Thermochemistry Dr Ron Rusay Energy Joules J calorie















![Hrxn° = [1 Hf (c) + 2 Hf (d)] [1 Hf (a) + Hrxn° = [1 Hf (c) + 2 Hf (d)] [1 Hf (a) +](https://slidetodoc.com/presentation_image_h/1ebbdb5c0be7468e66f64e88e7e2cb3f/image-38.jpg)












- Slides: 64

Thermochemistry Dr. Ron Rusay

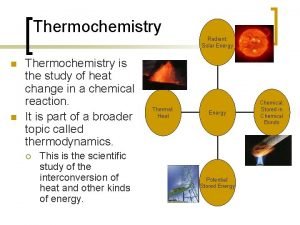
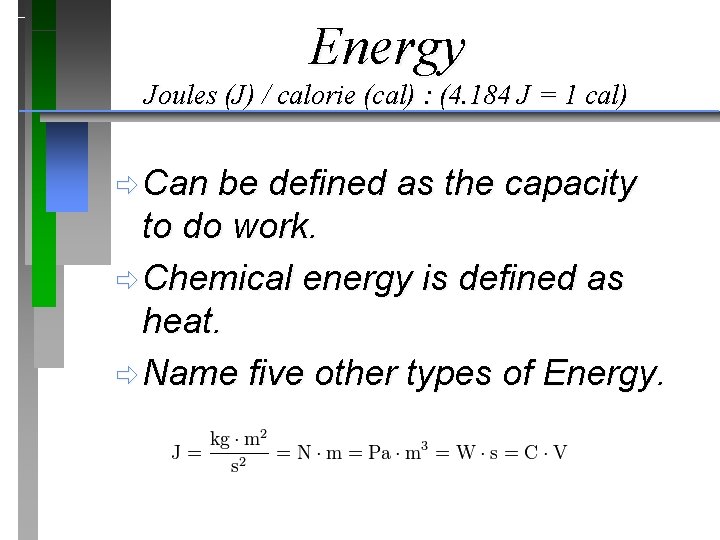
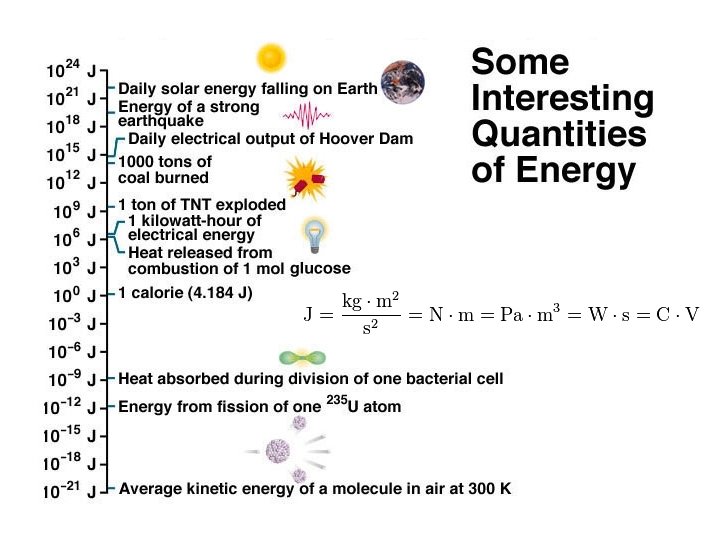
Energy Joules (J) / calorie (cal) : (4. 184 J = 1 cal) ð Can be defined as the capacity to do work. ð Chemical energy is defined as heat. ð Name five other types of Energy.

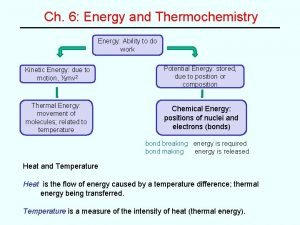
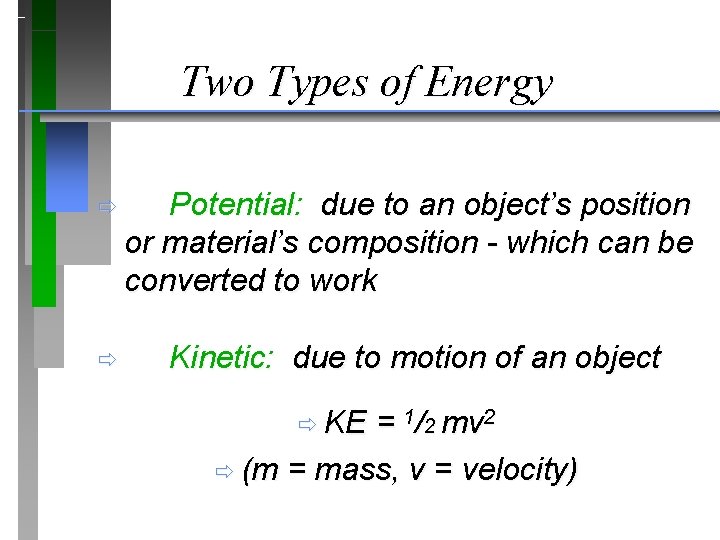
Two Types of Energy ð Potential: due to an object’s position or material’s composition - which can be converted to work ð Kinetic: due to motion of an object ð KE = 1/2 mv 2 ð (m = mass, v = velocity)


Law of Conservation of Energy ð Different forms of energy can be inter -converted but can neither be created nor destroyed. ð (Euniverse ð Describe is constant) three inter-conversions of energy.

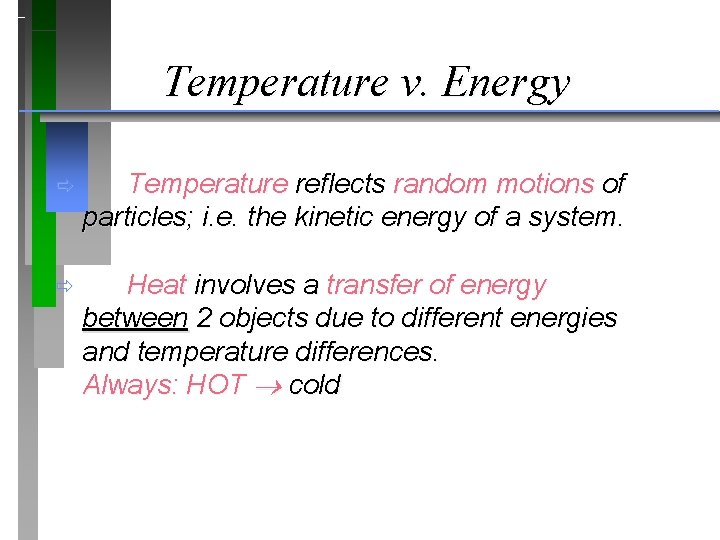
Temperature v. Energy ð Temperature reflects random motions of particles; i. e. the kinetic energy of a system. ð Heat involves a transfer of energy between 2 objects due to different energies and temperature differences. Always: HOT cold

Heat (Energy) Loss

Energy: A State Function ð ð Depends only on the state of the system - not the path of how it arrived at that state. It is independent of pathway.

