sum of the average atomic masses of all the atoms represented in the formula. Book example Average atomic mass of H : 1. 01 amu Average atomic mass of O : 16. 00 amu H 2 O (2 x 1. 01 amu) + 16 amu = 18. 02 amu

Section 7. 3 - Using Chemical Formulas Molar Masses A compound’s molar mass is numerically equal to its formula mass. Book example Formula mass of H 2 O = 18. 02 amu Which is also the molar mass of water 18. 02 g/mol.

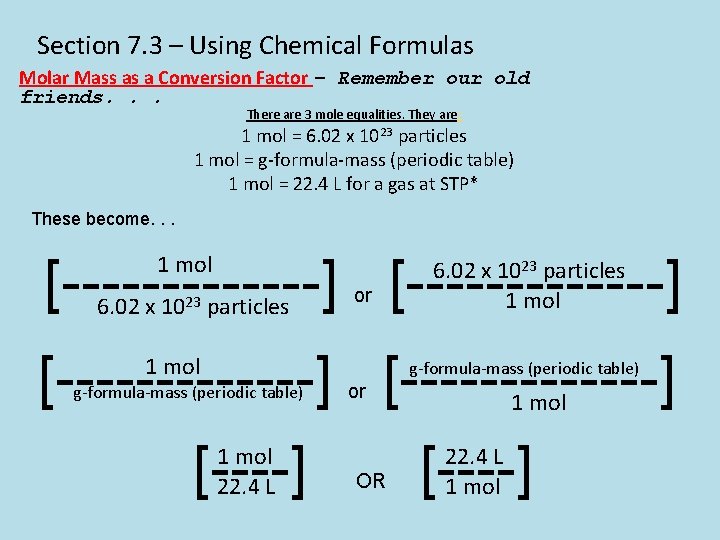
Section 7. 3 – Using Chemical Formulas Molar Mass as a Conversion Factor – Remember our old friends. . . There are 3 mole equalities. They are: 1 mol = 6. 02 x 1023 particles 1 mol = g-formula-mass (periodic table) 1 mol = 22. 4 L for a gas at STP* These become. . . [-------------] 1 mol 6. 02 x 1023 particles or 6. 02 x 1023 particles 1 mol [-------------] [----] 1 mol g-formula-mass (periodic table) 1 mol 22. 4 L or OR 1 mol 22. 4 L 1 mol

Section 7. 3 - Using Chemical Formulas Percentage Composition The percentage by mass of each element in a compound is known as the percentage composition of the compound. Mass of X in sample of compound Mass of sample of compound X 100 % = Mass % X in compound

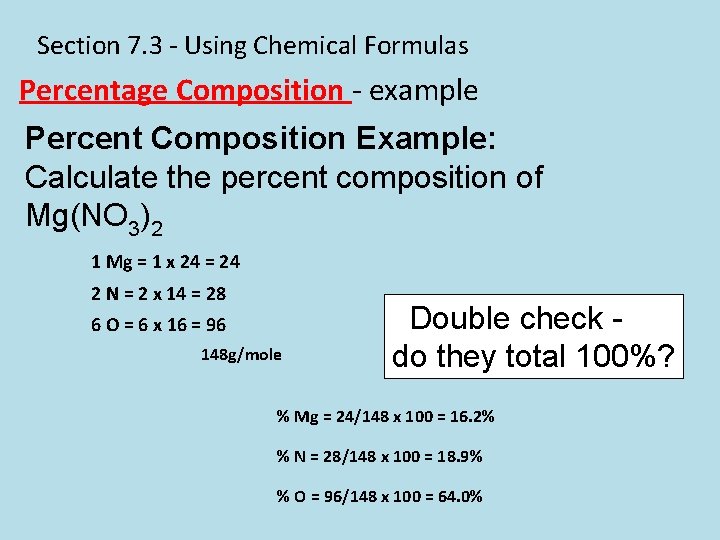
Section 7. 3 - Using Chemical Formulas Percentage Composition - example Percent Composition Example: Calculate the percent composition of Mg(NO 3)2 1 Mg = 1 x 24 = 24 2 N = 2 x 14 = 28 6 O = 6 x 16 = 96 148 g/mole Double check do they total 100%? % Mg = 24/148 x 100 = 16. 2% % N = 28/148 x 100 = 18. 9% % O = 96/148 x 100 = 64. 0%

Section 7. 3 - Using Chemical Formulas Percentage Composition - practice Quiz Break Key

