Chemical Reactions Physical Change Chemical Change Chemical Change




- Slides: 10

Chemical Reactions

Physical Change Chemical Change

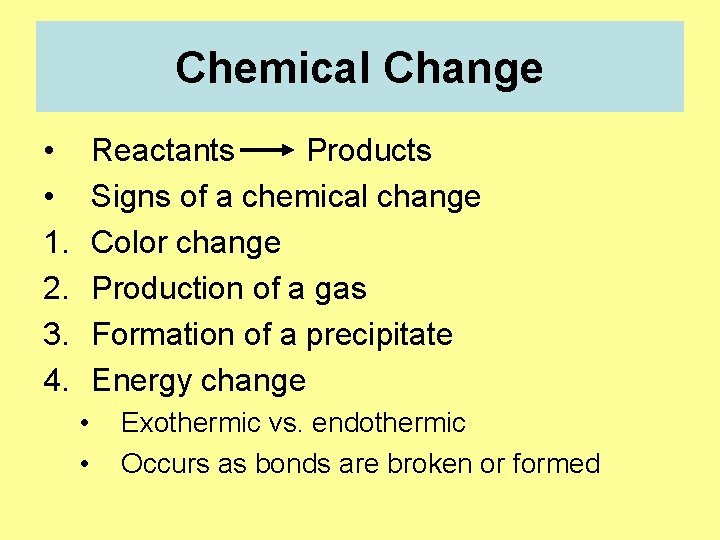
Chemical Change • • 1. 2. 3. 4. Reactants Products Signs of a chemical change Color change Production of a gas Formation of a precipitate Energy change • • Exothermic vs. endothermic Occurs as bonds are broken or formed

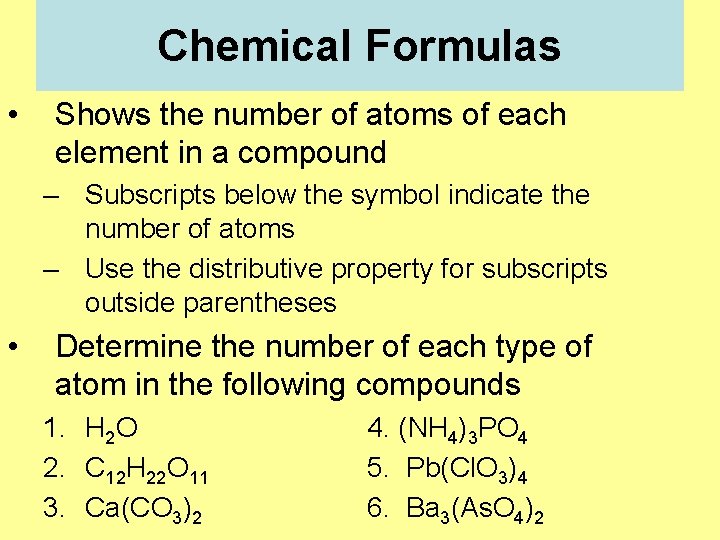
Chemical Formulas • Shows the number of atoms of each element in a compound – Subscripts below the symbol indicate the number of atoms – Use the distributive property for subscripts outside parentheses • Determine the number of each type of atom in the following compounds 1. H 2 O 2. C 12 H 22 O 11 3. Ca(CO 3)2 4. (NH 4)3 PO 4 5. Pb(Cl. O 3)4 6. Ba 3(As. O 4)2

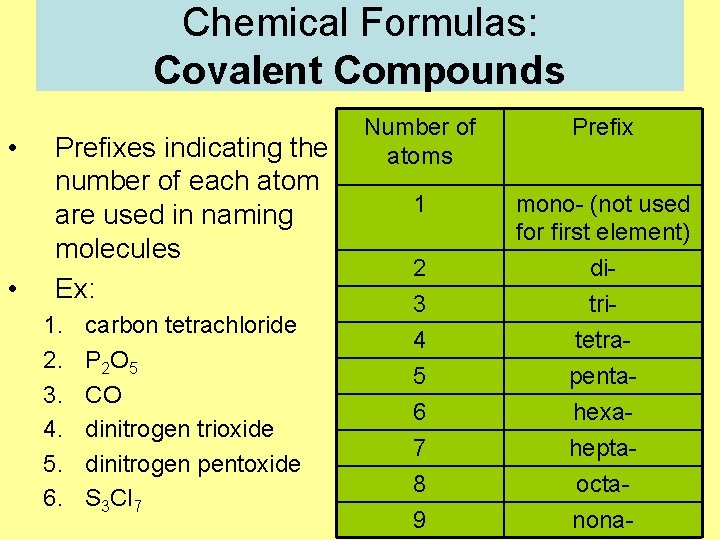
Chemical Formulas: Covalent Compounds • • Prefixes indicating the number of each atom are used in naming molecules Ex: 1. 2. 3. 4. 5. 6. carbon tetrachloride P 2 O 5 CO dinitrogen trioxide dinitrogen pentoxide S 3 Cl 7 Number of atoms Prefix 1 mono- (not used for first element) 2 3 4 5 6 7 8 9 ditritetrapentahexaheptaoctanona-

Chemical Formulas: Covalent Compounds • 1. 2. 3. 4. • 5. 6. 7. Write the prefix for the number indicated. 9 7 8 6 Write the name or the formula. NO 8. dinitrogen tetroxide Si. O 2 9. sulfur hexafluoride P 3 Cl 5 10. dihydrogen monoxide

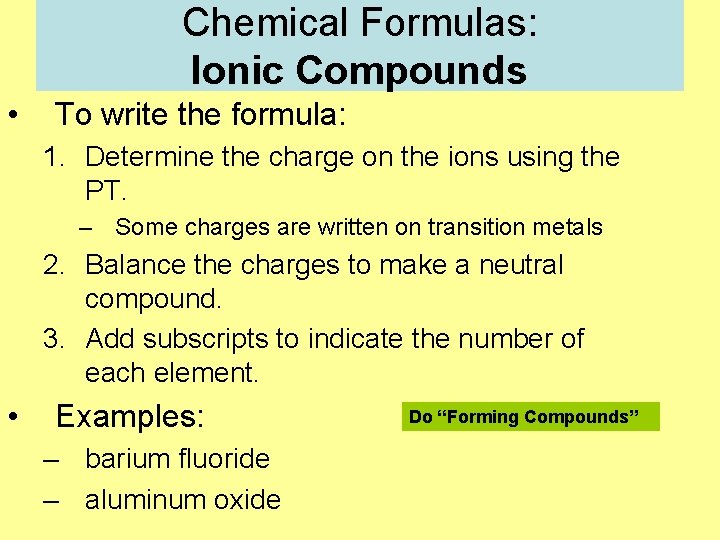
Chemical Formulas: Ionic Compounds • To write the formula: 1. Determine the charge on the ions using the PT. – Some charges are written on transition metals 2. Balance the charges to make a neutral compound. 3. Add subscripts to indicate the number of each element. • Examples: – barium fluoride – aluminum oxide Do “Forming Compounds”

Chemical Equations • Reactions are written for chemical changes – Use correct chemical formulas – Reactants Products • Due to the Law of Conservation of Mass, the number of atoms of each element on the reactant side = number of atoms of each element on the product side – Use coefficients to balance equations – Do not change subscripts (they’re determined by neutralizing charges when writing formulas) Use “balancing chemical equations” handout

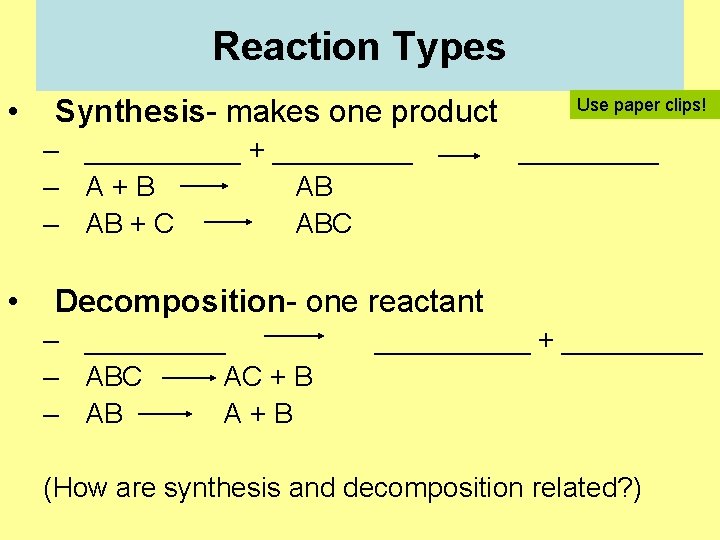
Reaction Types • Synthesis- makes one product – _____ + _____ – A+B AB – AB + C ABC • Use paper clips! _____ Decomposition- one reactant – _____ – ABC AC + B – AB A+B _____ + _____ (How are synthesis and decomposition related? )

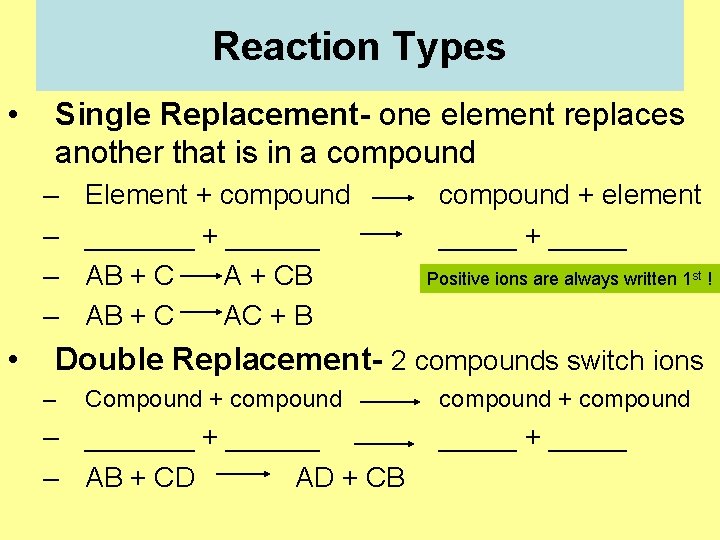
Reaction Types • Single Replacement- one element replaces another that is in a compound – – • Element + compound _______ + ______ AB + C A + CB AB + C AC + B compound + element _____ + _____ Positive ions are always written 1 st ! Double Replacement- 2 compounds switch ions – Compound + compound – _______ + ______ – AB + CD AD + CB compound + compound _____ + _____
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Is painting a wall a physical change
Is painting a wall a physical change Chemical change and physical change
Chemical change and physical change Differences between chemical and physical changes
Differences between chemical and physical changes What is example of physical change
What is example of physical change Spare change physical versus chemical change
Spare change physical versus chemical change Whats a chemical change
Whats a chemical change How does a physical change differ from a chemical change? *
How does a physical change differ from a chemical change? * Chemical change in baking
Chemical change in baking



