System and Surroundings ð System: That on which we focus attention ð Surroundings: Everything else in the universe ð Universe = System + Surroundings


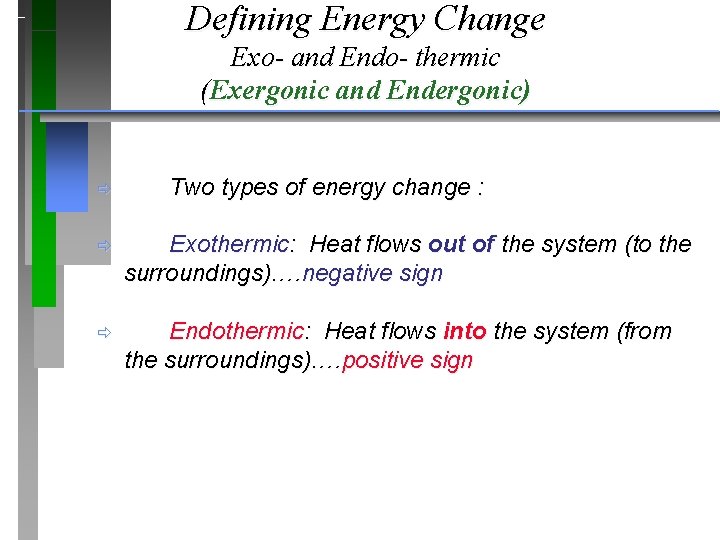
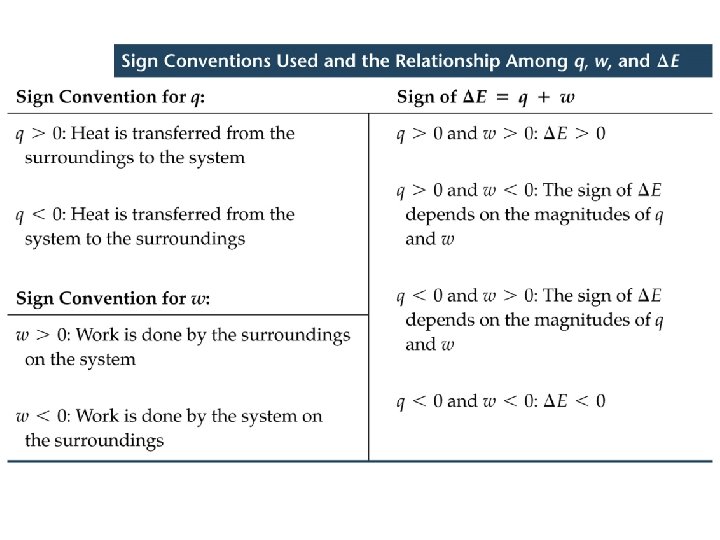
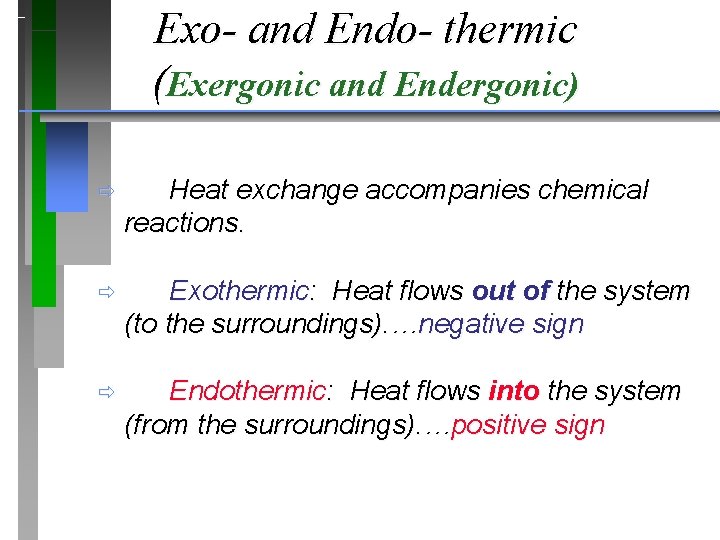
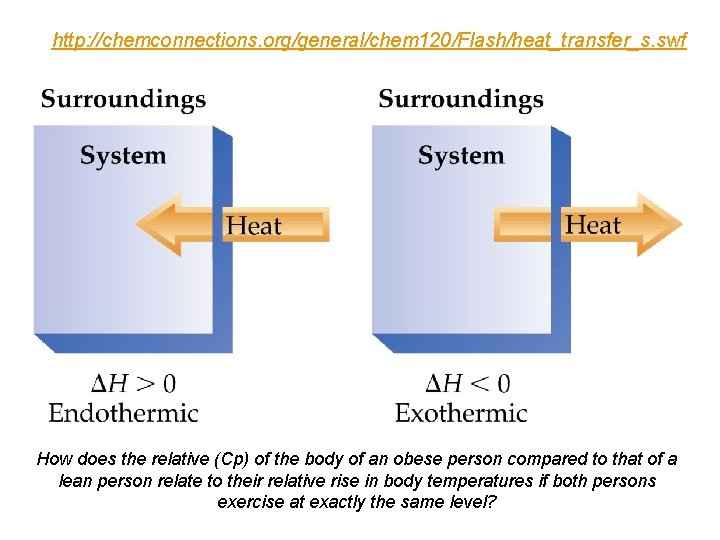
Defining Energy Change Exo- and Endo- thermic (Exergonic and Endergonic) ð Two types of energy change : ð Exothermic: Heat flows out of the system (to the surroundings). …negative sign ð Endothermic: Heat flows into the system (from the surroundings). …positive sign

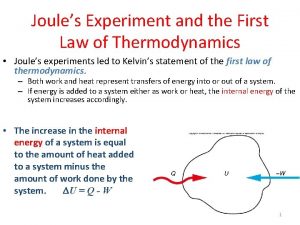
First of Three Laws of Thermodynamics First Law of Thermodynamics: The energy of the universe is constant or “energy is conserved”. ð

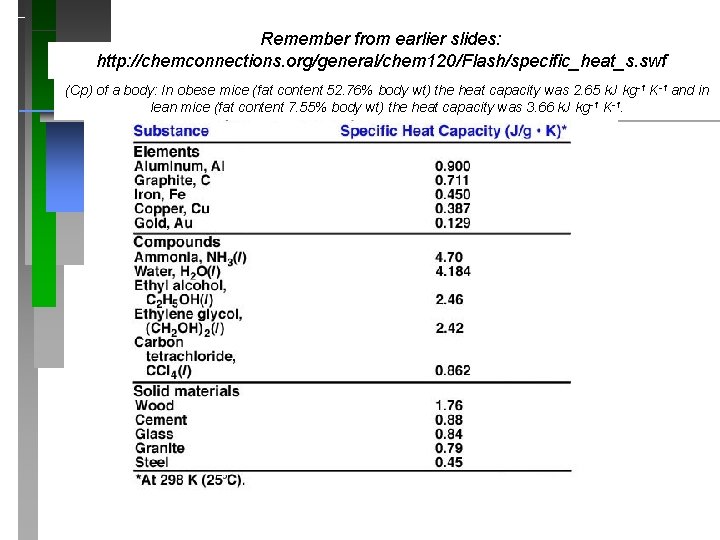
Heat Capacity (Specific Heat) P O http: //chemconnections. org/general/chem 120/Flash/specific_heat_s. swf

