Thermochemistry Thermo heat Chemistry study of matter Thermochemistry







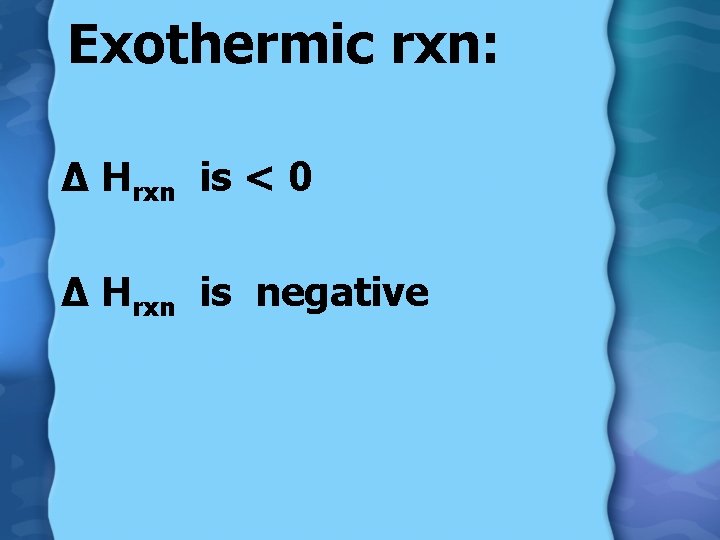
- Slides: 24

Thermochemistry Thermo = heat Chemistry = study of matter

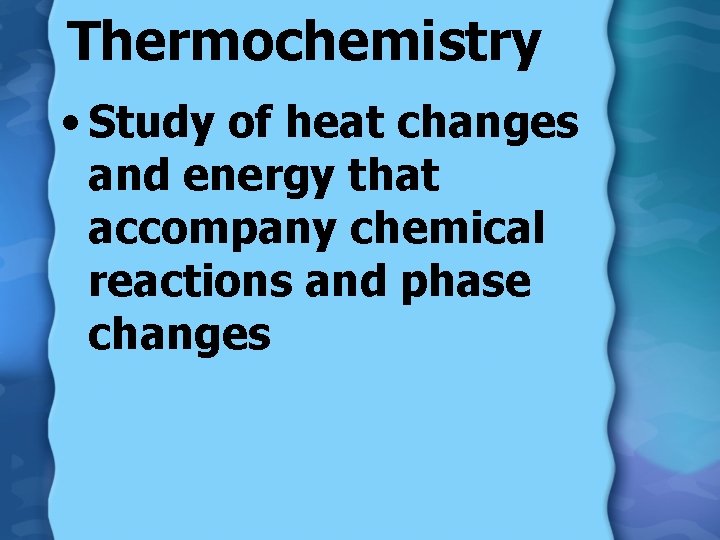
Thermochemistry • Study of heat changes and energy that accompany chemical reactions and phase changes

Review of Energy • Capacity to do work or to create heat and or generate electricity • Types: • Chemical • Nuclear • Thermal • Radiant (light) • Electrical • Mechanical

Law of conservation of Energy • Energy can be converted from one form to another but it cannot be created or destroyed

Forms of energy: • Potential – Stored energy • Kinetic – Energy of motion

Chemical potential energy • Energy stored within the structural units of chemical compounds

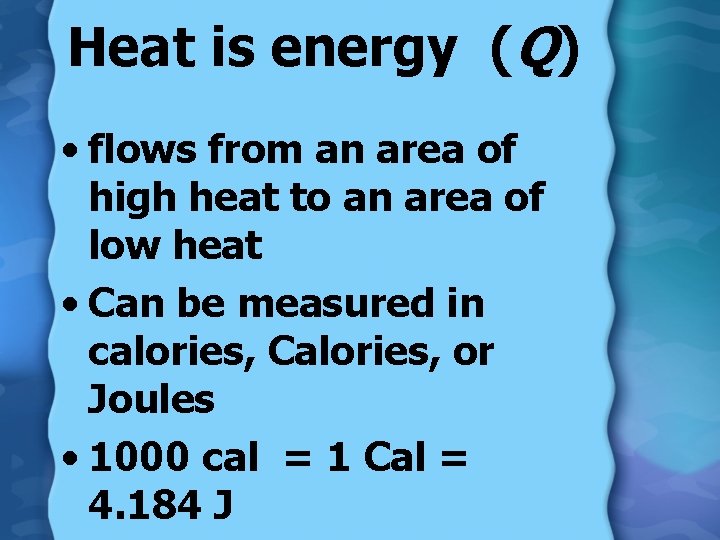
Heat is energy (Q) • flows from an area of high heat to an area of low heat • Can be measured in calories, Calories, or Joules • 1000 cal = 1 Cal = 4. 184 J

