Thermochemistry Chapters 6 and 16 Thermochemistry Energy The













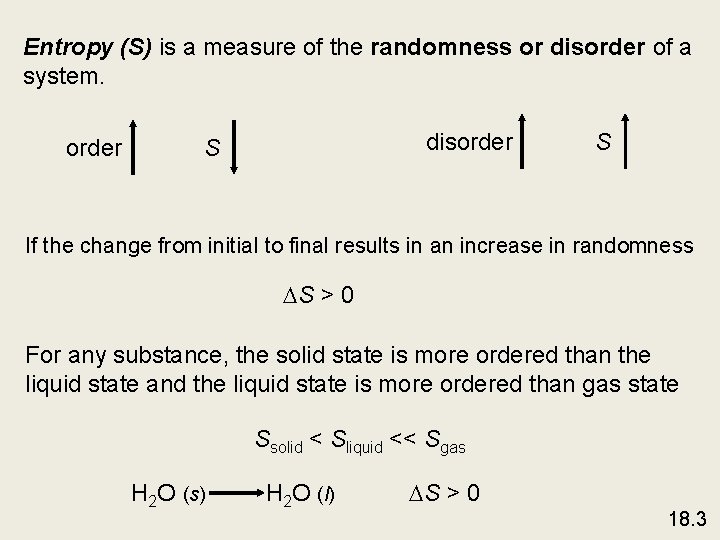


![The specific heat (s) [most books use lower case c] of a substance is The specific heat (s) [most books use lower case c] of a substance is](https://slidetodoc.com/presentation_image_h2/abeb172a6b910b76451f092c3feb5974/image-57.jpg)
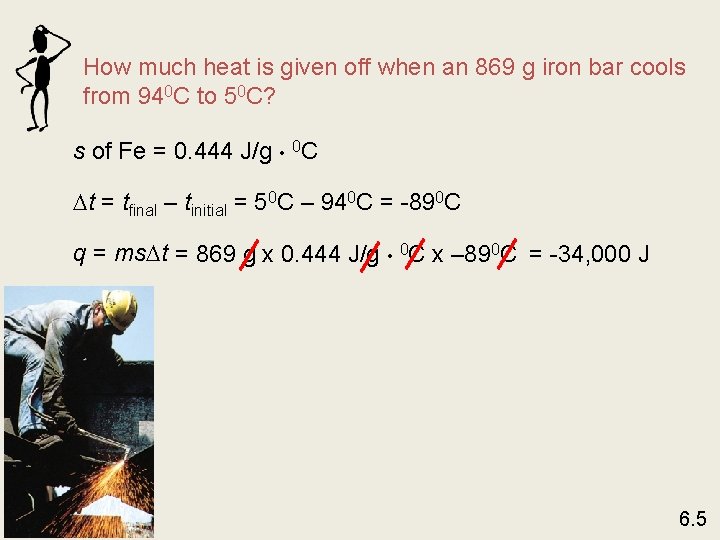
- Slides: 69

Thermochemistry Chapters 6 and 16

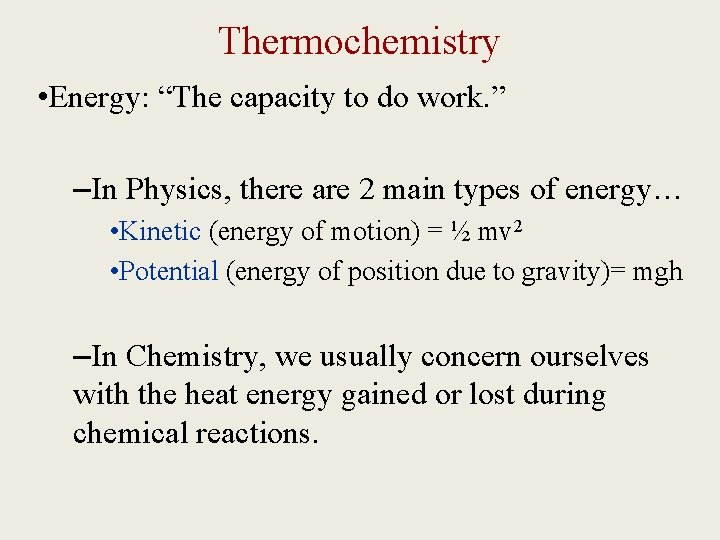
Thermochemistry • Energy: “The capacity to do work. ” –In Physics, there are 2 main types of energy… • Kinetic (energy of motion) = ½ mv 2 • Potential (energy of position due to gravity)= mgh –In Chemistry, we usually concern ourselves with the heat energy gained or lost during chemical reactions.

