Thermochemistry Thermochemistry is concerned with the heat changes





- Slides: 31

Thermochemistry

Thermochemistry is concerned with the heat changes that occur during chemical reactions. Can deal with gaining or losing heat

Energy The capacity for doing work or supplying heat. - Energy is only detected because of its effects Energy stored within the structural units of chemical substances is called Chemical Potential Energy (Gasoline)

Heat (q) Energy that transfers from one object to another because of a temperature difference between them Heat is not detectable, only changes caused by heat Heat always flows from a warmer object to a cooler object

Universe The system and its surroundings make up the universe System – part of the universe you focus on Surroundings – everything else in the universe

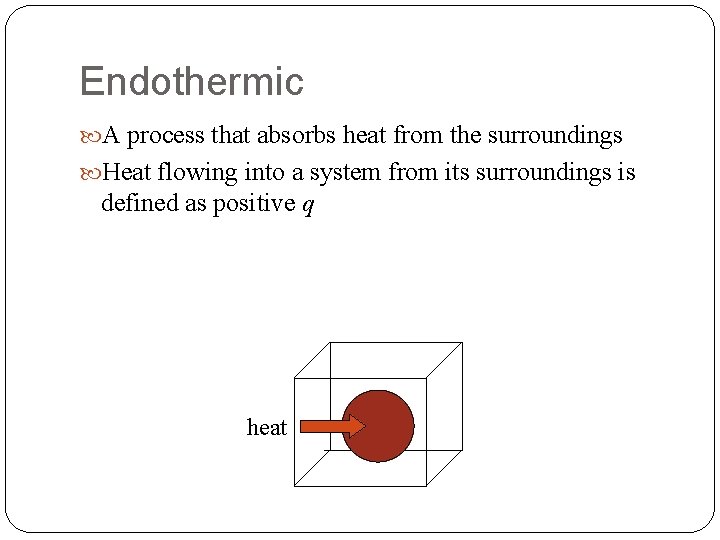
Endothermic A process that absorbs heat from the surroundings Heat flowing into a system from its surroundings is defined as positive q heat

syste m

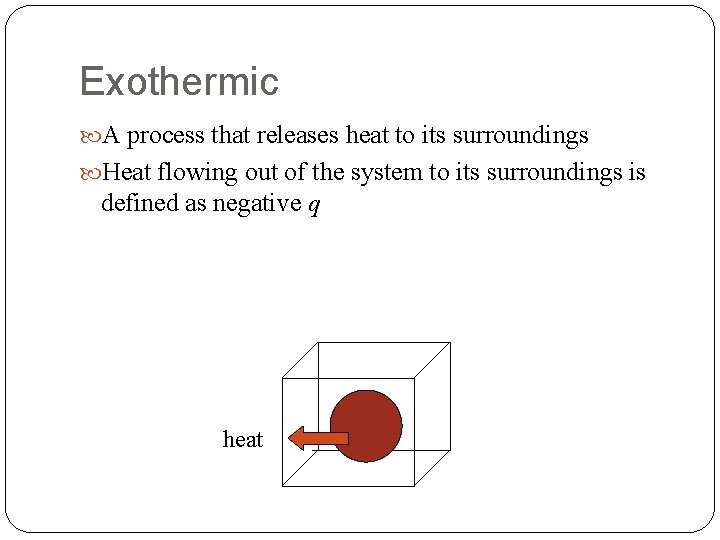
Exothermic A process that releases heat to its surroundings Heat flowing out of the system to its surroundings is defined as negative q heat