Section 7. 4 - Determining Chemical Formulas Objectives HW – Notes on section 7. 4 pgs 229 -233 Students will be able to : • Define empirical formula, and explain how the terms applies to ionic and molecular compounds. • Determine an empirical formula from either a percentage or a mass composition. • Explain the relationship between the empirical formula and the molecular formula of a given compound. • Determine a molecular formula from an empirical formula

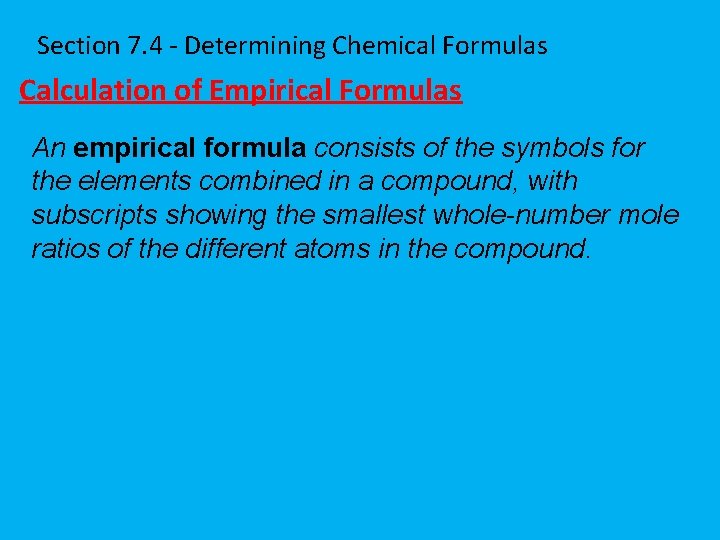
Section 7. 4 - Determining Chemical Formulas Calculation of Empirical Formulas An empirical formula consists of the symbols for the elements combined in a compound, with subscripts showing the smallest whole-number mole ratios of the different atoms in the compound.

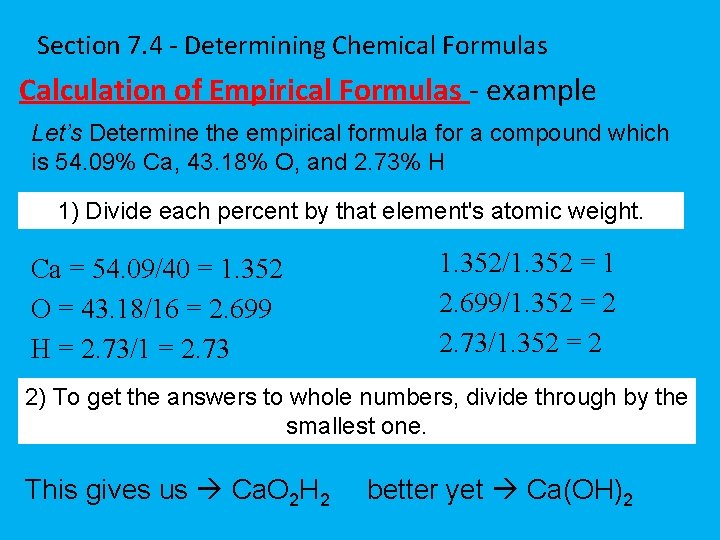
Section 7. 4 - Determining Chemical Formulas Calculation of Empirical Formulas - example Let’s Determine the empirical formula for a compound which is 54. 09% Ca, 43. 18% O, and 2. 73% H 1) Divide each percent by that element's atomic weight. Ca = 54. 09/40 = 1. 352 O = 43. 18/16 = 2. 699 H = 2. 73/1 = 2. 73 1. 352/1. 352 = 1 2. 699/1. 352 = 2 2. 73/1. 352 = 2 2) To get the answers to whole numbers, divide through by the smallest one. This gives us Ca. O 2 H 2 better yet Ca(OH)2

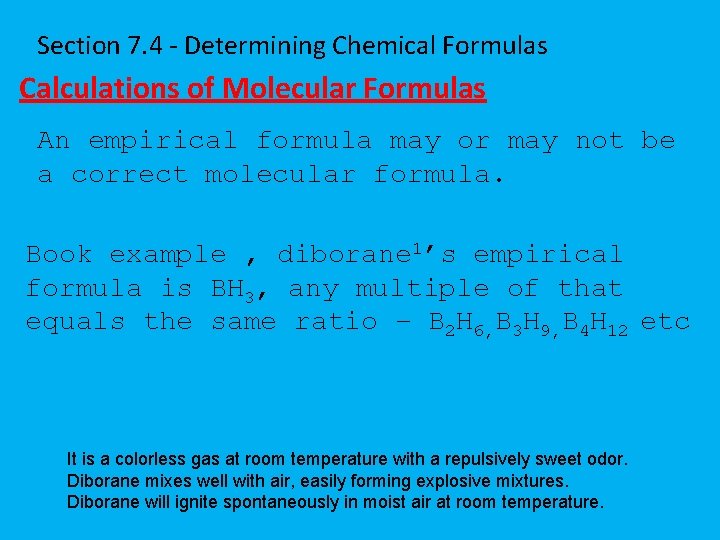
Section 7. 4 - Determining Chemical Formulas Calculations of Molecular Formulas An empirical formula may or may not be a correct molecular formula. Book example , diborane 1’s empirical formula is BH 3, any multiple of that equals the same ratio – B 2 H 6, B 3 H 9, B 4 H 12 etc It is a colorless gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature.

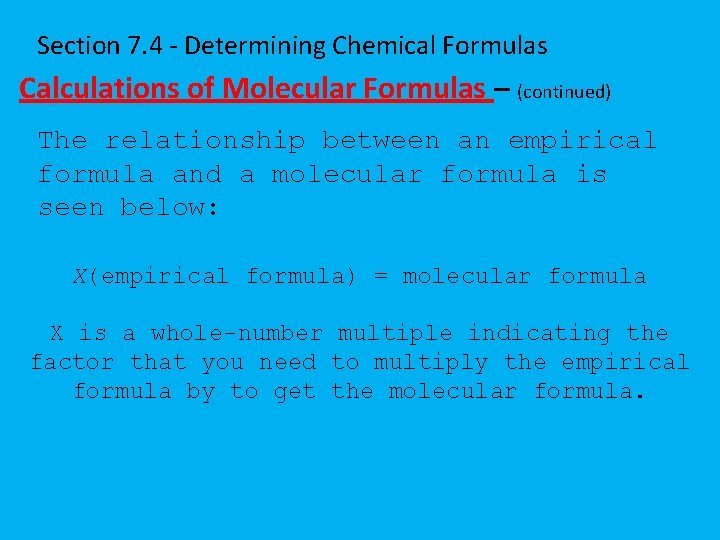
Section 7. 4 - Determining Chemical Formulas Calculations of Molecular Formulas – (continued) The relationship between an empirical formula and a molecular formula is seen below: X(empirical formula) = molecular formula X is a whole-number multiple indicating the factor that you need to multiply the empirical formula by to get the molecular formula.

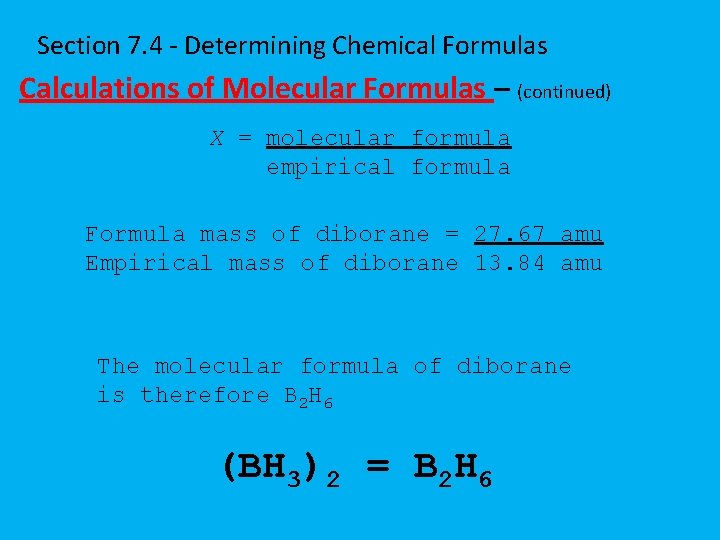
Section 7. 4 - Determining Chemical Formulas Calculations of Molecular Formulas – (continued) X = molecular formula empirical formula Formula mass of diborane = 27. 67 amu Empirical mass of diborane 13. 84 amu The molecular formula of diborane is therefore B 2 H 6 (BH 3)2 = B 2 H 6

Section 7. 4 - Determining Chemical Formulas PRACTICE Empirical Formula Practice Molecular Formula Practice