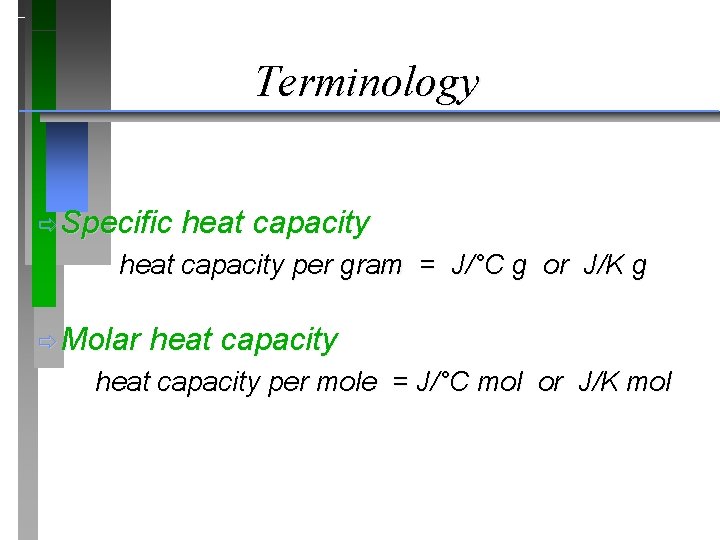
Terminology ð Specific heat capacity per gram = J/°C g or J/K g ð Molar heat capacity per mole = J/°C mol or J/K mol

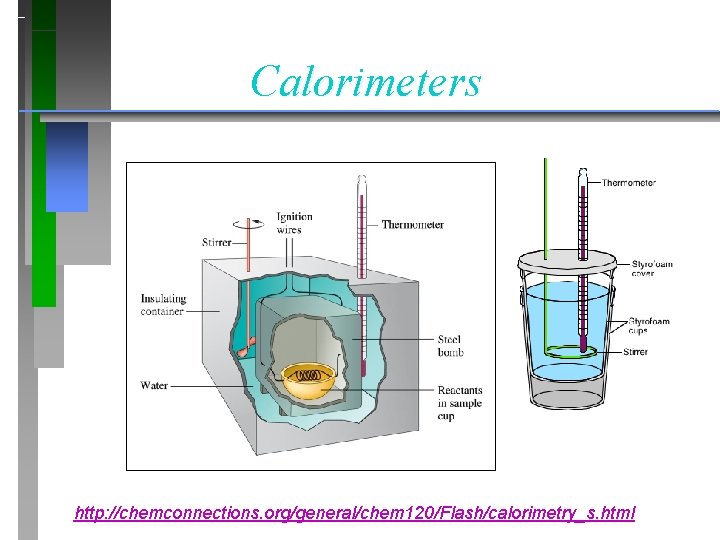
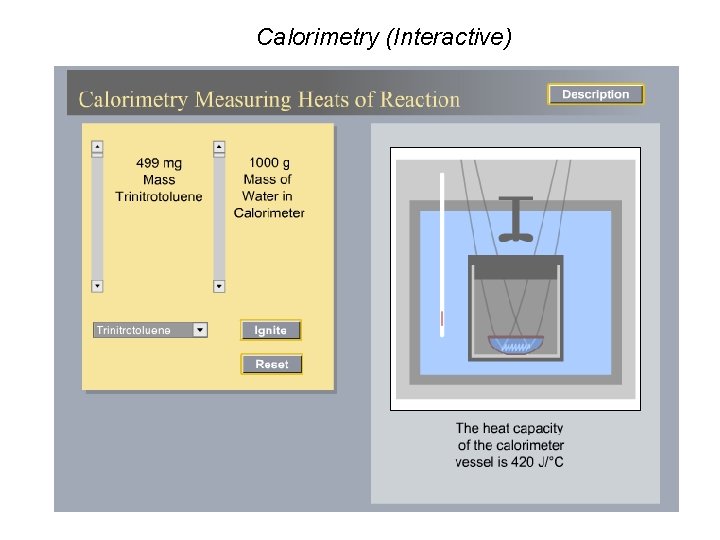
Calorimeters http: //chemconnections. org/general/chem 120/Flash/calorimetry_s. html

QUESTION

Heat Capacities

QUESTION

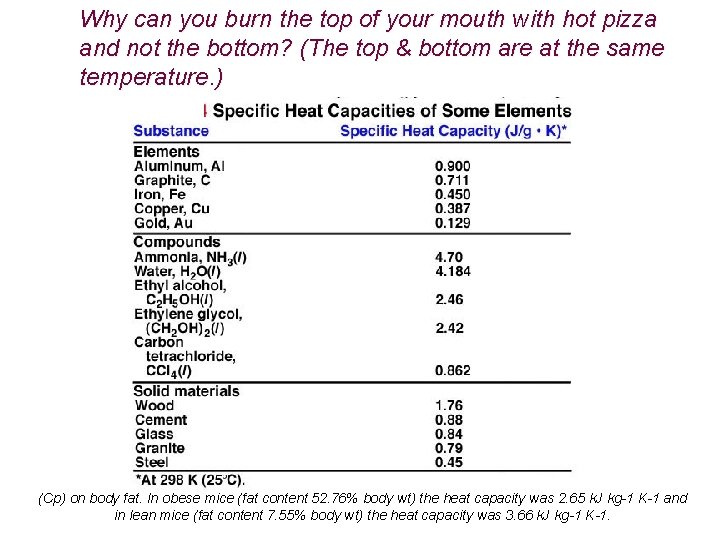
Why can you burn the top of your mouth with hot pizza and not the bottom? (The top & bottom are at the same temperature!) http: //www. dailymotion. com/video/x 3 hfwx_the-science-of-pizza_people

Why can you burn the top of your mouth with hot pizza and not the bottom? (The top & bottom are at the same temperature. ) (Cp) on body fat. In obese mice (fat content 52. 76% body wt) the heat capacity was 2. 65 k. J kg-1 K-1 and in lean mice (fat content 7. 55% body wt) the heat capacity was 3. 66 k. J kg-1 K-1.

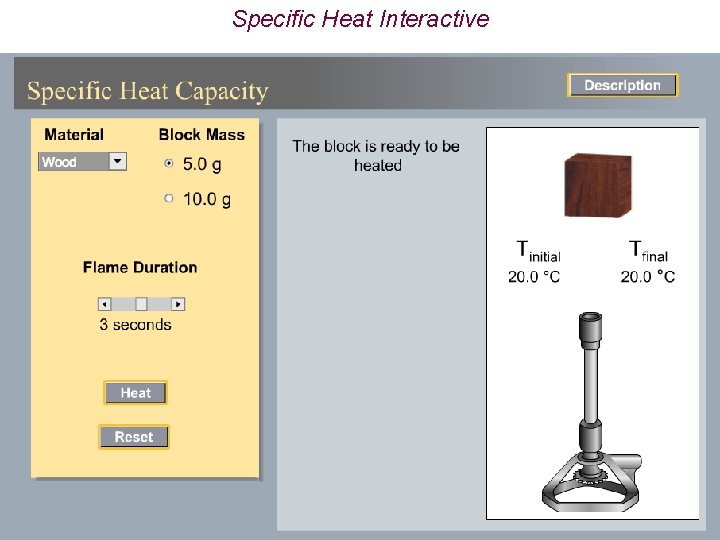
Specific Heat Interactive

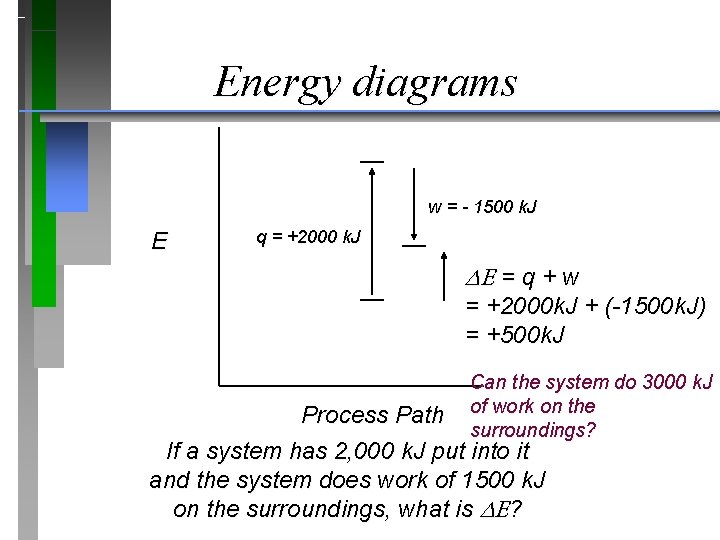
Energy diagrams w = - 1500 k. J E q = +2000 k. J = q + w = +2000 k. J + (-1500 k. J) = +500 k. J Can the system do 3000 k. J of work on the surroundings? Process Path If a system has 2, 000 k. J put into it and the system does work of 1500 k. J on the surroundings, what is ?

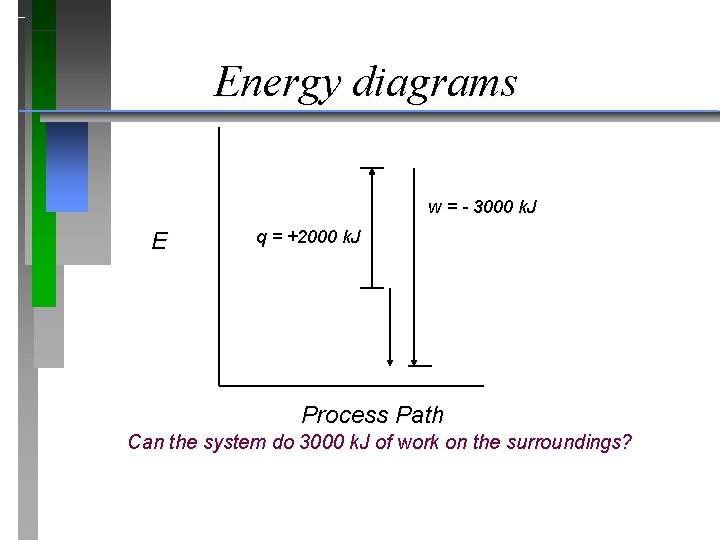
Energy diagrams w = - 3000 k. J E q = +2000 k. J Process Path Can the system do 3000 k. J of work on the surroundings?

QUESTION


QUESTION


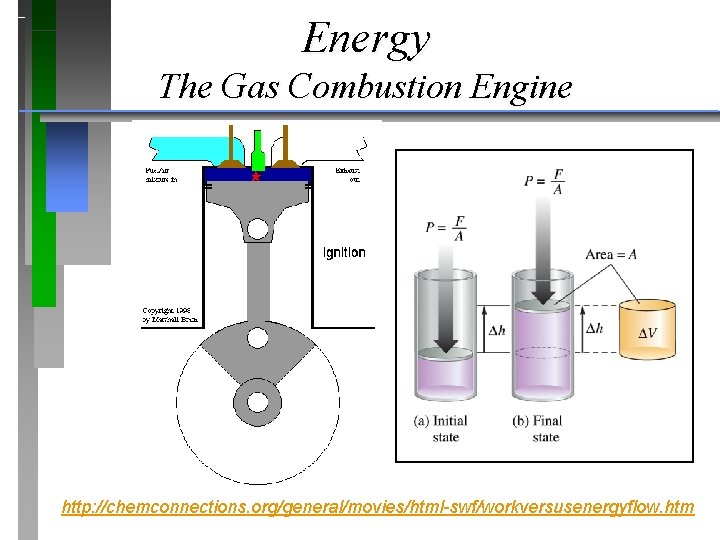
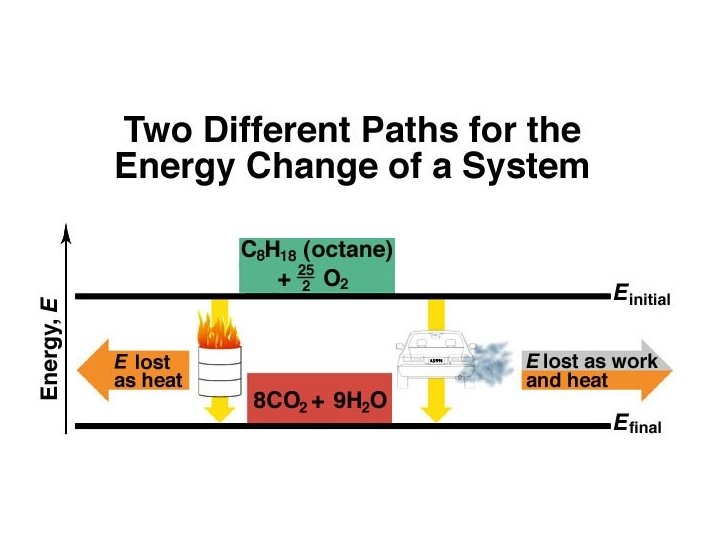
Energy The Gas Combustion Engine http: //chemconnections. org/general/movies/html-swf/workversusenergyflow. htm


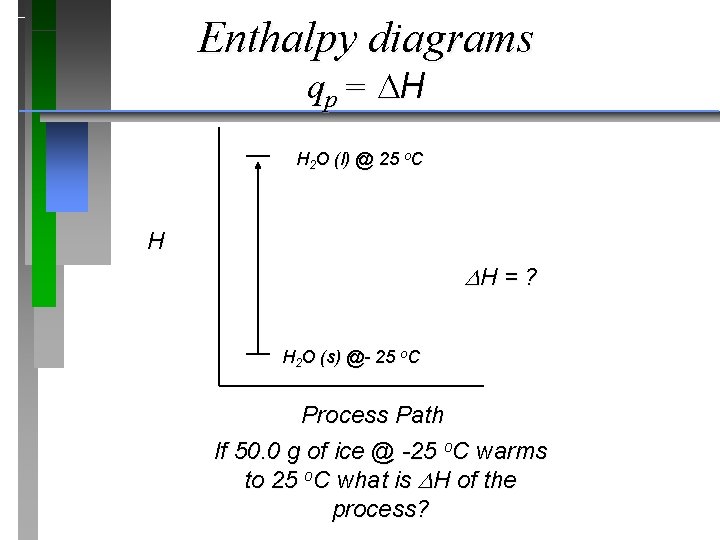
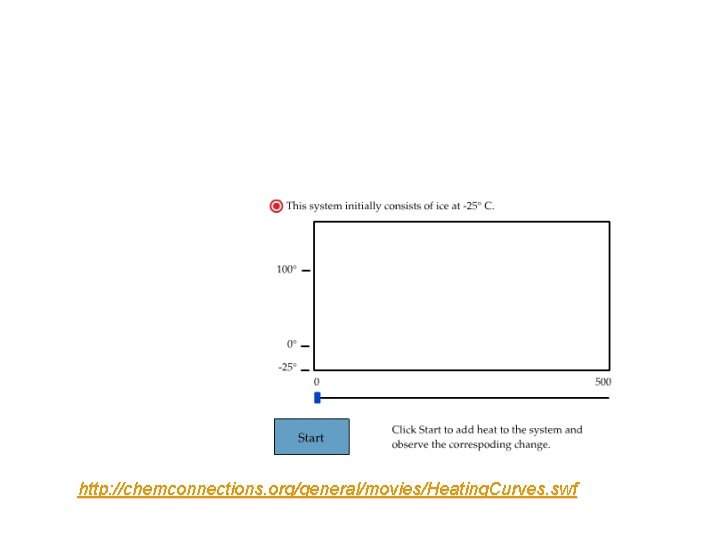
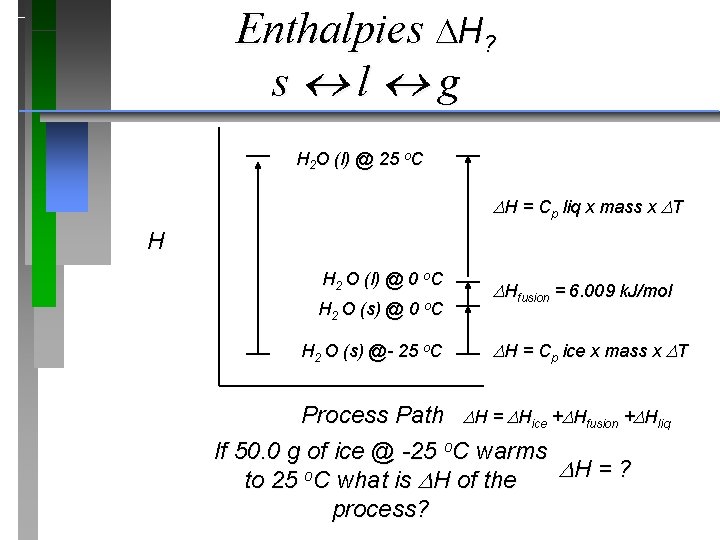
Enthalpy diagrams qp = H H 2 O (l) @ 25 o. C H H = ? H 2 O (s) @- 25 o. C Process Path If 50. 0 g of ice @ -25 o. C warms to 25 o. C what is H of the process?

http: //chemconnections. org/general/movies/Heating. Curves. swf

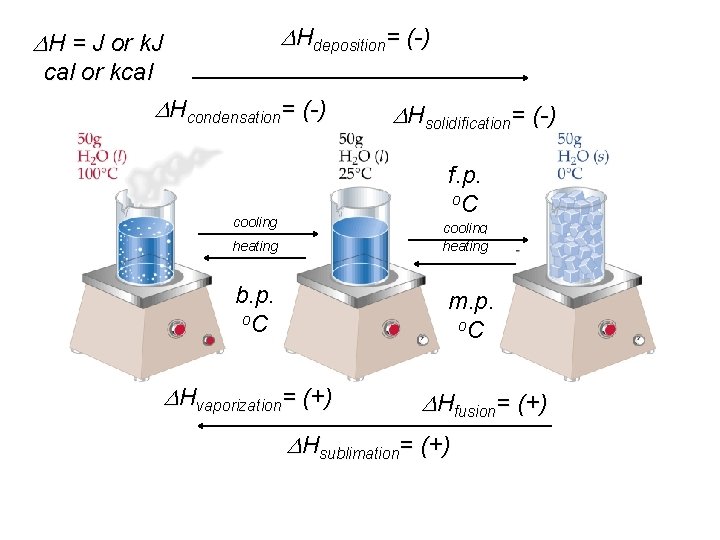
Hdeposition= (-) H = J or k. J cal or kcal Hcondensation= (-) Hsolidification= (-) f. p. o. C cooling heating b. p. o. C m. p. o. C Hvaporization= (+) Hfusion= (+) Hsublimation= (+)

Enthalpies H? s l g H 2 O (l) @ 25 o. C H = Cp liq x mass x T H H 2 O (l) @ 0 o. C H 2 O (s) @- 25 o. C Hfusion = 6. 009 k. J/mol H = Cp ice x mass x T Process Path H = Hice + Hfusion + Hliq If 50. 0 g of ice @ -25 o. C warms H = ? to 25 o. C what is H of the process?

QUESTION

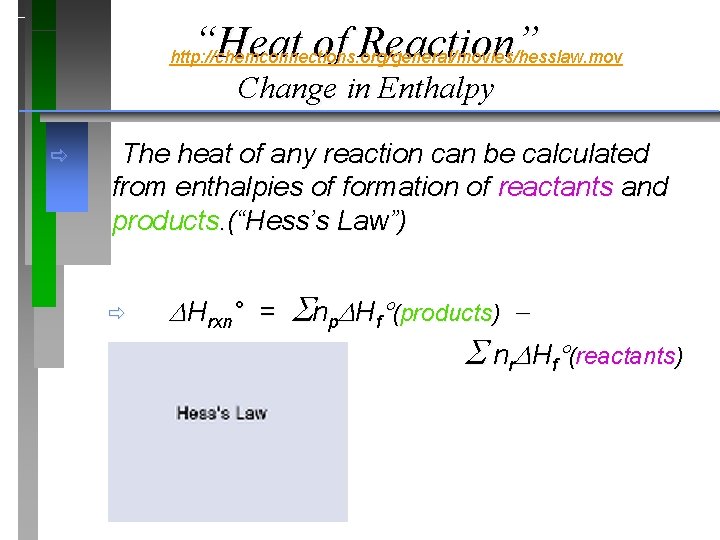
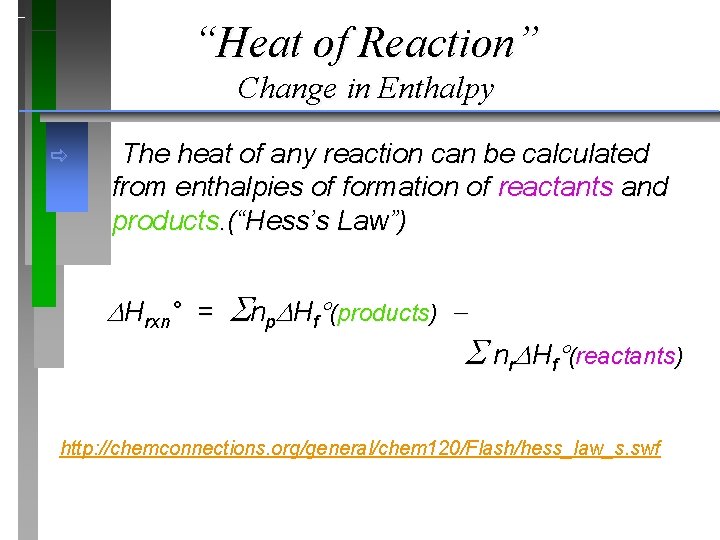
“Heat of Reaction” http: //chemconnections. org/general/movies/hesslaw. mov Change in Enthalpy ð The heat of any reaction can be calculated from enthalpies of formation of reactants and products. (“Hess’s Law”) ð Hrxn° = np Hf (products) nr Hf (reactants)

QUESTION A) 4675 k. J B) -1545 k. J D) -1720 k. J C) -290 k. J

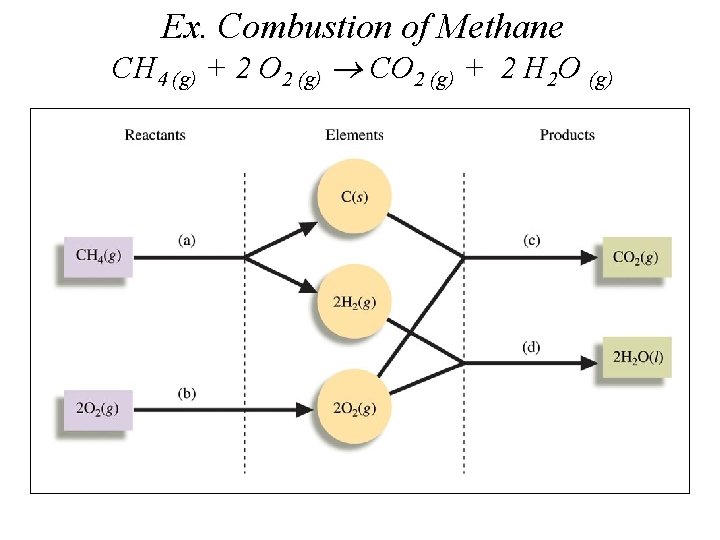
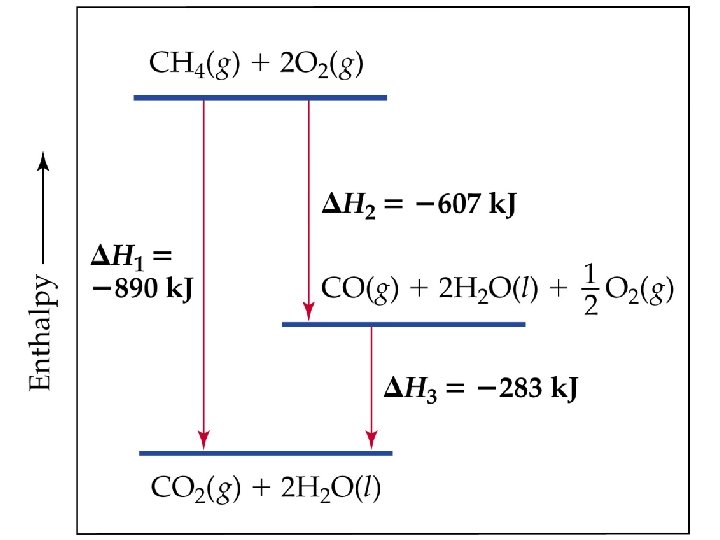
Ex. Combustion of Methane CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g)
![Hrxn 1 Hf c 2 Hf d 1 Hf a Hrxn° = [1 Hf (c) + 2 Hf (d)] [1 Hf (a) +](https://slidetodoc.com/presentation_image_h/1ebbdb5c0be7468e66f64e88e7e2cb3f/image-38.jpg)
Hrxn° = [1 Hf (c) + 2 Hf (d)] [1 Hf (a) + 2 Hf (b)] - Hrxn°= [-394 k. J+(-572 k. J)]-[-75 k. J+0 k. J]= -891 k. J

QUESTION

Exo- and Endo- thermic (Exergonic and Endergonic) ð Heat exchange accompanies chemical reactions. ð Exothermic: Heat flows out of the system (to the surroundings). …negative sign ð Endothermic: Heat flows into the system (from the surroundings). …positive sign

QUESTION

Remember from earlier slides: http: //chemconnections. org/general/chem 120/Flash/specific_heat_s. swf (Cp) of a body: In obese mice (fat content 52. 76% body wt) the heat capacity was 2. 65 k. J kg -1 K-1 and in lean mice (fat content 7. 55% body wt) the heat capacity was 3. 66 k. J kg-1 K-1.

http: //chemconnections. org/general/chem 120/Flash/heat_transfer_s. swf How does the relative (Cp) of the body of an obese person compared to that of a lean person relate to their relative rise in body temperatures if both persons exercise at exactly the same level?

“Heat of Reaction” Change in Enthalpy ð The heat of any reaction can be calculated from enthalpies of formation of reactants and products. (“Hess’s Law”) Hrxn° = np Hf (products) nr Hf (reactants) http: //chemconnections. org/general/chem 120/Flash/hess_law_s. swf

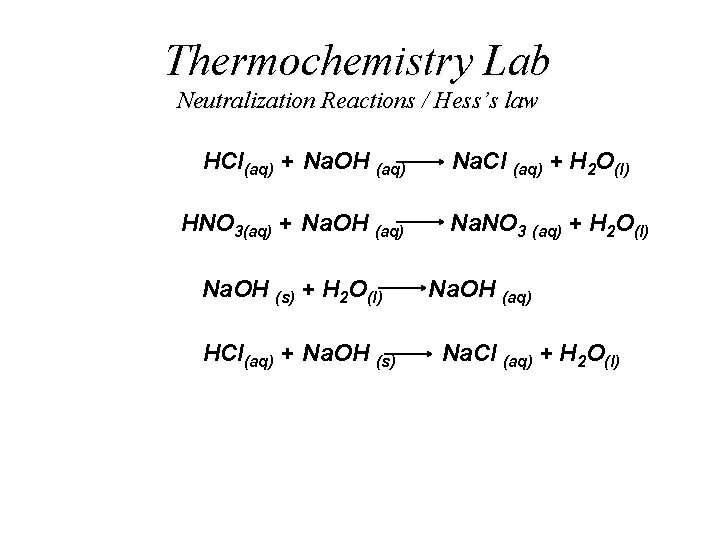
Thermochemistry Lab Neutralization Reactions / Hess’s law HCl(aq) + Na. OH (aq) HNO 3(aq) + Na. OH (aq) Na. OH (s) + H 2 O(l) HCl(aq) + Na. OH (s) Na. Cl (aq) + H 2 O(l) Na. NO 3 (aq) + H 2 O(l) Na. OH (aq) Na. Cl (aq) + H 2 O(l)

Calorimetry (Interactive)

Thermochemistry Lab Neutralization Reactions / Hess’s law Tfinal Tinitial

QUESTION

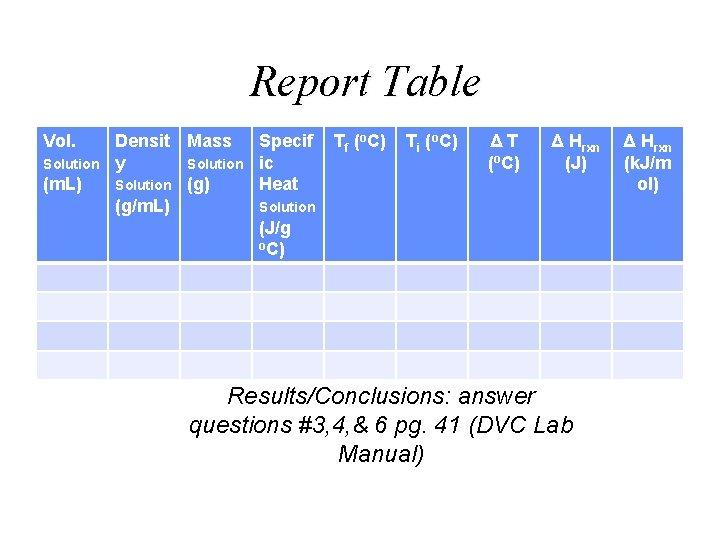
Report Table Vol. Solution (m. L) Densit Mass Specif y Solution ic Solution (g) Heat (g/m. L) Solution (J/g o. C) Tf (o. C) Ti (o. C) ΔT (o. C) Δ Hrxn (J) Results/Conclusions: answer questions #3, 4, & 6 pg. 41 (DVC Lab Manual) Δ Hrxn (k. J/m ol)

QUESTION

QUESTION


Exothermic Reaction http: //www. youtube. com/watch? v=rd. Csb. Zf 1_Ng


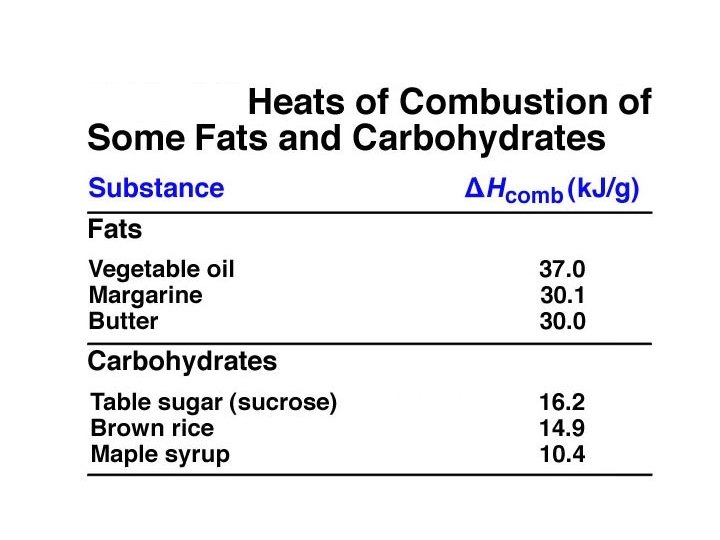
Heats of Combustion of octane releases 5, 470 k. J per mole of octane ( Hcomb = -5, 470 k. J/mol) ð How many gallons of water can be boiled by burning 1 gallon of gasoline? (Assume the water is at 25 o. C) ð How many grams of fat have the equivalent combustion energy as 1 gallon of gasoline? ð How many pounds of CO 2 are added to atmosphere from burning 1 gallon of gasoline? (This question relates to the Greenhouse Gas ð Workshop. )

Endothermic Reaction

QUESTION http: //chemconnections. org/general/chem 120/Flash/calorimetry_s. swf

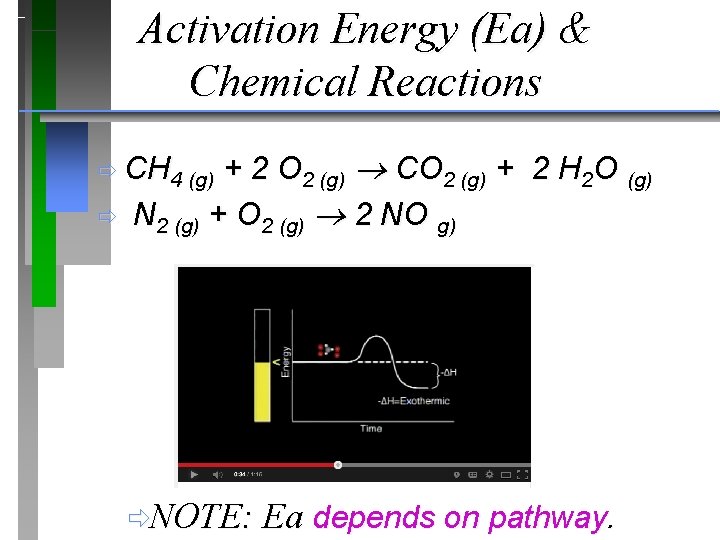
Activation Energy (Ea) & Chemical Reactions + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) N 2 (g) + O 2 (g) 2 NO g) ð CH 4 (g) ð ðNOTE: Ea depends on pathway.

Hess’s Law Continued ð Reactants ð Products H = + (endothermic); H = - (exothermic) ð The change in enthalpy is the same whether the reaction takes place in one step or a series of steps.



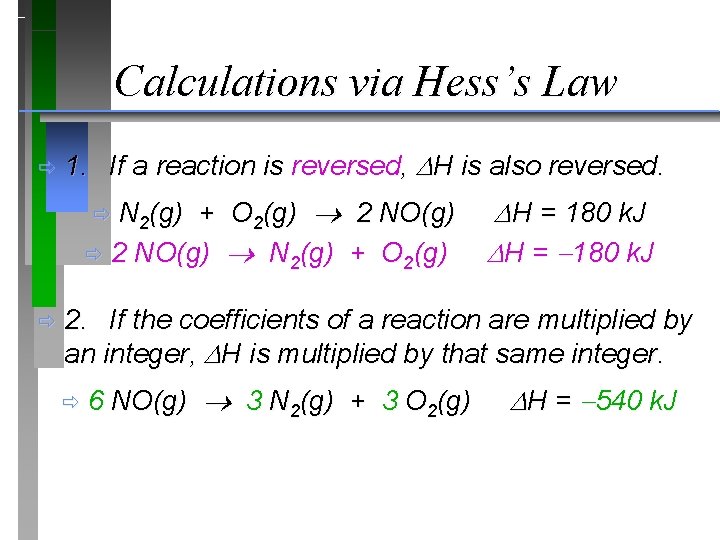
Calculations via Hess’s Law ð 1. If a reaction is reversed, H is also reversed. N 2(g) + O 2(g) 2 NO(g) ð 2 NO(g) N 2(g) + O 2(g) ð ð H = 180 k. J 2. If the coefficients of a reaction are multiplied by an integer, H is multiplied by that same integer. ð 6 NO(g) 3 N 2(g) + 3 O 2(g) H = 540 k. J

QUESTION

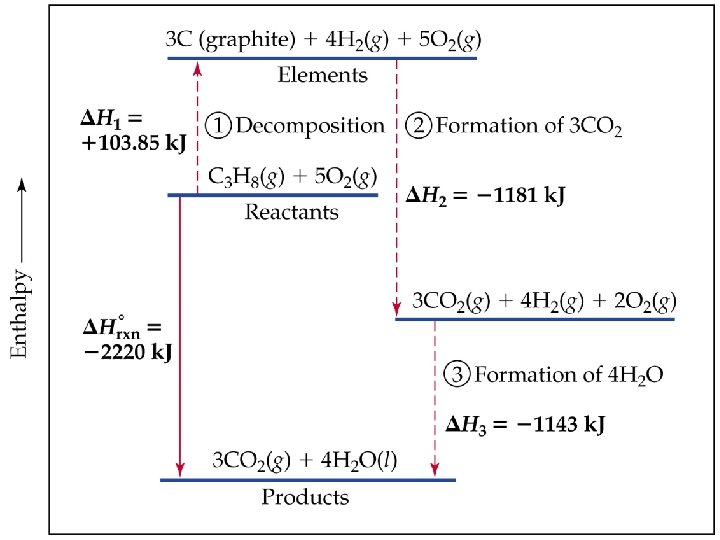
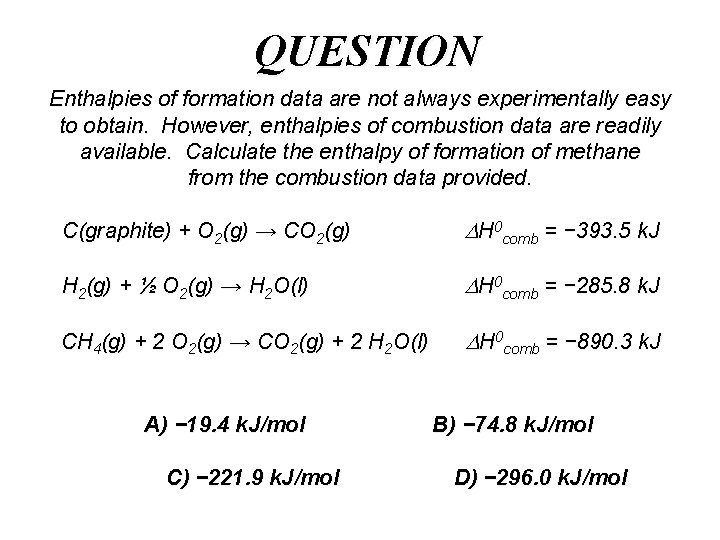
QUESTION Enthalpies of formation data are not always experimentally easy to obtain. However, enthalpies of combustion data are readily available. Calculate the enthalpy of formation of methane from the combustion data provided. C(graphite) + O 2(g) → CO 2(g) H 0 comb = − 393. 5 k. J H 2(g) + ½ O 2(g) → H 2 O(l) H 0 comb = − 285. 8 k. J CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l) H 0 comb = − 890. 3 k. J A) − 19. 4 k. J/mol C) − 221. 9 k. J/mol B) − 74. 8 k. J/mol D) − 296. 0 k. J/mol
 Rusay
Rusay Joule energy examples
Joule energy examples Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry Cronies calorie restriction
Cronies calorie restriction Différence entre calorie et kilocalorie
Différence entre calorie et kilocalorie How big is 3 oz of chicken
How big is 3 oz of chicken My plate livestrong
My plate livestrong Calorie dragibus
Calorie dragibus Sesso calorie
Sesso calorie Raw milk fat percentage
Raw milk fat percentage Calorie carote
Calorie carote Calorie restriction
Calorie restriction Noci calorie
Noci calorie Laf nutrizione
Laf nutrizione Every calorie counts
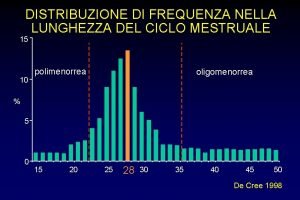
Every calorie counts Oligomenorrea
Oligomenorrea Calcolo calorie
Calcolo calorie Calorie is defined as
Calorie is defined as Perdite di sangue in menopausa
Perdite di sangue in menopausa Harris-benedict -basal metabolic rate calorie calc
Harris-benedict -basal metabolic rate calorie calc Diet chart for diabetic patients
Diet chart for diabetic patients Equivalente meccanico del calore
Equivalente meccanico del calore Enfamil gentlease 24 calorie formula recipe
Enfamil gentlease 24 calorie formula recipe Endotermico
Endotermico Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis The diagram below shows a toy cart possessing 16 joules
The diagram below shows a toy cart possessing 16 joules Angela uses a force of 25 newtons
Angela uses a force of 25 newtons Joule experiment for first law of thermodynamics
Joule experiment for first law of thermodynamics Joules to newtons
Joules to newtons Igcse physics circuit symbols
Igcse physics circuit symbols Monophasic vs biphasic defibrillator joules
Monophasic vs biphasic defibrillator joules Standard conditions vs stp
Standard conditions vs stp Scalar units
Scalar units Momentum to joules
Momentum to joules 4 184 joules
4 184 joules Ritmos chocaveis
Ritmos chocaveis Electric potential from electric field
Electric potential from electric field Joules poore
Joules poore Colocacion palas desfibrilador
Colocacion palas desfibrilador W=fxd
W=fxd Qxa=e
Qxa=e Atomo cargado negativamente
Atomo cargado negativamente Formula of energy
Formula of energy Chapter 14 review physical science
Chapter 14 review physical science Gibbs free energy unit
Gibbs free energy unit Chapter 17 thermochemistry practice problems answers
Chapter 17 thermochemistry practice problems answers Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is study of
Thermochemistry is study of Thermochemical equation
Thermochemical equation Thermochemistry
Thermochemistry Gaussian thermochemistry
Gaussian thermochemistry Thermochemical equation
Thermochemical equation Thermochemistry clipart
Thermochemistry clipart Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Thermochemistry is a study of
Thermochemistry is a study of Thermochemistry equations
Thermochemistry equations Thermochemistry one pager
Thermochemistry one pager Thermochemical equation examples
Thermochemical equation examples Kirchhoff's law of thermochemistry
Kirchhoff's law of thermochemistry Chapter 17 thermochemistry
Chapter 17 thermochemistry Thermochemistry equations
Thermochemistry equations Thermochemistry cartoon
Thermochemistry cartoon Thermodynamics ap chemistry
Thermodynamics ap chemistry