system

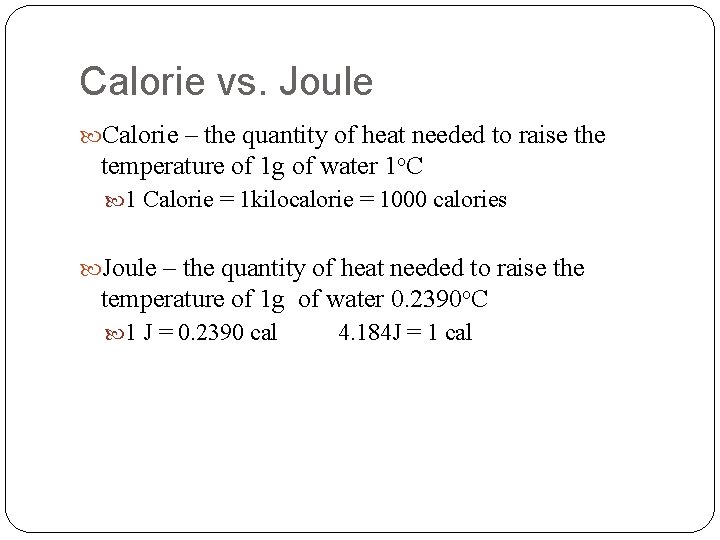
Calorie vs. Joule Calorie – the quantity of heat needed to raise the temperature of 1 g of water 1 o. C 1 Calorie = 1 kilocalorie = 1000 calories Joule – the quantity of heat needed to raise the temperature of 1 g of water 0. 2390 o. C 1 J = 0. 2390 cal 4. 184 J = 1 cal

Heat Capacity The amount of heat needed to increase the temperature of an object exactly 1 o. C The greater the mass of the object, the greater its heat capacity. Which has more heat capacity, a drop of water, or an entire pool?

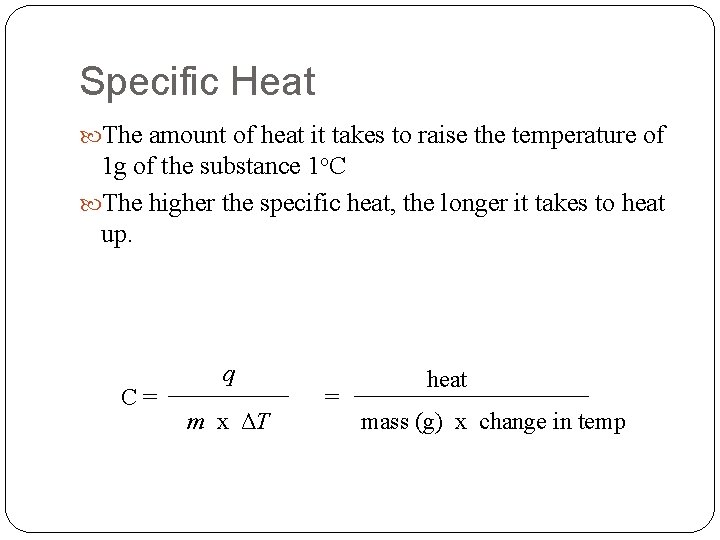
Specific Heat The amount of heat it takes to raise the temperature of 1 g of the substance 1 o. C The higher the specific heat, the longer it takes to heat up. C= q m x ΔT = heat mass (g) x change in temp

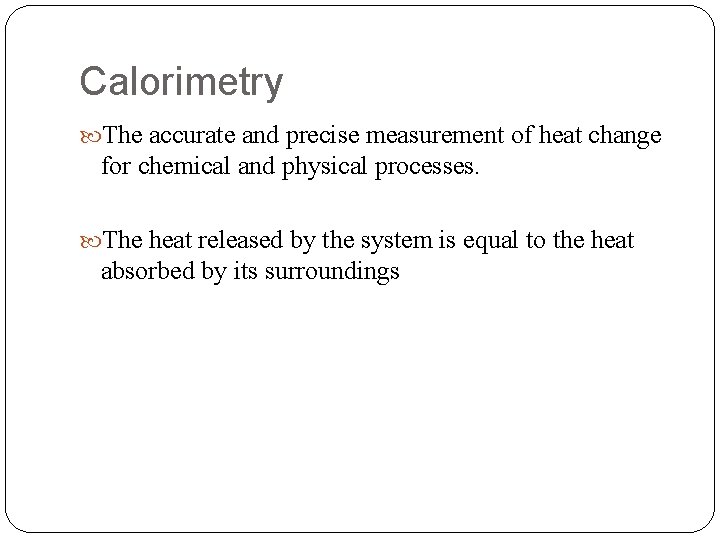
Calorimetry The accurate and precise measurement of heat change for chemical and physical processes. The heat released by the system is equal to the heat absorbed by its surroundings