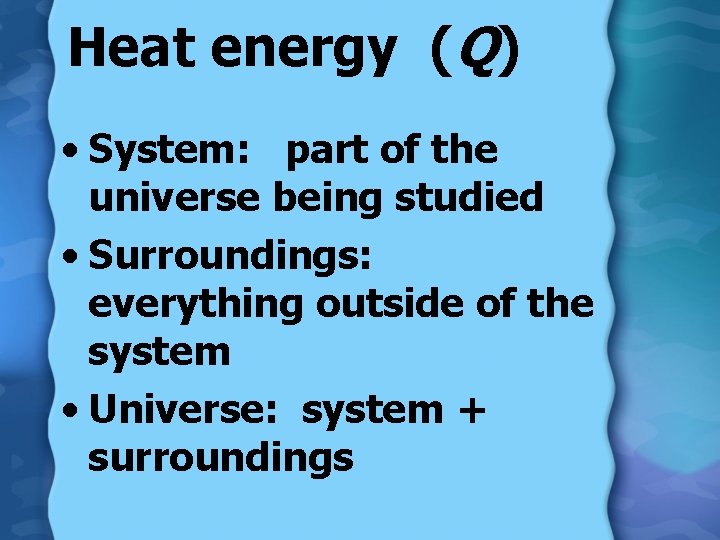
Heat energy (Q) • System: part of the universe being studied • Surroundings: everything outside of the system • Universe: system + surroundings

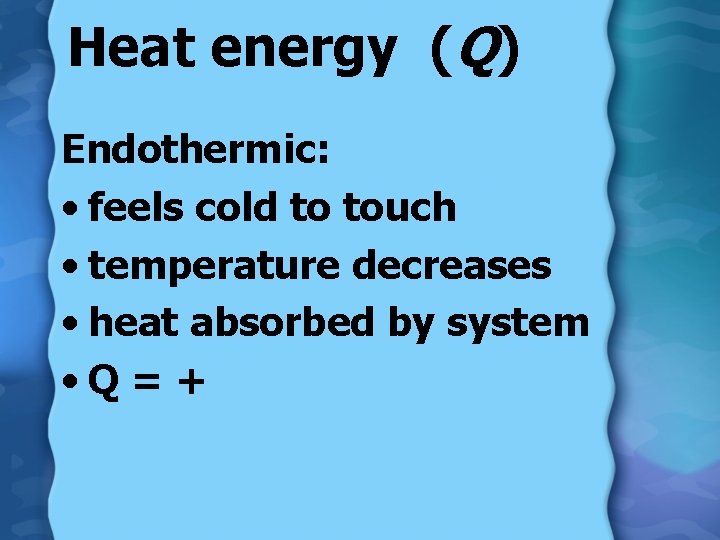
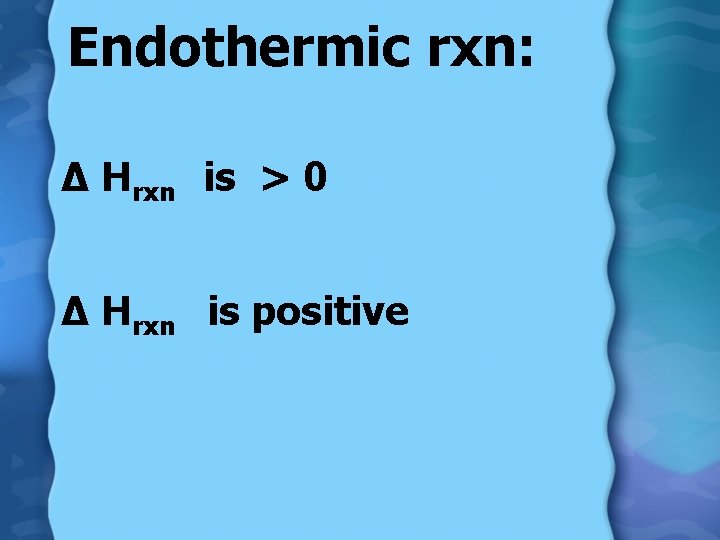
Heat energy (Q) Endothermic: • feels cold to touch • temperature decreases • heat absorbed by system • Q = +