Law of Conservation of Energy: Energy can neither be created nor destroyed, but can be converted between forms The First Law of Thermodynamics: The total energy content of the universe is constant

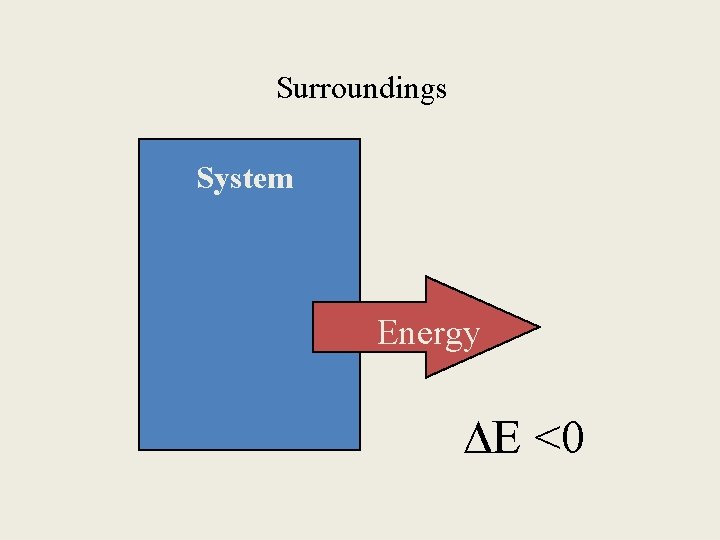
The universe • • is divided into two halves. the system and the surroundings. The system is the part you are concerned with. The surroundings are the rest.

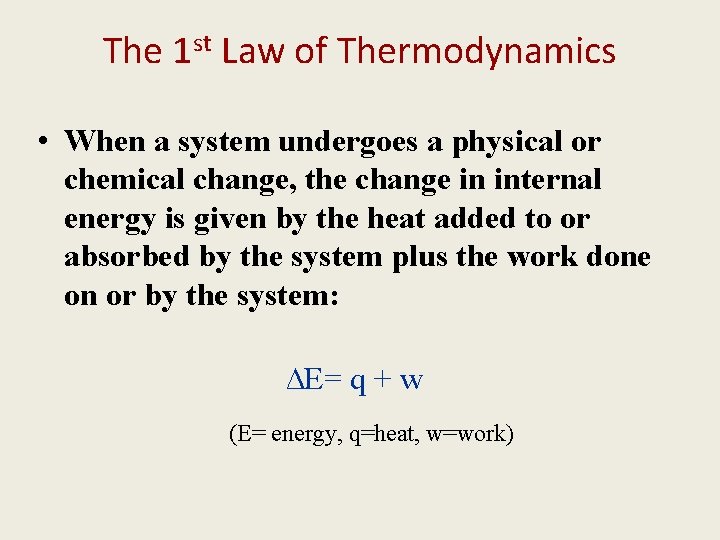
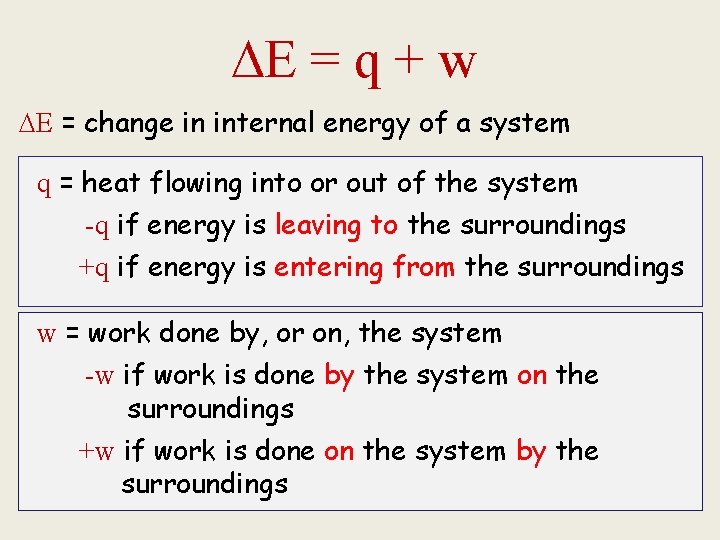
The 1 st Law of Thermodynamics • When a system undergoes a physical or chemical change, the change in internal energy is given by the heat added to or absorbed by the system plus the work done on or by the system: ∆E= q + w (E= energy, q=heat, w=work)