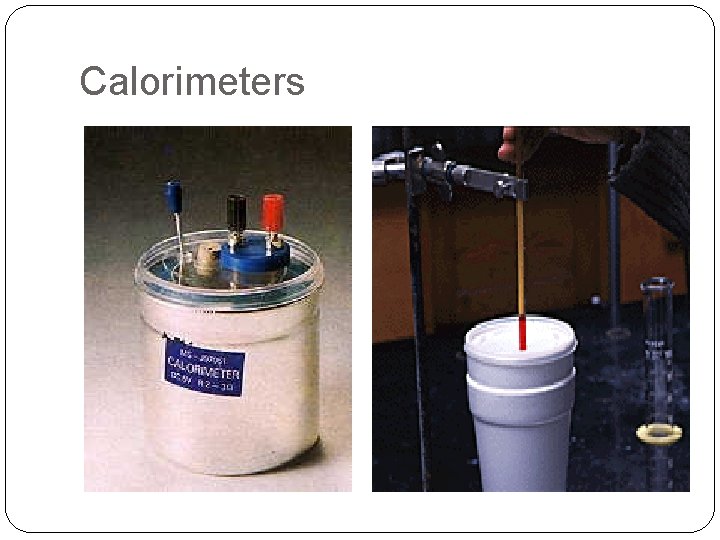
Calorimeters

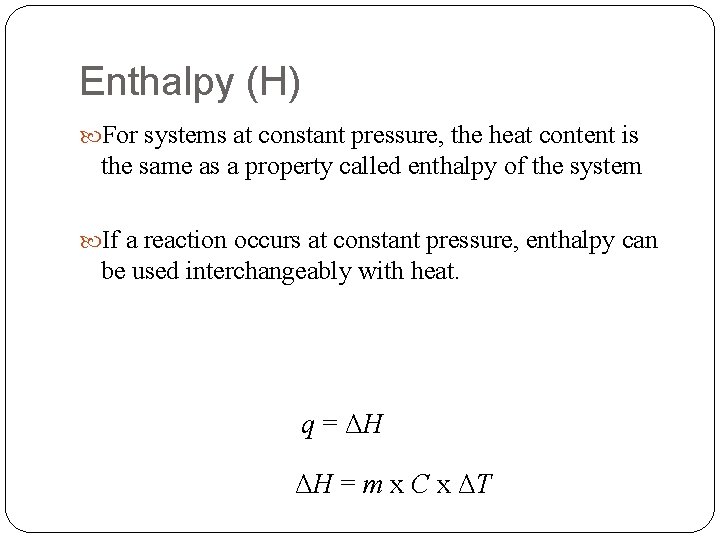
Enthalpy (H) For systems at constant pressure, the heat content is the same as a property called enthalpy of the system If a reaction occurs at constant pressure, enthalpy can be used interchangeably with heat. q = ΔH ΔH = m x C x ΔT

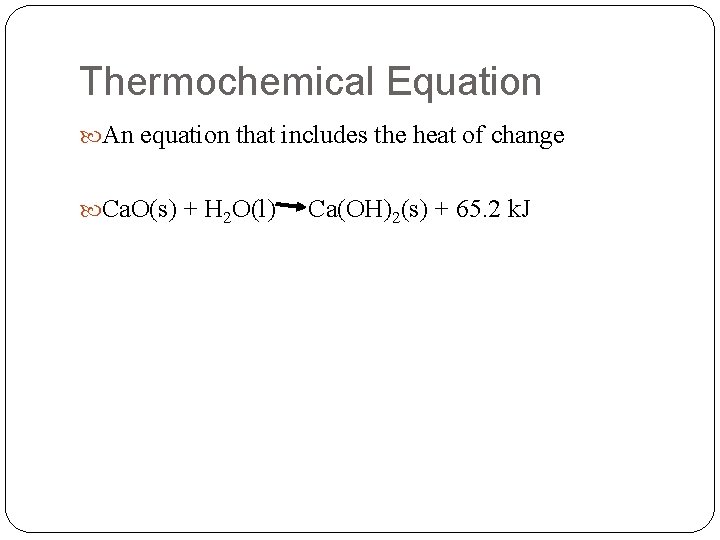
Thermochemical Equation An equation that includes the heat of change Ca. O(s) + H 2 O(l) Ca(OH)2(s) + 65. 2 k. J