Heat energy (Q) Exothermic: • feels warm to touch • temperature increases • heat released by system • Q = -

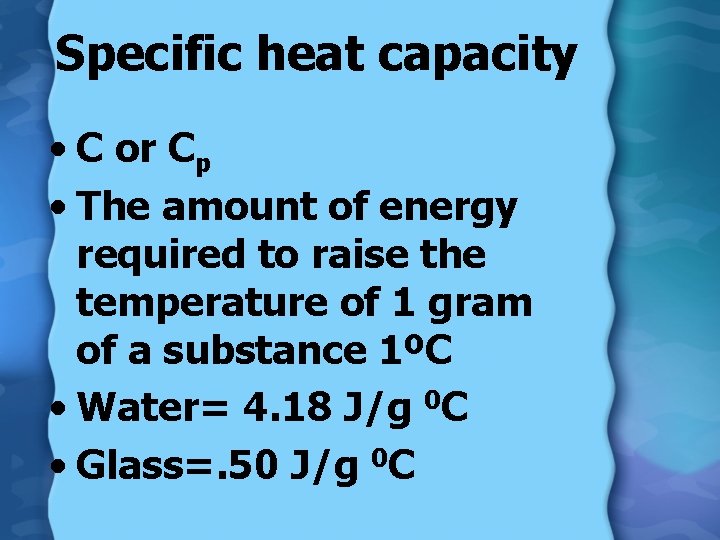
Specific heat capacity • C or Cp • The amount of energy required to raise the temperature of 1 gram of a substance 1ºC • Water= 4. 18 J/g 0 C • Glass=. 50 J/g 0 C

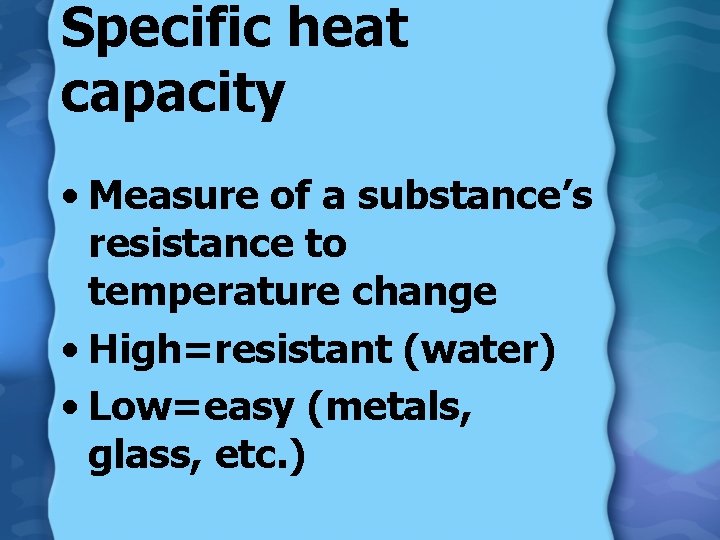
Specific heat capacity • Measure of a substance’s resistance to temperature change • High=resistant (water) • Low=easy (metals, glass, etc. )

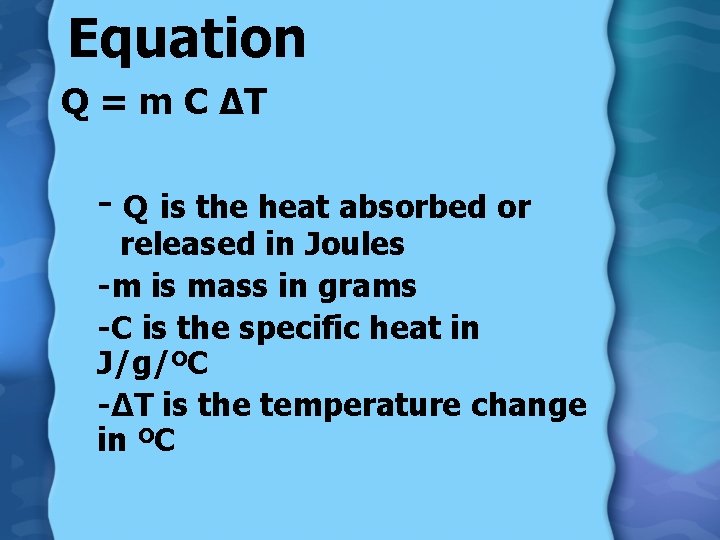
Equation Q = m C ∆T - Q is the heat absorbed or released in Joules -m is mass in grams -C is the specific heat in J/g/ºC -∆T is the temperature change in ºC