E = q + w E = change in internal energy of a system q = heat flowing into or out of the system -q if energy is leaving to the surroundings +q if energy is entering from the surroundings w = work done by, or on, the system -w if work is done by the system on the surroundings +w if work is done on the system by the surroundings

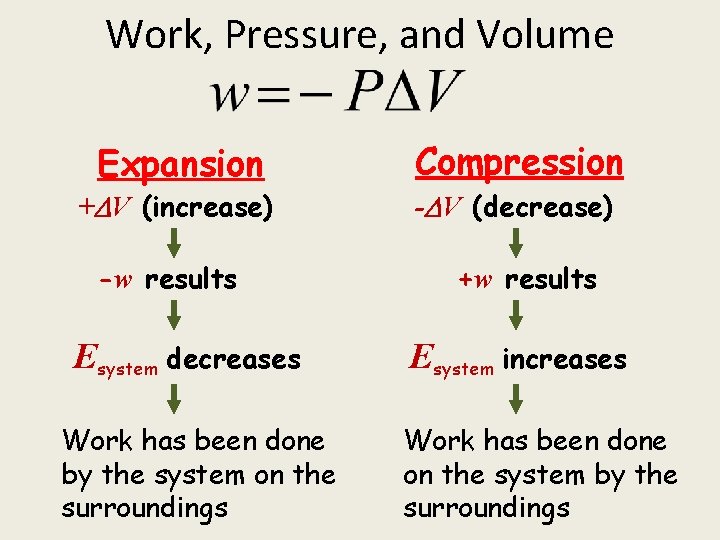
Work, Pressure, and Volume Expansion + V (increase) -w results Esystem decreases Work has been done by the system on the surroundings Compression - V (decrease) +w results Esystem increases Work has been done on the system by the surroundings

State Functions depend ONLY on the present state of the system ENERGY IS A STATE FUNCTION A person standing at the top of Mt. Everest has the same potential energy whether they got there by hiking up, or by falling down from a plane WORK IS NOT A STATE FUNCTION

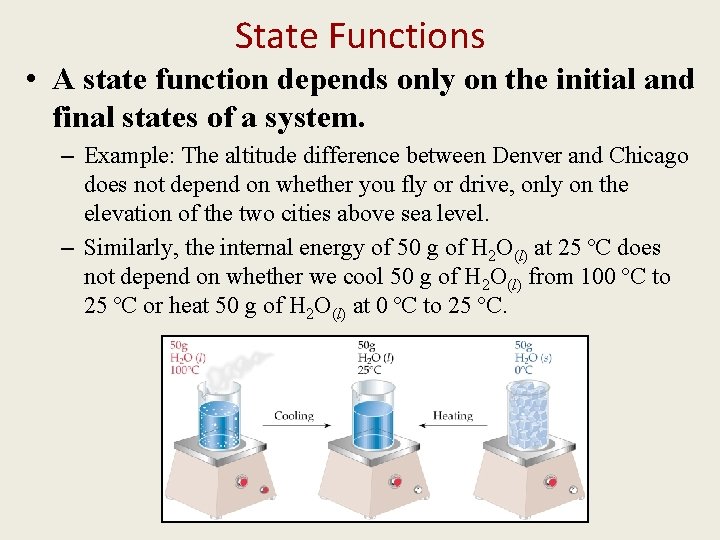
State Functions • A state function depends only on the initial and final states of a system. – Example: The altitude difference between Denver and Chicago does not depend on whether you fly or drive, only on the elevation of the two cities above sea level. – Similarly, the internal energy of 50 g of H 2 O(l) at 25 ºC does not depend on whether we cool 50 g of H 2 O(l) from 100 ºC to 25 ºC or heat 50 g of H 2 O(l) at 0 ºC to 25 ºC.