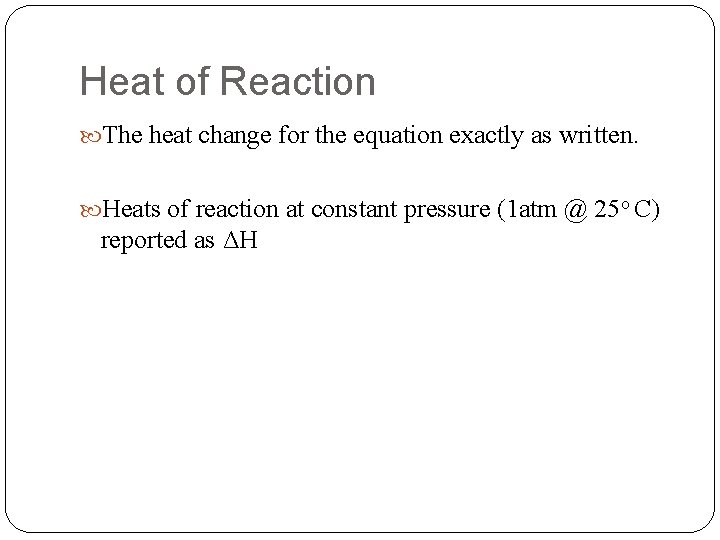
Heat of Reaction The heat change for the equation exactly as written. Heats of reaction at constant pressure (1 atm @ 25 o C) reported as ΔH

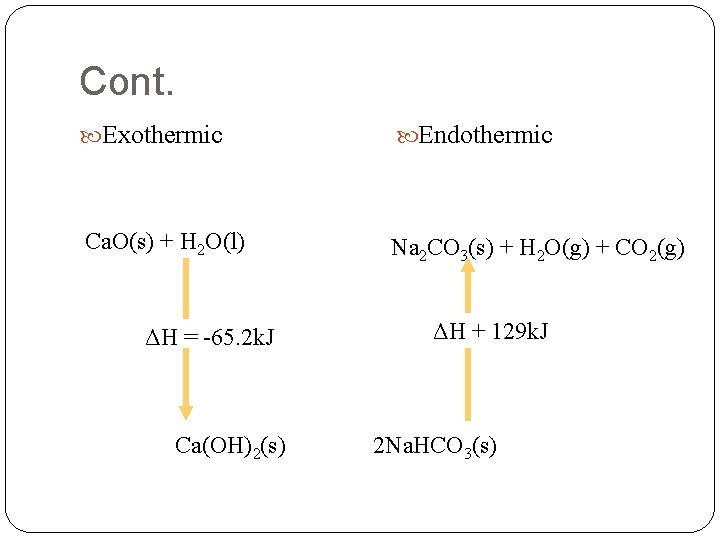
Cont. Exothermic Endothermic Ca. O(s) + H 2 O(l) Na 2 CO 3(s) + H 2 O(g) + CO 2(g) ΔH = -65. 2 k. J Ca(OH)2(s) ΔH + 129 k. J 2 Na. HCO 3(s)