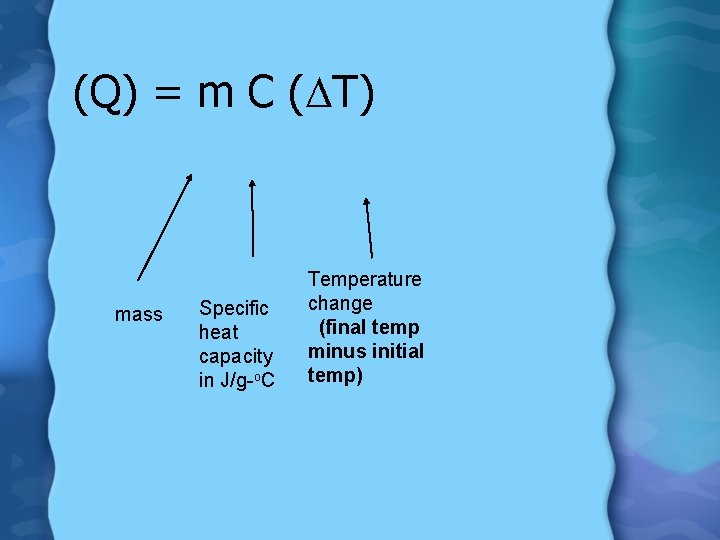
(Q) = m C (DT) mass Specific heat capacity in J/g-o. C Temperature change (final temp minus initial temp)

When 1982 g of water underwent a temperature change from 23. 677 o. C to 27. 482 o. C, how much energy in k. J did the water absorb? The specific heat of water is 4. 184 J/(go. C). Show all work!

How much energy in J is required to raise the temperature of 500. 0 g of copper from 22. 8 o. C to 100. 0 o. C? The specific heat of copper is 0. 387 J/g/o. C. Show all work!

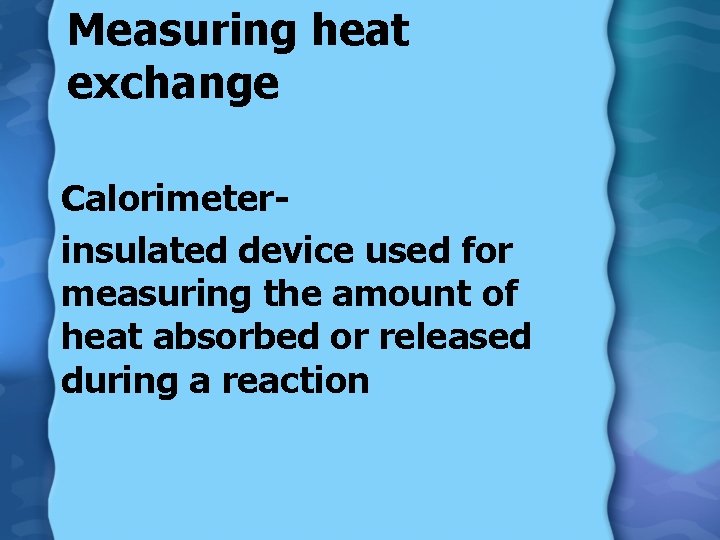
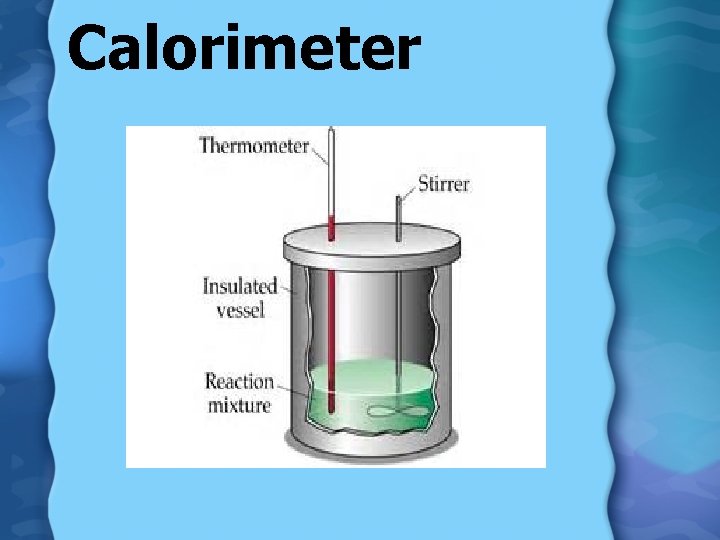
Measuring heat exchange Calorimeterinsulated device used for measuring the amount of heat absorbed or released during a reaction

