Do Now Answer these in your notes 1


- Slides: 14

Do Now • Answer these in your notes: 1. Draw a coffee cup calorimeter in your notes. 2. How do we use a coffee cup calorimeter? 3. When looking at q (heat) how can we tell is a value is endothermic or exothermic?

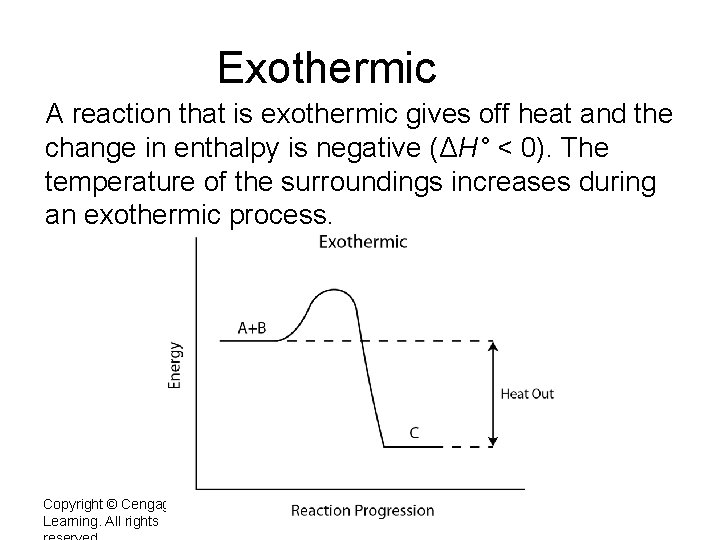
Exothermic A reaction that is exothermic gives off heat and the change in enthalpy is negative (ΔH° < 0). The temperature of the surroundings increases during an exothermic process. Copyright © Cengage Learning. All rights 2

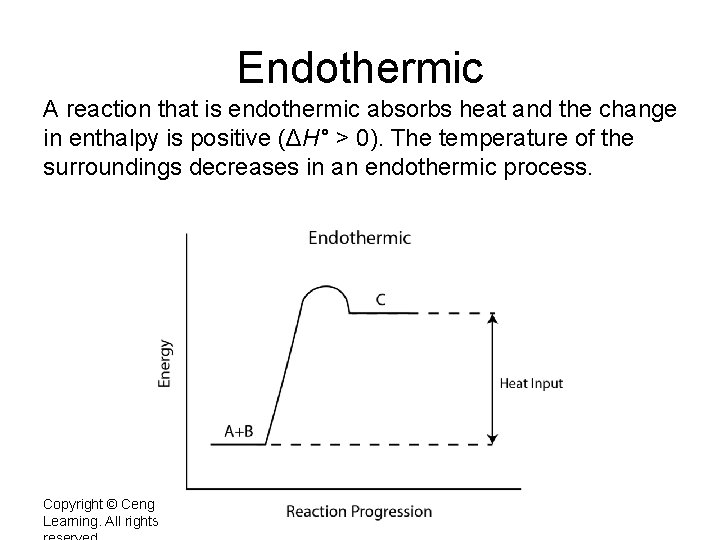
Endothermic A reaction that is endothermic absorbs heat and the change in enthalpy is positive (ΔH° > 0). The temperature of the surroundings decreases in an endothermic process. Copyright © Cengage Learning. All rights 3

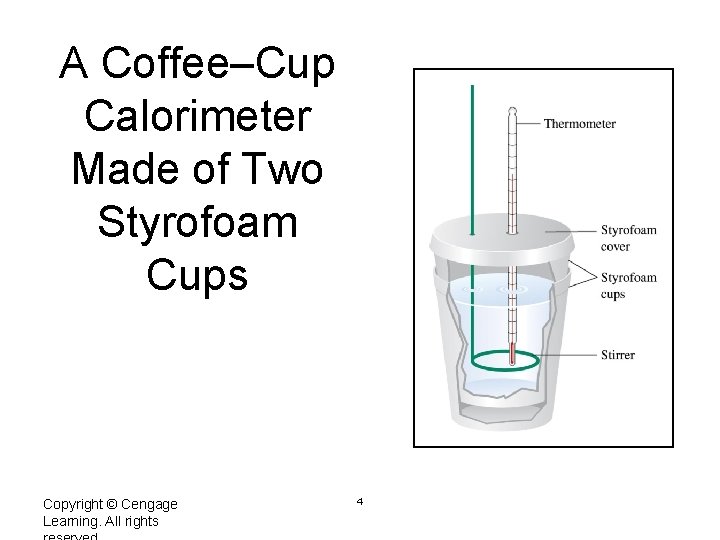
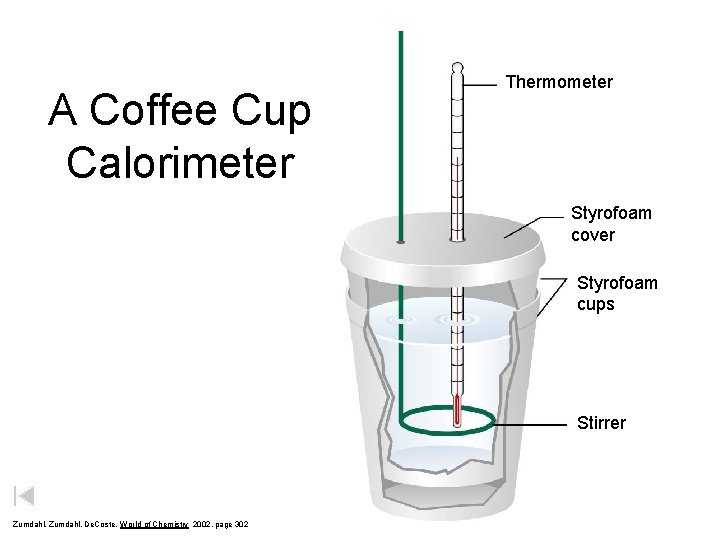
A Coffee–Cup Calorimeter Made of Two Styrofoam Cups Copyright © Cengage Learning. All rights 4

Calorimetry • Calorimetry is the measurement of heat flow. • It allows us to calculate the amount of energy required to heat up a substance or to make a substance change states.

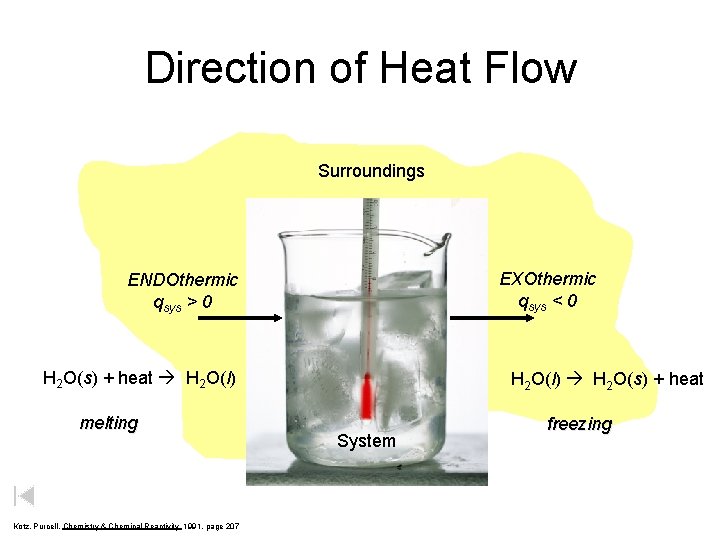
Direction of Heat Flow Surroundings ENDOthermic qsys > 0 System H 2 O(s) + heat H 2 O(l) melting Kotz, Purcell, Chemistry & Chemical Reactivity 1991, page 207 EXOthermic qsys < 0 H 2 O(l) H 2 O(s) + heat System freezing

Experimental Determination of Specific Heat of a Metal Typical apparatus used in this activity include a boiler (such as large glass beaker), a heat source (Bunsen burner or hot plate), a stand or tripod for the boiler, a calorimeter, thermometers, samples (typically samples of copper, aluminum, zinc, tin, or lead), tongs (or forceps or string) to handle samples, and a balance.

A Coffee Cup Calorimeter Thermometer Styrofoam cover Styrofoam cups Stirrer Zumdahl, De. Coste, World of Chemistry 2002, page 302

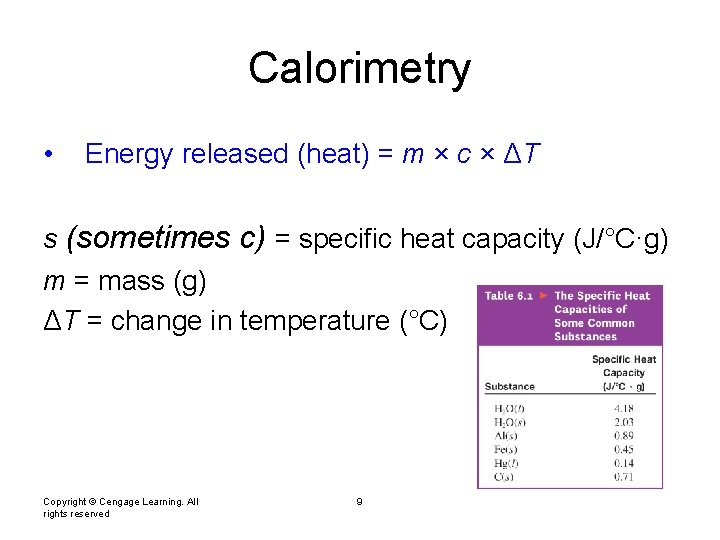
Calorimetry • Energy released (heat) = m × c × ΔT s (sometimes c) = specific heat capacity (J/°C·g) m = mass (g) ΔT = change in temperature (°C) Copyright © Cengage Learning. All rights reserved 9

Calorimetry • Experimental way of measuring heat generation/consumption by essentially catching all the heat energy in a water bath or water bath + metal apparatus. • Coffee Cup Calorimetry • Styrofoam cup insulates the contents so any heat generated or consumed in the water can be measured by the temperature change • -qmetal = m. CH 2 O∆T

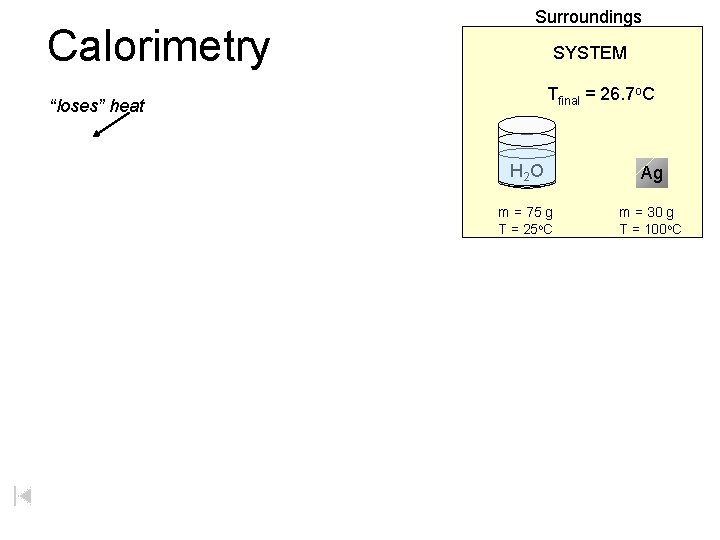
Calorimetry Surroundings SYSTEM Tfinal = 26. 7 o. C “loses” heat H 2 O Ag m = 75 g T = 25 o. C m = 30 g T = 100 o. C

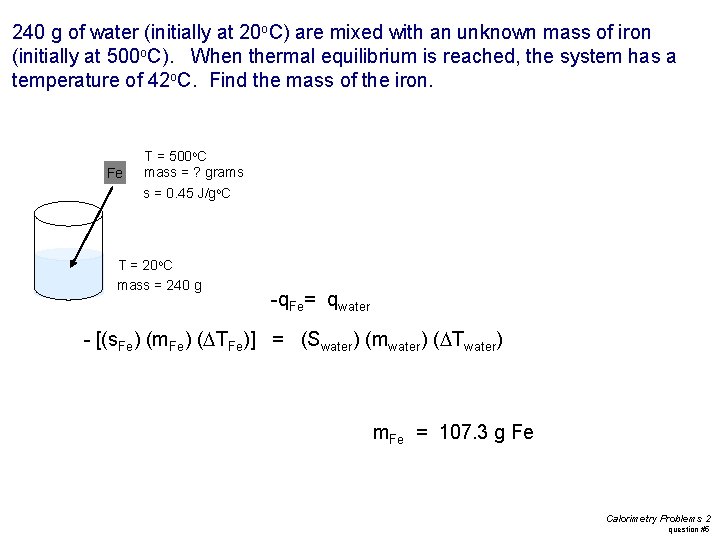
240 g of water (initially at 20 o. C) are mixed with an unknown mass of iron (initially at 500 o. C). When thermal equilibrium is reached, the system has a temperature of 42 o. C. Find the mass of the iron. Fe T = 500 o. C mass = ? grams s = 0. 45 J/go. C T = 20 o. C mass = 240 g -q. Fe= qwater - [(s. Fe) (m. Fe) (DTFe)] = (Swater) (mwater) (DTwater) m. Fe = 107. 3 g Fe Calorimetry Problems 2 question #5

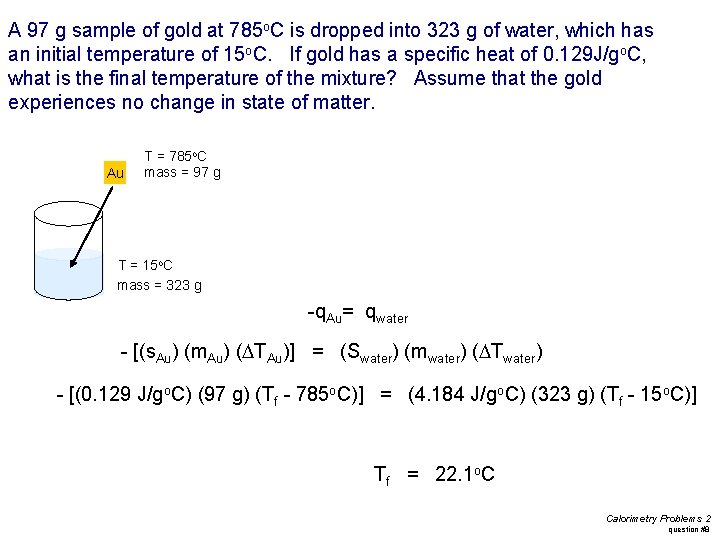
A 97 g sample of gold at 785 o. C is dropped into 323 g of water, which has an initial temperature of 15 o. C. If gold has a specific heat of 0. 129 J/go. C, what is the final temperature of the mixture? Assume that the gold experiences no change in state of matter. Au T = 785 o. C mass = 97 g T = 15 o. C mass = 323 g -q. Au= qwater - [(s. Au) (m. Au) (DTAu)] = (Swater) (mwater) (DTwater) - [(0. 129 J/go. C) (97 g) (Tf - 785 o. C)] = (4. 184 J/go. C) (323 g) (Tf - 15 o. C)] Tf = 22. 1 o. C Calorimetry Problems 2 question #8

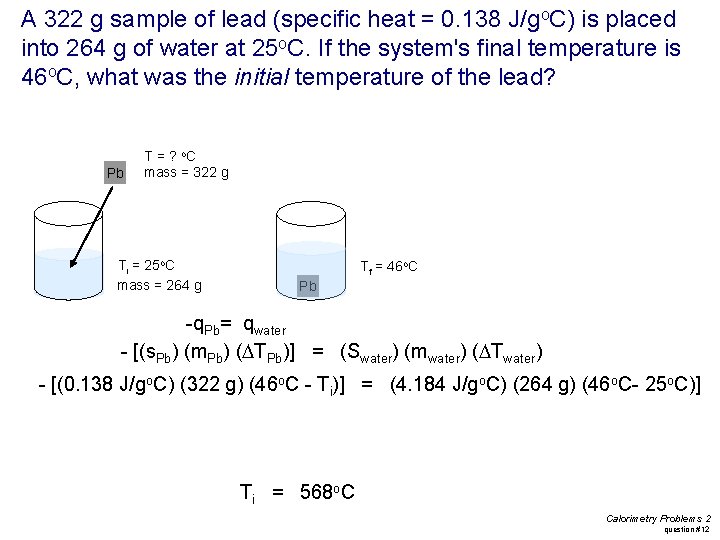
A 322 g sample of lead (specific heat = 0. 138 J/go. C) is placed into 264 g of water at 25 o. C. If the system's final temperature is 46 o. C, what was the initial temperature of the lead? Pb T = ? o. C mass = 322 g Ti = 25 o. C mass = 264 g Tf = 46 o. C Pb -q. Pb= qwater - [(s. Pb) (m. Pb) (DTPb)] = (Swater) (mwater) (DTwater) - [(0. 138 J/go. C) (322 g) (46 o. C - Ti)] = (4. 184 J/go. C) (264 g) (46 o. C- 25 o. C)] Ti = 568 o. C Calorimetry Problems 2 question #12