Exothermic Ca. Cl 2(s) H 2 O(l) Ca 2+(aq) + 2 Cl-(aq) ΔHsoln = -82. 8 k. J/mol

Endothermic NH 4 NO 3(s) H 2 O(l) NH 4+(aq) + NO 3 -(aq) ΔHsoln = 25. 7 k. J/mol

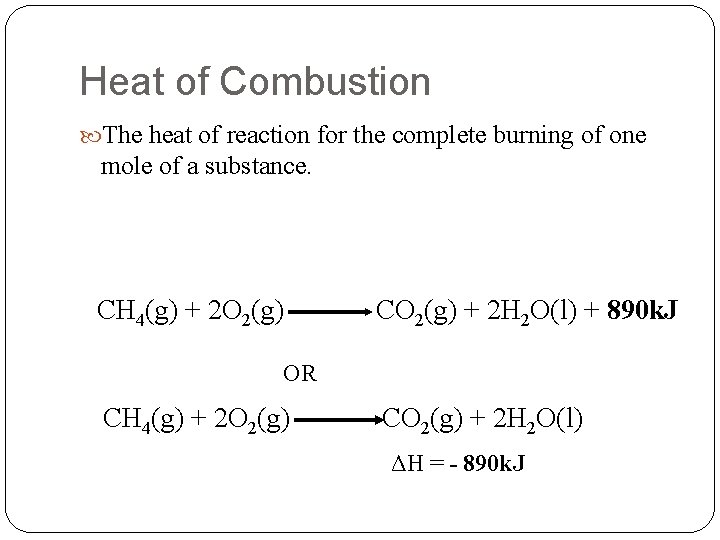
Heat of Combustion The heat of reaction for the complete burning of one mole of a substance. CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) + 890 k. J OR CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) ΔH = - 890 k. J

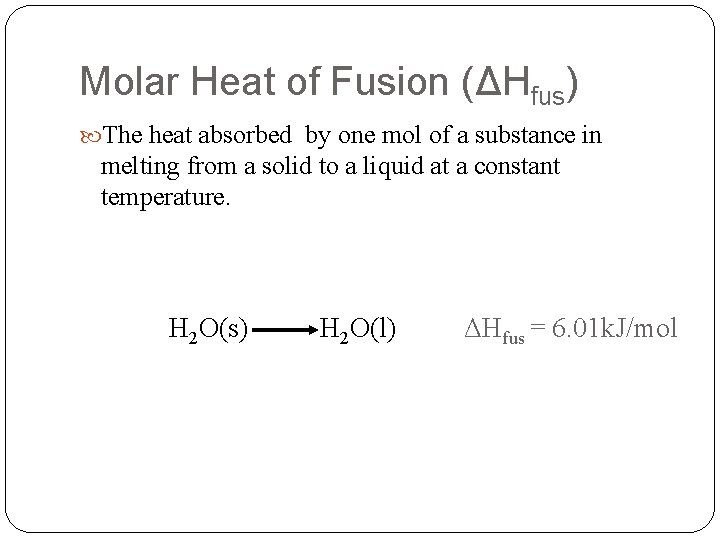
Molar Heat of Fusion (ΔHfus) The heat absorbed by one mol of a substance in melting from a solid to a liquid at a constant temperature. H 2 O(s) H 2 O(l) ΔHfus = 6. 01 k. J/mol

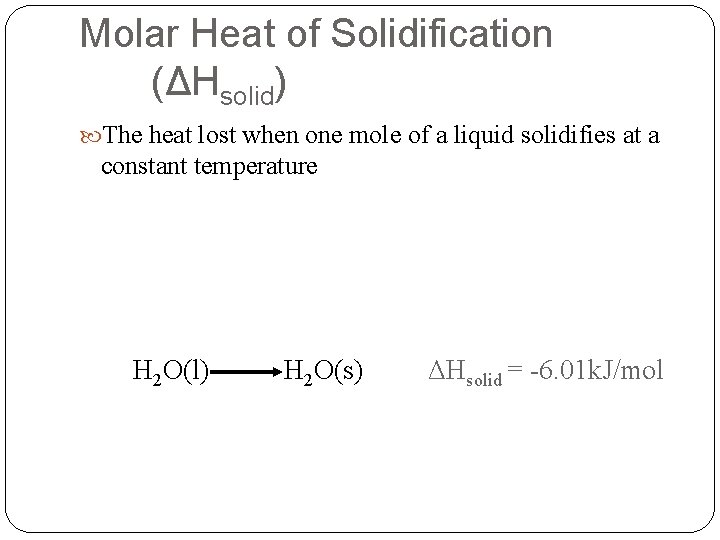
Molar Heat of Solidification (ΔHsolid) The heat lost when one mole of a liquid solidifies at a constant temperature H 2 O(l) H 2 O(s) ΔHsolid = -6. 01 k. J/mol