Calorimeter

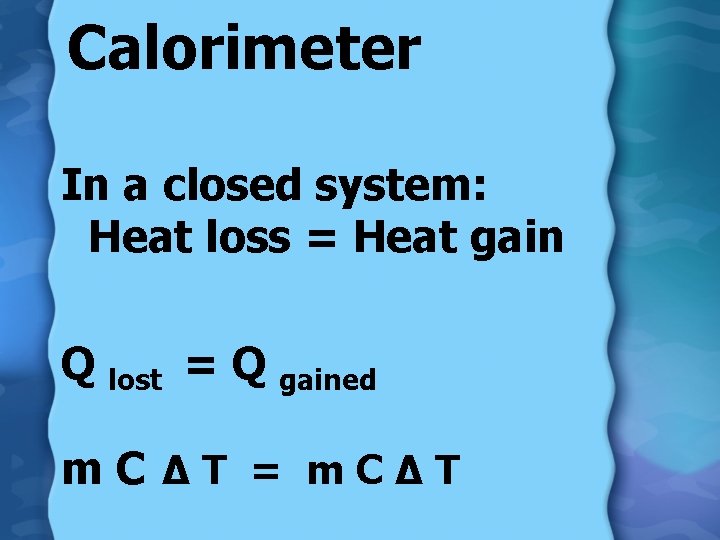
Calorimeter In a closed system: Heat loss = Heat gain Q lost = Q gained m. C ∆T = m. C∆T

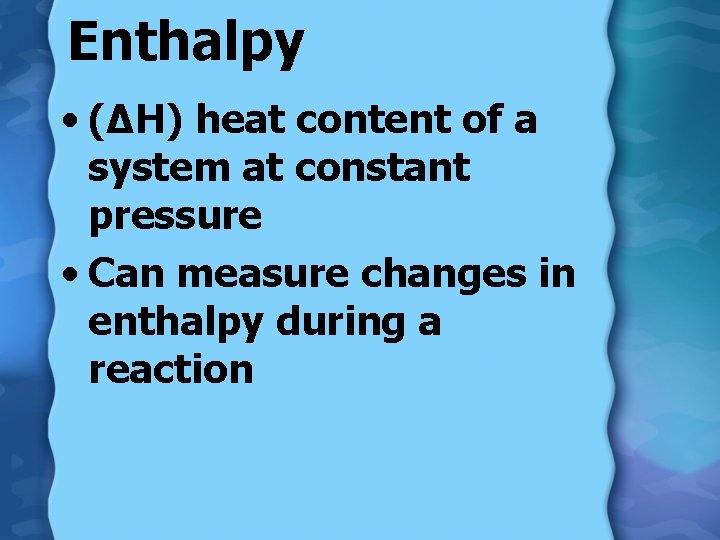
Enthalpy • (∆H) heat content of a system at constant pressure • Can measure changes in enthalpy during a reaction

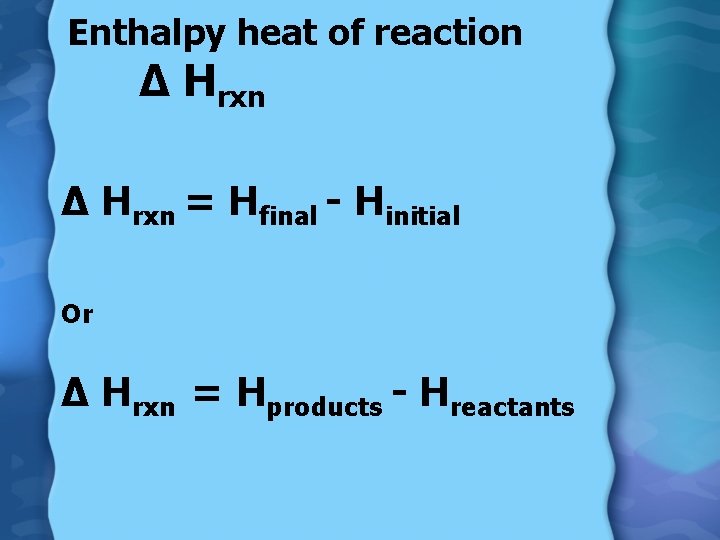
Enthalpy heat of reaction ∆ Hrxn = Hfinal - Hinitial Or ∆ Hrxn = Hproducts - Hreactants

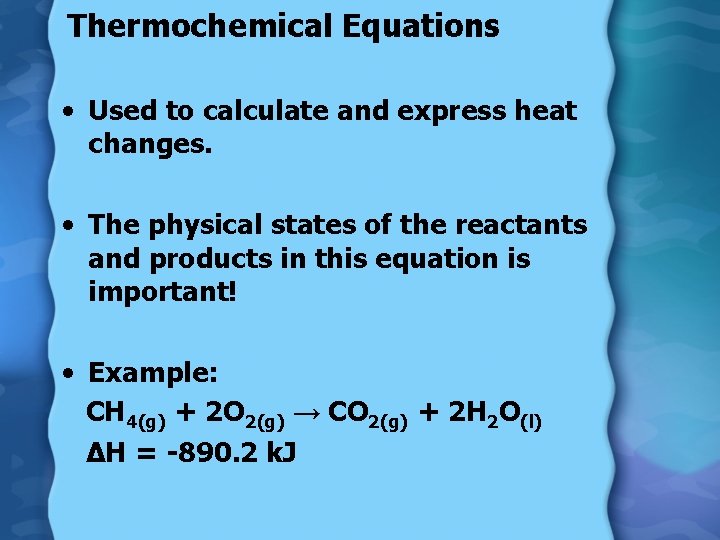
Thermochemical Equations • Used to calculate and express heat changes. • The physical states of the reactants and products in this equation is important! • Example: CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l) ∆H = -890. 2 k. J
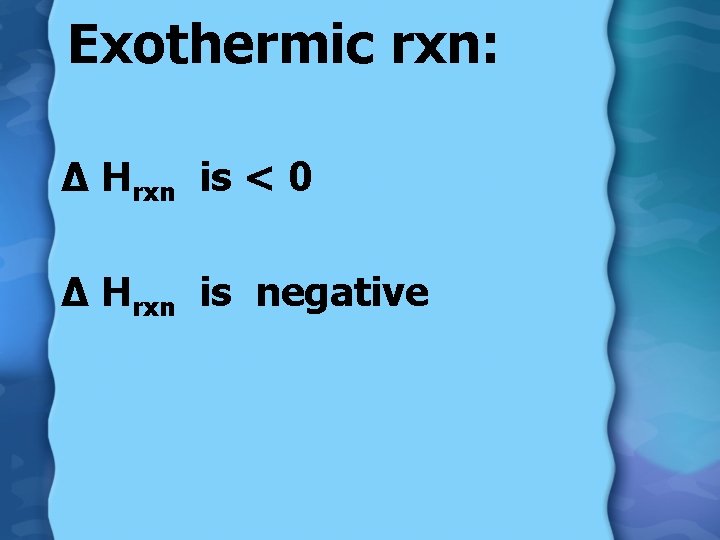
Exothermic rxn: ∆ Hrxn is < 0 ∆ Hrxn is negative

Endothermic rxn: ∆ Hrxn is > 0 ∆ Hrxn is positive
 Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Ap chemistry thermodynamics
Ap chemistry thermodynamics Ap chem unit 7
Ap chem unit 7 General chemistry thermochemistry
General chemistry thermochemistry Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is study of
Thermochemistry is study of Thermochemistry is study of
Thermochemistry is study of Study of energy transformations
Study of energy transformations Ilmu kimia yang mempelajari tentang panas atau suhu disebut
Ilmu kimia yang mempelajari tentang panas atau suhu disebut Thermochemistry is the study of
Thermochemistry is the study of Q = m x cp x ∆t
Q = m x cp x ∆t Section 1 composition of matter
Section 1 composition of matter What is white matter made of
What is white matter made of Section 1 composition of matter
Section 1 composition of matter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Brain falx
Brain falx Composition of matter section 1
Composition of matter section 1 Gray matter and white matter
Gray matter and white matter Telecephalon
Telecephalon Energy naturally flows from warmer matter to cooler matter.
Energy naturally flows from warmer matter to cooler matter. Prefix of thermo
Prefix of thermo Thermo solver
Thermo solver Haake mars iii
Haake mars iii Ascoli thermo precipitation test
Ascoli thermo precipitation test