Grade 10 Chemistry Knowledge area Chemical change Reactions


- Slides: 6

Grade 10 Chemistry Knowledge area: Chemical change

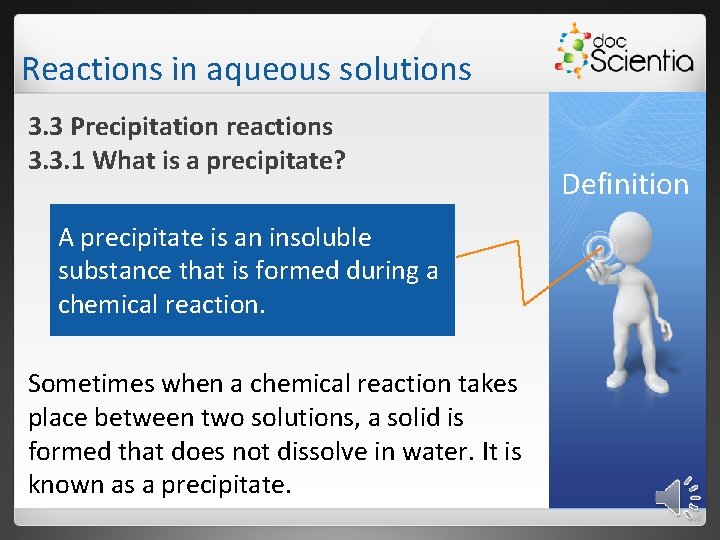
Reactions in aqueous solutions 3. 3 Precipitation reactions 3. 3. 1 What is a precipitate? A precipitate is an insoluble substance that is formed during a chemical reaction. Sometimes when a chemical reaction takes place between two solutions, a solid is formed that does not dissolve in water. It is known as a precipitate. Definition

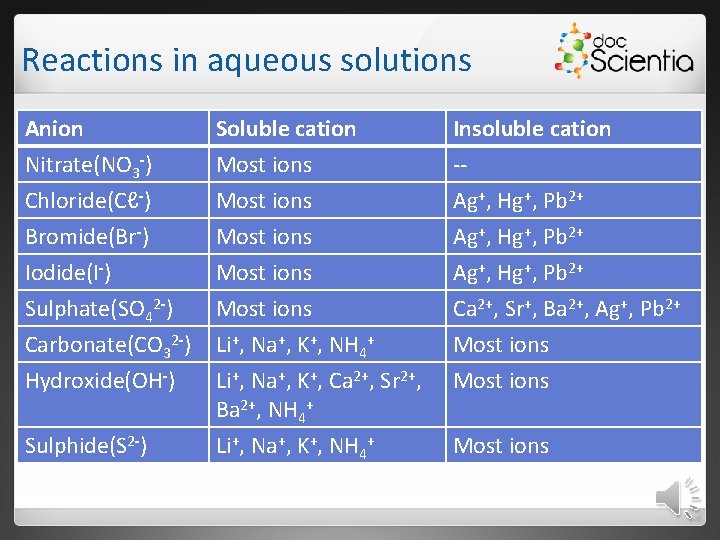
Reactions in aqueous solutions Anion Nitrate(NO 3 -) Chloride(Cℓ-) Bromide(Br-) Iodide(I-) Sulphate(SO 42 -) Carbonate(CO 32 -) Soluble cation Most ions Most ions Li+, Na+, K+, NH 4+ Insoluble cation -Ag+, Hg+, Pb 2+ Ca 2+, Sr+, Ba 2+, Ag+, Pb 2+ Most ions Hydroxide(OH-) Li+, Na+, K+, Ca 2+, Sr 2+, Ba 2+, NH 4+ Li+, Na+, K+, NH 4+ Most ions Sulphide(S 2 -) Most ions