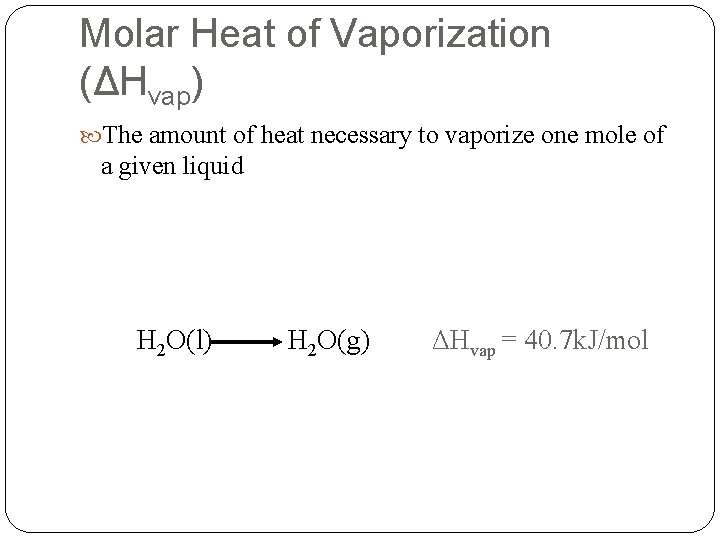
Molar Heat of Vaporization (ΔHvap) The amount of heat necessary to vaporize one mole of a given liquid H 2 O(l) H 2 O(g) ΔHvap = 40. 7 k. J/mol

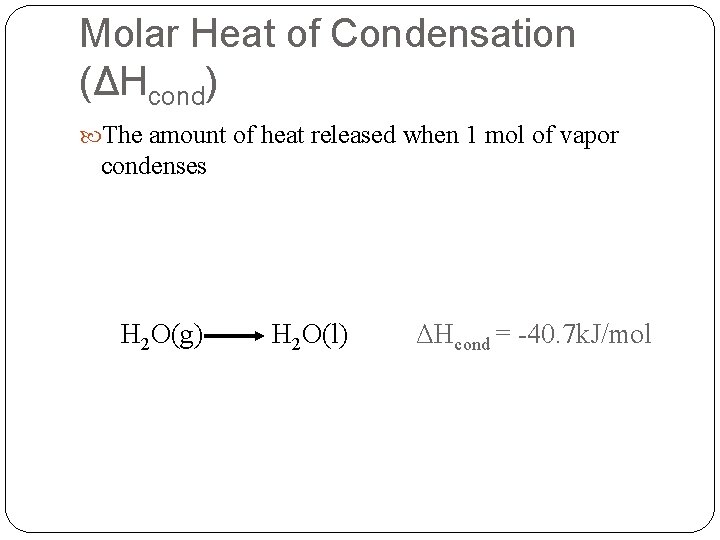
Molar Heat of Condensation (ΔHcond) The amount of heat released when 1 mol of vapor condenses H 2 O(g) H 2 O(l) ΔHcond = -40. 7 k. J/mol

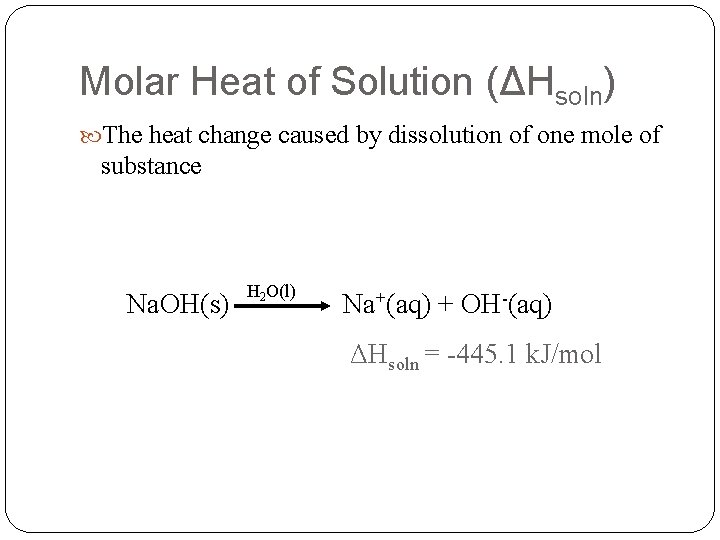
Molar Heat of Solution (ΔHsoln) The heat change caused by dissolution of one mole of substance Na. OH(s) H 2 O(l) Na+(aq) + OH-(aq) ΔHsoln = -445. 1 k. J/mol