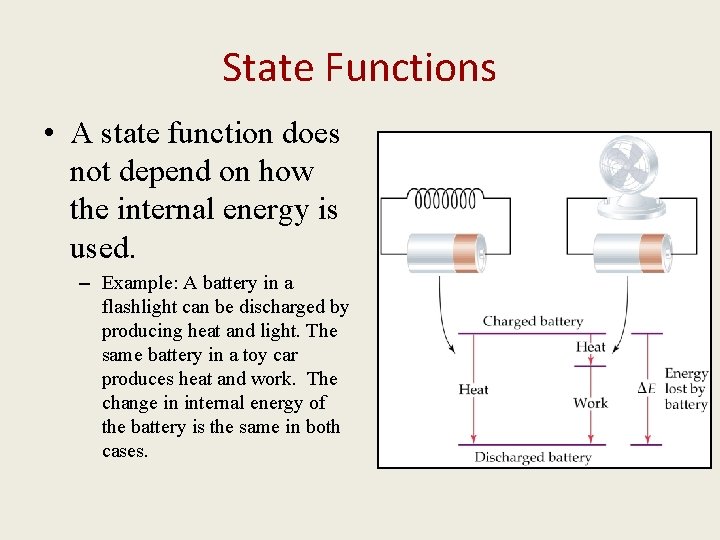
State Functions • A state function does not depend on how the internal energy is used. – Example: A battery in a flashlight can be discharged by producing heat and light. The same battery in a toy car produces heat and work. The change in internal energy of the battery is the same in both cases.

State Functions • ∆E is a state function. Enthalpy • Enthalpy, H: -Enthalpy is heat transferred between the system and surroundings carried out under constant pressure. (Open containers are under constant pressure!) -Enthalpy is also a state function. ∆H is (+) for endothermic reactions…just like q! ∆H is (−) for exothermic reactions…just like q!

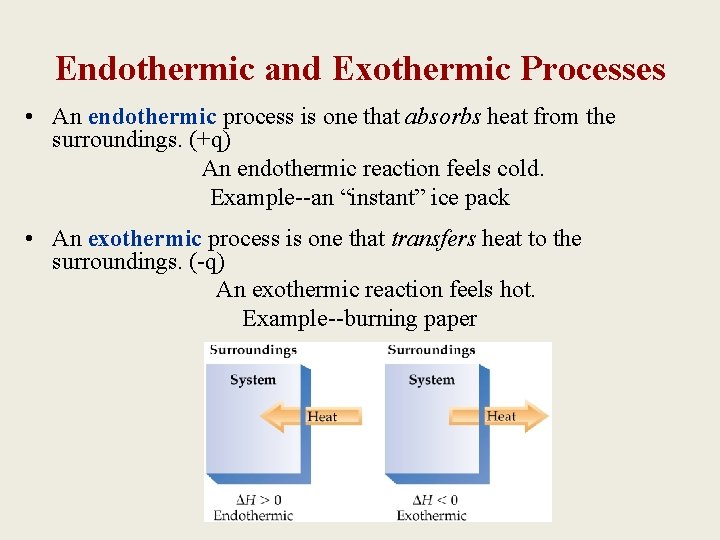
Endothermic and Exothermic Processes • An endothermic process is one that absorbs heat from the surroundings. (+q) An endothermic reaction feels cold. Example--an “instant” ice pack • An exothermic process is one that transfers heat to the surroundings. (-q) An exothermic reaction feels hot. Example--burning paper