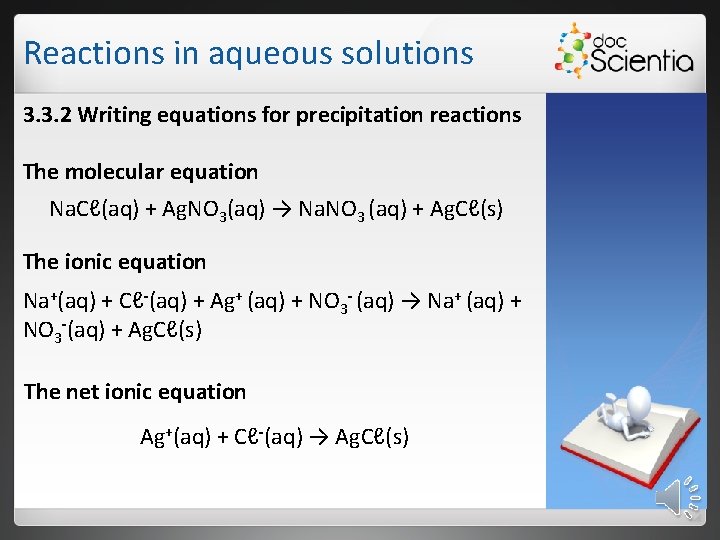
Reactions in aqueous solutions 3. 3. 2 Writing equations for precipitation reactions The molecular equation Na. Cℓ(aq) + Ag. NO 3(aq) → Na. NO 3 (aq) + Ag. Cℓ(s) The ionic equation Na+(aq) + Cℓ-(aq) + Ag+ (aq) + NO 3 - (aq) → Na+ (aq) + NO 3 -(aq) + Ag. Cℓ(s) The net ionic equation Ag+(aq) + Cℓ-(aq) → Ag. Cℓ(s)

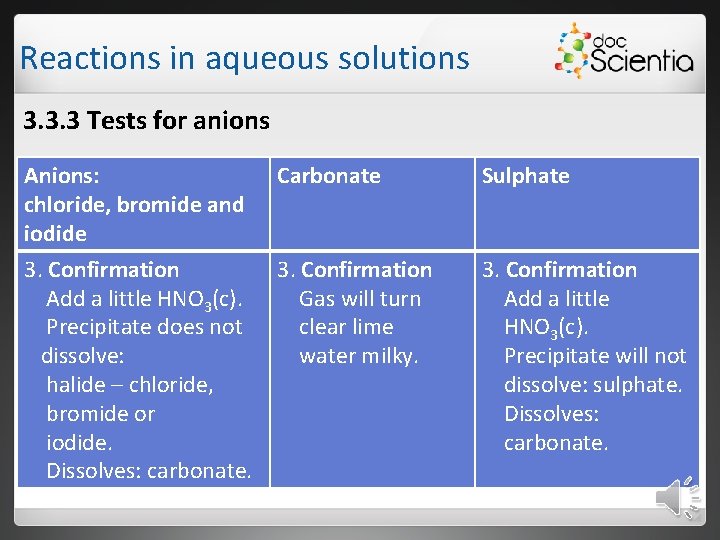
Reactions in aqueous solutions 3. 3. 3 Tests for anions Anions: Carbonate Sulphate chloride, bromide and iodide 1. Add a few drops Ag. NO 3 1. Add a little bit 1. Add a few drops (silver nitrate ) to of diluted acid, Ba. Cℓ 2 unknown e. g. (barium chloride) or halide solutions. hydrochloric Ba(OH)2. acid. 2. If the following 2. Bubbles form, precipitates because form: it effervesces. white: chloride cream-coloured: bromid 2. If a white precipitate forms SO 42 -.

Reactions in aqueous solutions 3. 3. 3 Tests for anions Anions: chloride, bromide and iodide 3. Confirmation Add a little HNO 3(c). Precipitate does not dissolve: halide – chloride, bromide or iodide. Dissolves: carbonate. Carbonate Sulphate 3. Confirmation Gas will turn clear lime water milky. 3. Confirmation Add a little HNO 3(c). Precipitate will not dissolve: sulphate. Dissolves: carbonate.