Hess’s Law Indirect method to measure heat reaction If you add two or more thermochemical equations to give a final equation, then you can also add the heats of reaction to give the final heat of reaction

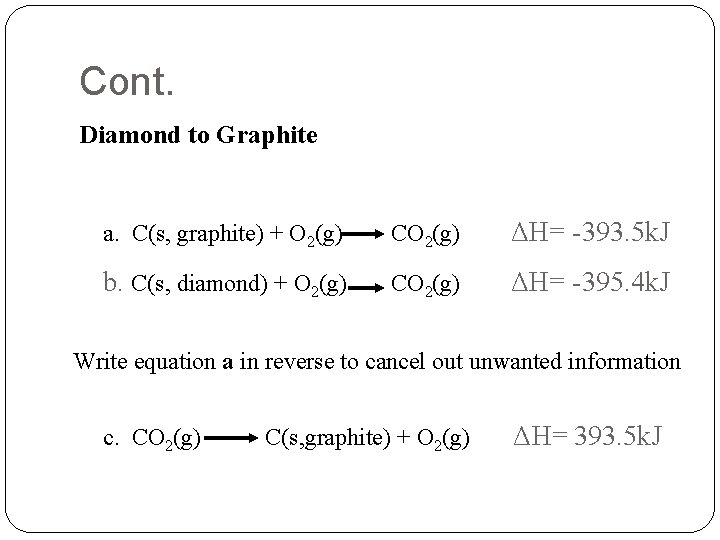
Cont. Diamond to Graphite a. C(s, graphite) + O 2(g) CO 2(g) ΔH= -393. 5 k. J b. C(s, diamond) + O 2(g) CO 2(g) ΔH= -395. 4 k. J Write equation a in reverse to cancel out unwanted information c. CO 2(g) C(s, graphite) + O 2(g) ΔH= 393. 5 k. J

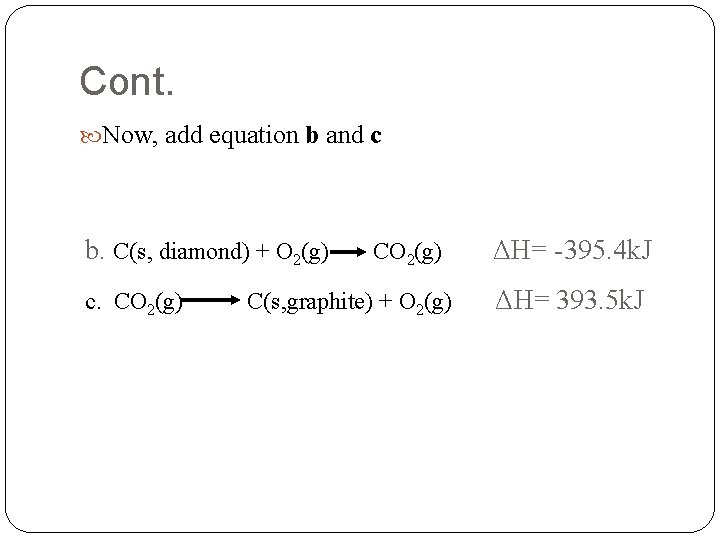
Cont. Now, add equation b and c b. C(s, diamond) + O 2(g) c. CO 2(g) C(s, graphite) + O 2(g) ΔH= -395. 4 k. J ΔH= 393. 5 k. J

