Calorie energy Calculations A calorie is defined as





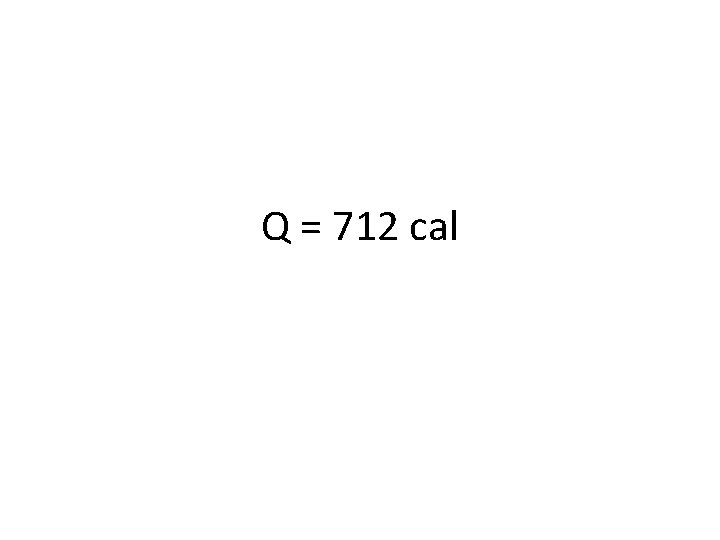








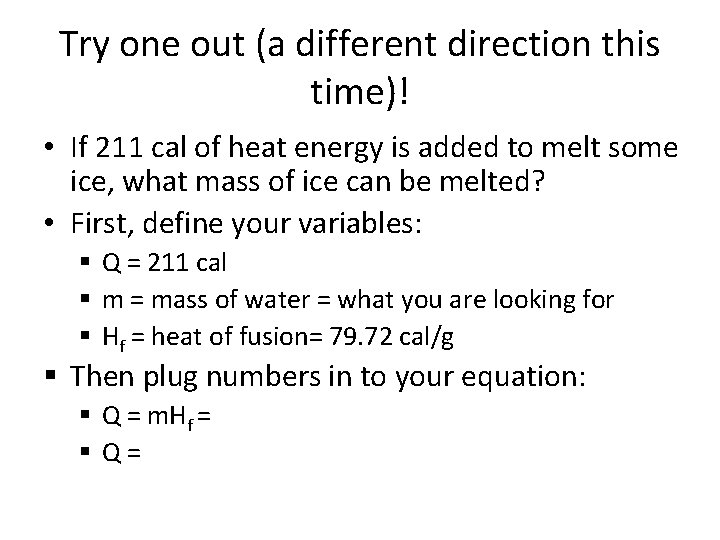




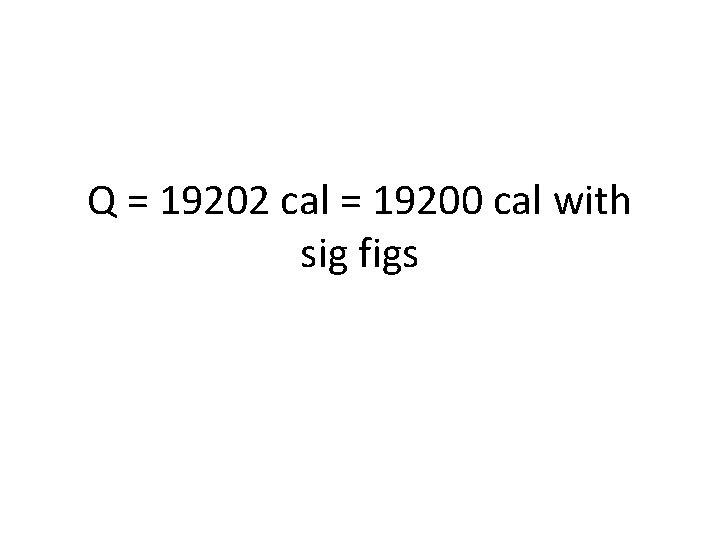

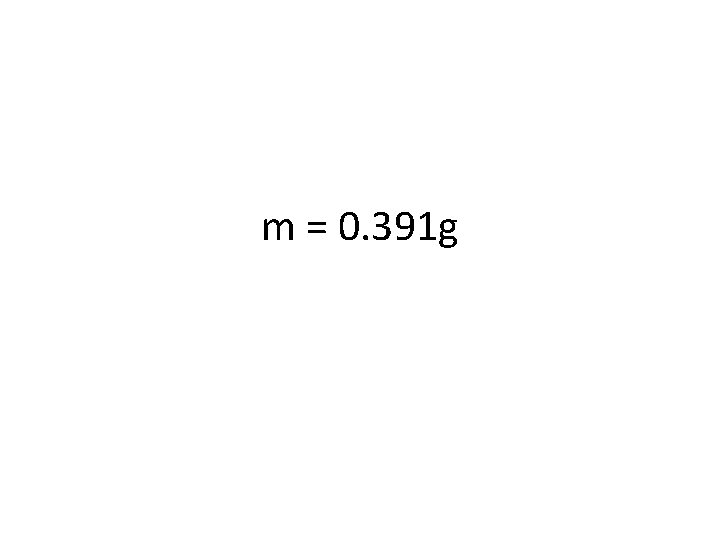
- Slides: 26

Calorie (energy) Calculations • A calorie is defined as the amount of energy it takes to raise the temperature of one gram of water by one degree Celsius. • So if we want to raise 10 grams of water by 1 degree Celsius, it requires ______ calories. • And if we want to raise 10 g of water by 10 ˚C, it requires _______ calories. • How did you do that math?

Calorie (energy) Calculations • There is an equation to streamline that math. ˚C Specific heat capacity, in cal/g˚C g , in Ma ss , in rgy , in mp e in t Ene nge Cha cal Q=mcΔT

What is c? ? • “c” is the specific heat capacity. • That is the amount of energy it takes to change the temperature of 1 gram of the substance by 1 ˚C. • Does that sound familiar? • In your yellow data book, find the table with physical constants for water. • What value (number and units) does “c” have for liquid water?

Try one out! • How much energy is absorbed if 12. 3 g of water is heated from 15. 0 ˚C to 35. 0 ˚C? • First, define your variables: § § Q = we don’t know, this is what you’re looking for m = mass of water = 12. 3 g c = specific heat capacity = 1. 000 cal/g ˚C ΔT = change in temp = 35 ˚C - 15 ˚C = 20. 0 ˚C § Then plug numbers in to your equation: § Q = mcΔT = (12. 3 g)*(1. 000 cal/g ˚C)*(20. 0 ˚C) § Q = 246 cal

Try one out! • How much energy is absorbed if 35. 6 g of water is heated from 15. 0 ˚C to 35. 0 ˚C? • First, define your variables: § § Q = we don’t know, this is what you’re looking for m = mass of water = c = specific heat capacity = 1. 000 cal/g ˚C ΔT = change in temp = § Then plug numbers in to your equation: § Q = mcΔT
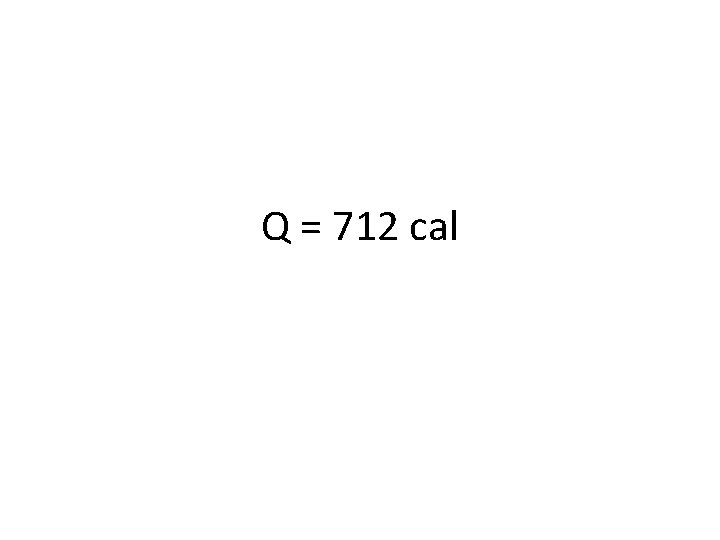
Q = 712 cal

Try one out (this time a different direction)! • If 211 cal of heat energy is added to 16. 0 grams of water, how much will the temperature increase? • First, define your variables: § § Q = 211 cal m = mass of water = 16. 0 g c = specific heat capacity = 1. 000 cal/g ˚C ΔT = change in temp = what we are looking for § Then plug numbers in to your equation: § Q = mcΔT

ΔT = 13. 2 °C

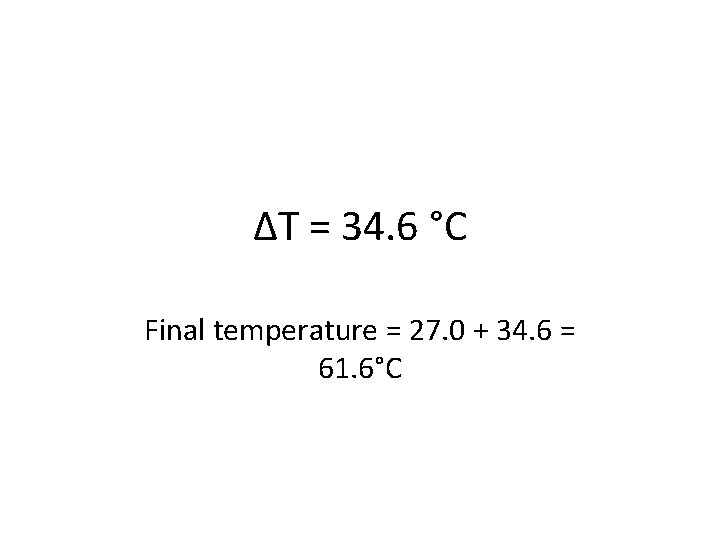
Try one out (this time a different direction)! • If 315 cal of heat energy is added to 9. 10 grams of water that starts at 27. 0 ˚C, what will the final temperature be? • First, define your variables: § § Q= m = mass of water = c = specific heat capacity = 1. 000 cal/g ˚C ΔT = change in temp = what we are looking for § Then plug numbers in to your equation: § Q = mcΔT § Once you have calculated the change, how can you find the final temperature? ?

ΔT = 34. 6 °C Final temperature = 27. 0 + 34. 6 = 61. 6°C

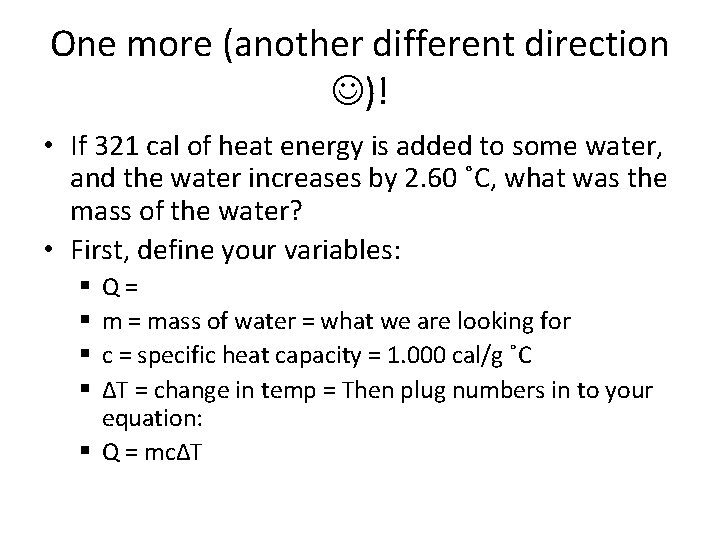
One more (another different direction )! • If 321 cal of heat energy is added to some water, and the water increases by 2. 60 ˚C, what was the mass of the water? • First, define your variables: Q= m = mass of water = what we are looking for c = specific heat capacity = 1. 000 cal/g ˚C ΔT = change in temp = Then plug numbers in to your equation: § Q = mcΔT § §

m = 123 g

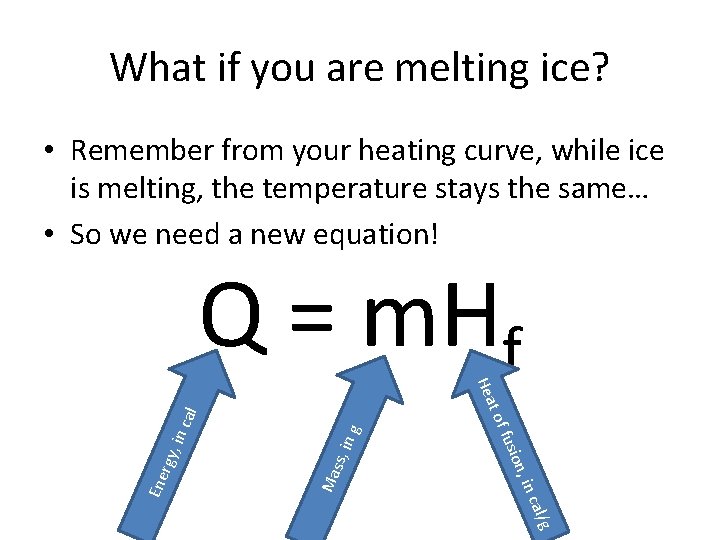
What if you are melting ice? • Remember from your heating curve, while ice is melting, the temperature stays the same… • So we need a new equation! l/g a in c in g ss, Ma cal , in rgy on, fusi Ene t of Hea Q = m. Hf

What is Hf? ? • “Hf” is the heat of fusion. • That is the amount of energy it takes to change 1 gram of the substance from a solid to a liquid (to melt it). • In your yellow data book, find the table with physical constants for water. • What value (number and units) does “Hf” have for water?

Try one out! • How much energy is absorbed if 12. 3 g of ice is melted? • First, define your variables: § Q = we don’t know, this is what you’re looking for § m = mass of water = 12. 3 g § Hf = heat of fusion= 79. 72 cal/g § Then plug numbers in to your equation: § Q = m. Hf = (12. 3 g)*(79. 72 cal/g) § Q = 981 cal

Try one out! • How much energy is absorbed if 35. 6 g of ice is melted? • First, define your variables: § Q = we don’t know, this is what you’re looking for § m = mass of water = § Hf = heat of fusion= 79. 72 cal/g § Then plug numbers in to your equation: § Q = m. Hf = § Q=

Q = 2838 cal = 2840 cal with sig figs
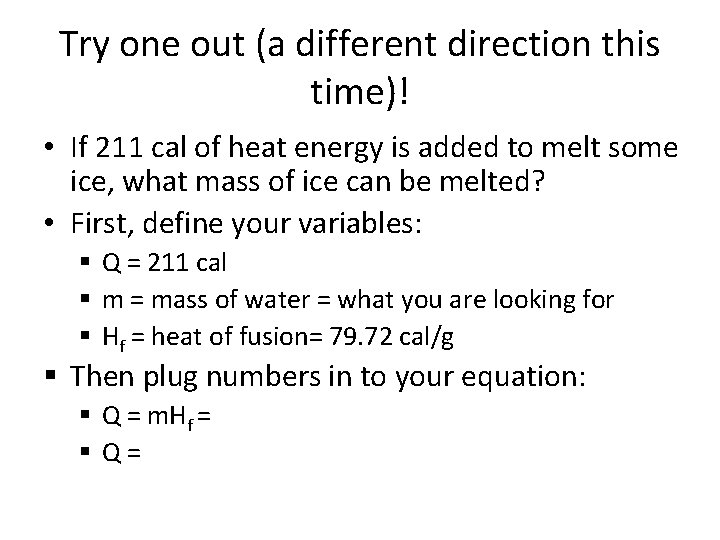
Try one out (a different direction this time)! • If 211 cal of heat energy is added to melt some ice, what mass of ice can be melted? • First, define your variables: § Q = 211 cal § m = mass of water = what you are looking for § Hf = heat of fusion= 79. 72 cal/g § Then plug numbers in to your equation: § Q = m. Hf = § Q=

m = 2. 65 g

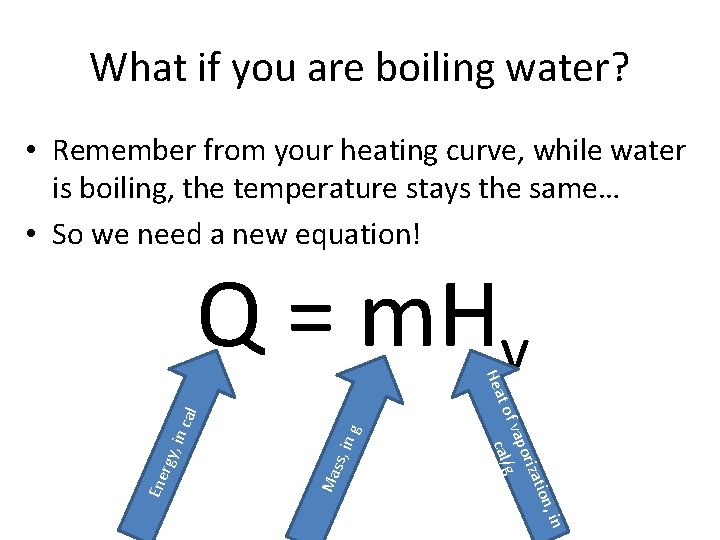
What if you are boiling water? • Remember from your heating curve, while water is boiling, the temperature stays the same… • So we need a new equation! n, in in g ss, Ma cal , in rgy atio oriz vap g cal/ Ene t of Hea Q = m. Hv

What is Hv? ? • “Hv” is the heat of vaporization. • That is the amount of energy it takes to change 1 gram of the substance from a liquid to a gas (to vaporize it). • In your yellow data book, find the table with physical constants for water. • What value (number and units) does “Hv” have for water?

Try one out! • How much energy is absorbed if 12. 3 g of water is vaporized (boiled)? • First, define your variables: § Q = we don’t know, this is what you’re looking for § m = mass of water = 12. 3 g § Hv = heat of vaporization= 539. 4 cal/g § Then plug numbers in to your equation: § Q = m. Hv = (12. 3 g)*(539. 4 cal/g) § Q = 6634. 62 cal = 6630 cal with sig figs

Try one out! • How much energy is absorbed if 35. 6 g of water is vaporized? • First, define your variables: § Q = we don’t know, this is what you’re looking for § m = mass of water = § Hv = heat of vaporization= 539. 4 cal/g § Then plug numbers in to your equation: § Q = m. Hv = § Q=
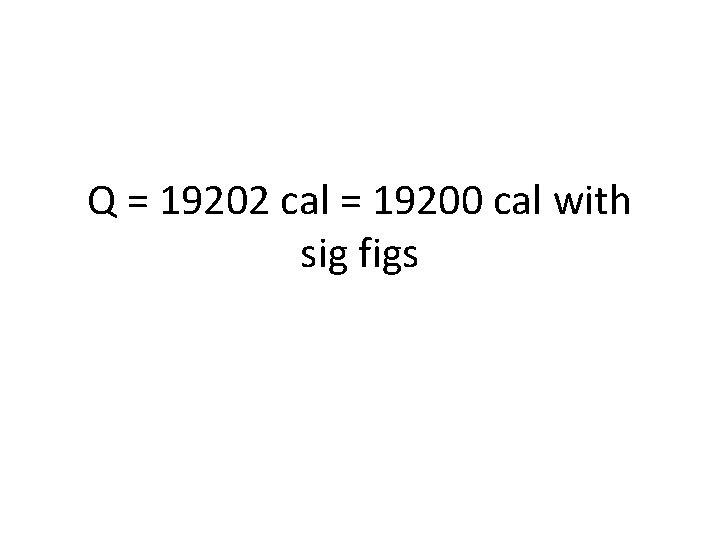
Q = 19202 cal = 19200 cal with sig figs

Try one out (a different direction this time)! • If 211 cal of heat energy is added to vaporize some water, what mass of water can be vaporized? • First, define your variables: § Q = 211 cal § m = mass of water = what you are looking for § Hv = heat of vaporization= § Then plug numbers in to your equation: § Q = m. Hv = § Q=
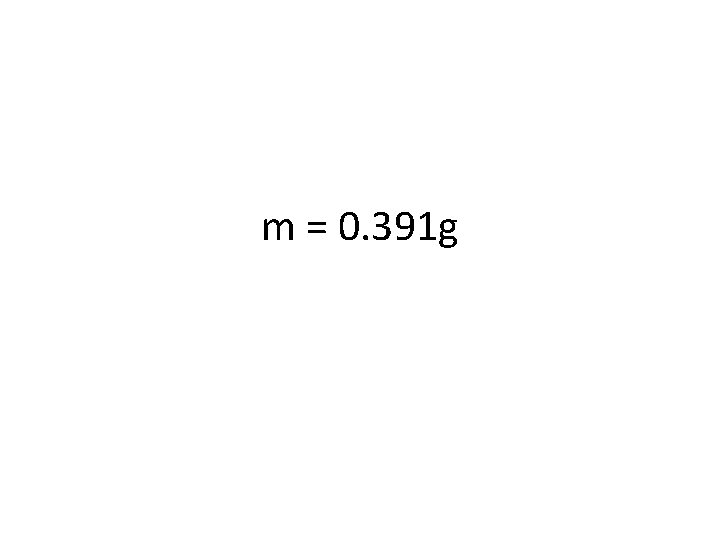
m = 0. 391 g