Grade 10 Chemistry Knowledge area Chemical change Physical


- Slides: 7

Grade 10 Chemistry Knowledge area: Chemical change

Physical and chemical change 1. 1 Separation of particles 1. 1. 1 Physical change • Types of changes: 1. State change 2. Change of shape 3. Colour change • Energy is required. Relatively small amounts of energy are necessary These are mainly reversible reactions. A change in shape is not always reversible.

Physical and chemical change 1. 1 Change of condition (state) Examples • Ice melts and forms water. • Water evaporates and forms water vapour. • Water freezes and forms ice. • Water vapour condenses and forms water. Sublimation is a process whereby a solid changes directly into a gas. Definition

Physical and chemical change Examples Dry ice (CO 2 solid) becomes CO 2(g). Quick facts Dry ice is typically used in ice-cream carts to keep the ice-cream iced. When it sublimates, it does not change into a liquid, which would make a mess.

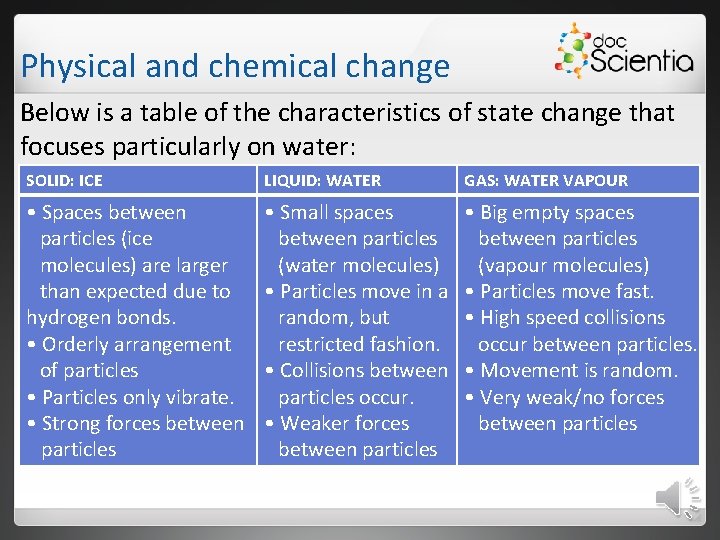
Physical and chemical change Below is a table of the characteristics of state change that focuses particularly on water: SOLID: ICE LIQUID: WATER GAS: WATER VAPOUR • Spaces between particles (ice molecules) are larger than expected due to hydrogen bonds. • Orderly arrangement of particles • Particles only vibrate. • Strong forces between particles • Small spaces between particles (water molecules) • Particles move in a random, but restricted fashion. • Collisions between particles occur. • Weaker forces between particles • Big empty spaces between particles (vapour molecules) • Particles move fast. • High speed collisions occur between particles. • Movement is random. • Very weak/no forces between particles

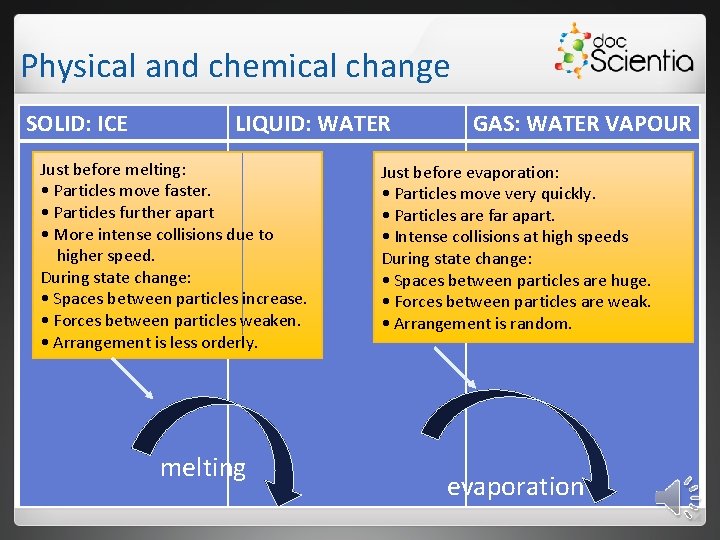
Physical and chemical change SOLID: ICE LIQUID: WATER Just before melting: • Particles move faster. • Particles further apart • More intense collisions due to higher speed. During state change: • Spaces between particles increase. • Forces between particles weaken. • Arrangement is less orderly. melting GAS: WATER VAPOUR Just before evaporation: • Particles move very quickly. • Particles are far apart. • Intense collisions at high speeds During state change: • Spaces between particles are huge. • Forces between particles are weak. • Arrangement is random. evaporation

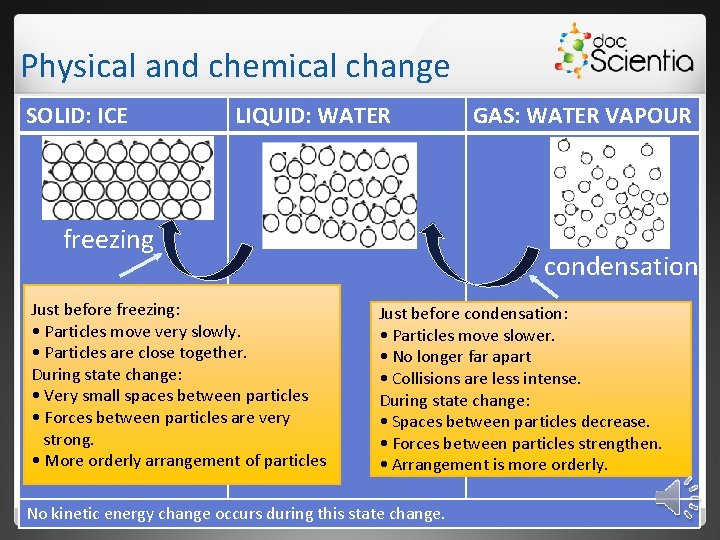
Physical and chemical change SOLID: ICE LIQUID: WATER freezing Just before freezing: • Particles move very slowly. • Particles are close together. During state change: • Very small spaces between particles • Forces between particles are very strong. • More orderly arrangement of particles GAS: WATER VAPOUR condensation Just before condensation: • Particles move slower. • No longer far apart • Collisions are less intense. During state change: • Spaces between particles decrease. • Forces between particles strengthen. • Arrangement is more orderly. No kinetic energy change occurs during this state change.
 Is mashing potatoes a chemical change
Is mashing potatoes a chemical change What is physical change and chemical change
What is physical change and chemical change Whats the difference between physical and chemical changes
Whats the difference between physical and chemical changes What is example of physical change
What is example of physical change Spare change physical versus chemical change
Spare change physical versus chemical change Whats the difference between physical and chemical changes
Whats the difference between physical and chemical changes How does a physical change differ from a chemical change? *
How does a physical change differ from a chemical change? * Baking chemical change
Baking chemical change