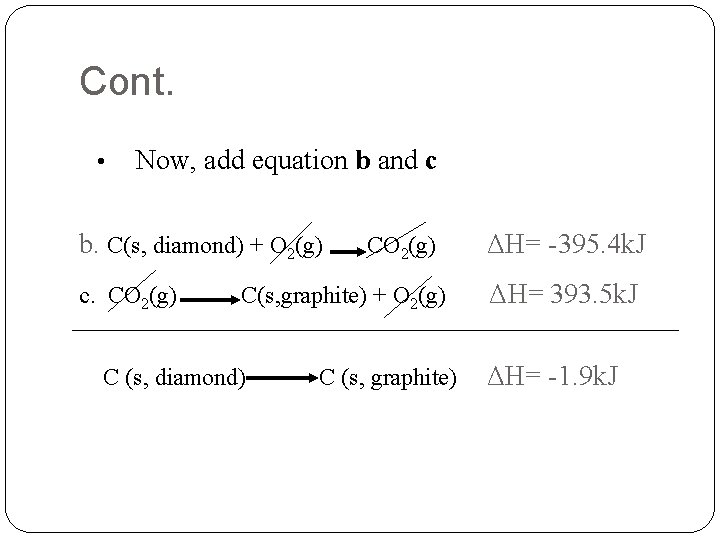
Cont. • Now, add equation b and c b. C(s, diamond) + O 2(g) c. CO 2(g) C(s, graphite) + O 2(g) C (s, diamond) C (s, graphite) ΔH= -395. 4 k. J ΔH= 393. 5 k. J ΔH= -1. 9 k. J

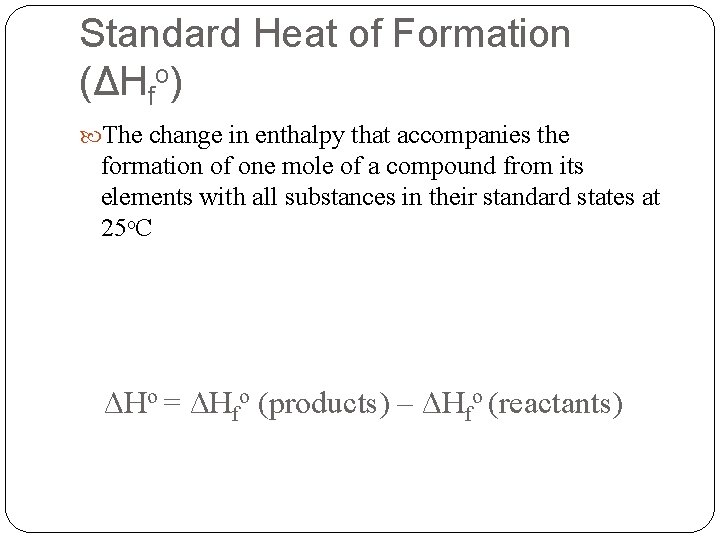
Standard Heat of Formation (ΔHfo) The change in enthalpy that accompanies the formation of one mole of a compound from its elements with all substances in their standard states at 25 o. C ΔHo = ΔHfo (products) – ΔHfo (reactants)
 Thermochemistry is study of: *
Thermochemistry is study of: * Thermochemistry is study of
Thermochemistry is study of Thermochemistry is concerned with the
Thermochemistry is concerned with the Elizabeth mulroney
Elizabeth mulroney Chemical vs physical change
Chemical vs physical change Specific latent heat of water
Specific latent heat of water Dry heat method examples
Dry heat method examples Thermal capacity
Thermal capacity Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công của trọng lực
Công của trọng lực Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phản ứng thế ankan
Phản ứng thế ankan Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi điện thế nghỉ
điện thế nghỉ Chúa sống lại
Chúa sống lại Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Phối cảnh
Phối cảnh Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất ưu thế lai là gì
ưu thế lai là gì Sơ đồ cơ thể người
Sơ đồ cơ thể người Tư thế ngồi viết
Tư thế ngồi viết Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể