Lesson 5 Changes in Matter The Law of
















- Slides: 20

Lesson 5: Changes in Matter

The Law of Conservation of Matter and Energy tells us that matter and energy cannot be created or destroyed. But matter can be changed!

Changes in matter can be classified as either physical changes or chemical changes.

A physical change is a change in matter that does not affect its chemical composition. After a physical change, a substance may look different, but it is still the same substance!

You can melt ice and then refreeze the water to make ice again. The ice changes states but it is always water. You can tear up a piece of paper and it’s still paper. You can mix sugar in water and still taste that the sugar is there. These are all physical changes. Physical changes mean that matter has changed states but remains the same.

Discovery Education Video: Physical Change Explores how matter can change physically without changing its state.

Discovery Education Video: Physical Changes Discover that when matter undergoes a physical change, its properties remain the same

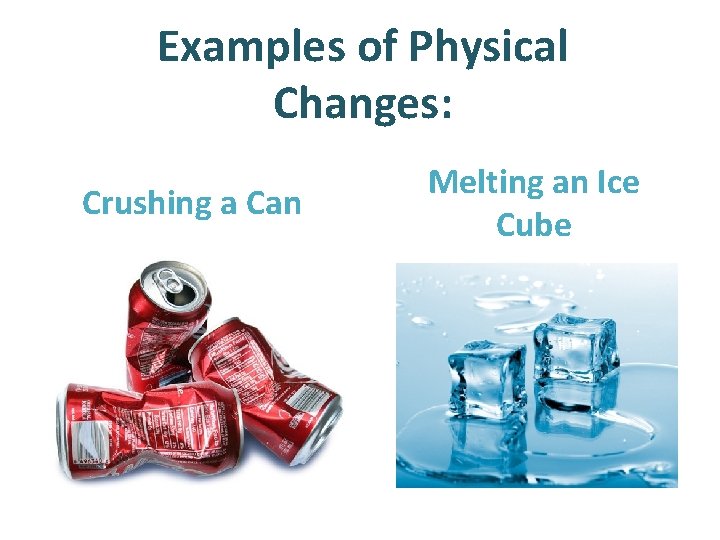
Examples of Physical Changes: Crushing a Can Melting an Ice Cube

More Examples of Physical Changes: Boiling Water Mixing Sand Water

More Examples of Physical Changes: Breaking a Glass Shredding Paper

More Examples of Physical Changes: Chopping Wood Mixing Red and Green Marbles

A chemical change is a process that changes a substance into a new substance through a chemical reaction. Thermite Reaction

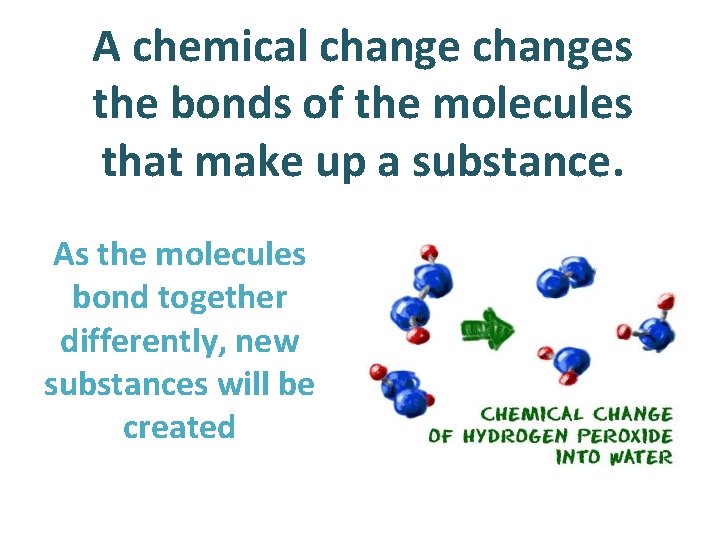
A chemical changes the bonds of the molecules that make up a substance. As the molecules bond together differently, new substances will be created

You light a fire while you camp to keep warm and to cook. You also see a chemical change. Once the wood turns to ashes, it cannot turn back into wood. The wood has become something different. That’s what happens in a chemical change.

Discovery Education Video: Chemical Change Establishes the difference between physical and chemical changes in matter by defining the term "chemical change. "

Examples of Chemical Changes: Rusting of Iron Burning of Wood

More Examples of Chemical Changes: Baking a Cake Exploding Fireworks

More Examples of Chemical Changes: Baking Soda and Vinegar Rotting Bananas

Discovery Education Video: Changes in the Properties of Matter Amanda’s friend Kyle is having trouble understanding matter, but she knows just the thing to do. She takes him to “A Matter of Fact, ” an interesting store full of scientific things—and a peculiar storeowner to help them! Kyle learns all about the physical and chemical properties of matter, such as conductivity, magnetism, and combustibility. He finds out what mixtures and solutions are. Last, he learns about the physical and chemical changes of matter like rusting, tarnishing, and burning.

Changes in Matter Key Questions: 1) What is the difference between a physical and a chemical change? 2) Can all changes in matter be undone? Explain your answer. 3) What are some examples of changes in matter that you have observed. Were these physical or chemical changes? How do you know?