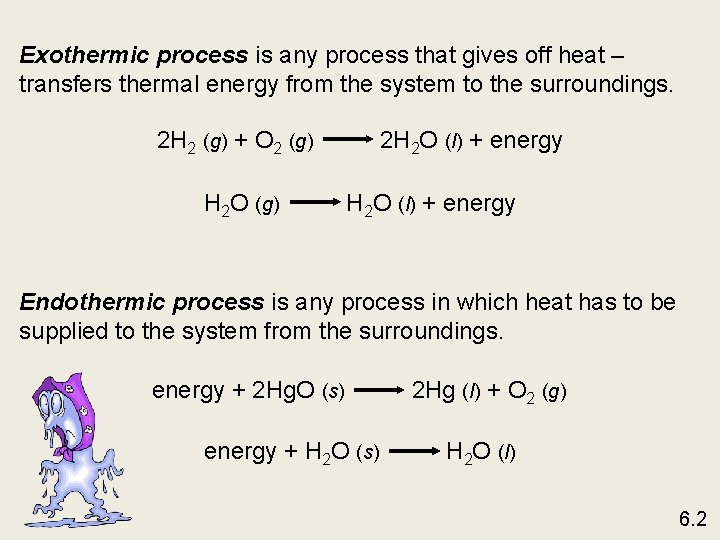
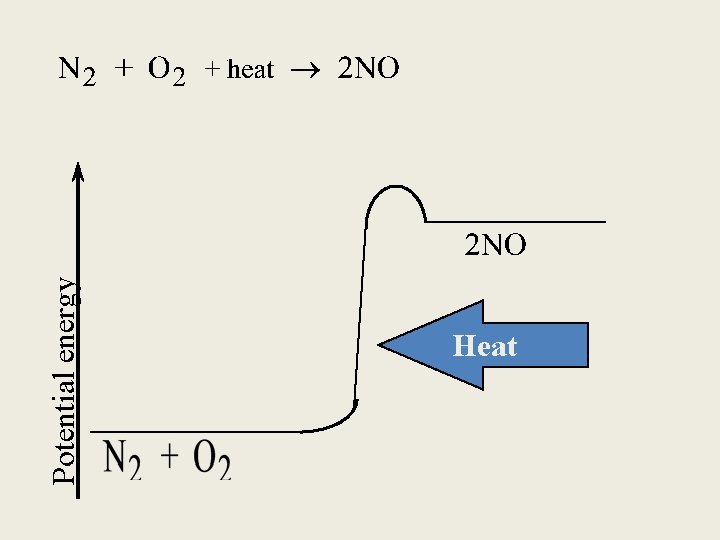
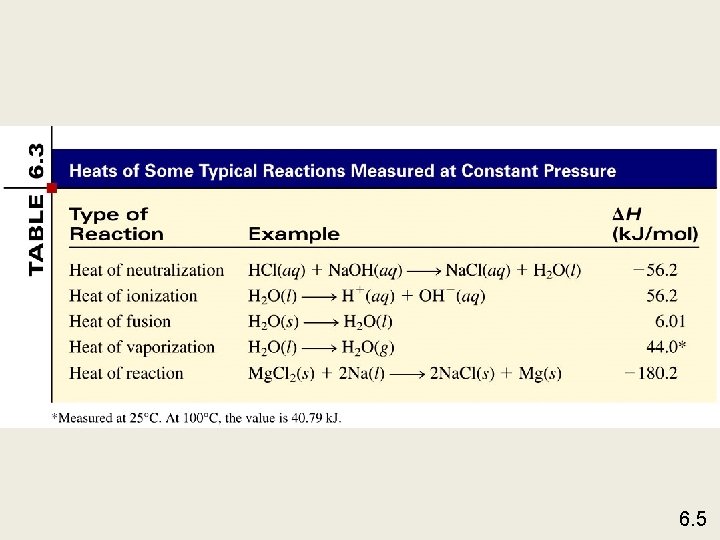
Exothermic process is any process that gives off heat – transfers thermal energy from the system to the surroundings. 2 H 2 (g) + O 2 (g) H 2 O (g) 2 H 2 O (l) + energy Endothermic process is any process in which heat has to be supplied to the system from the surroundings. energy + 2 Hg. O (s) energy + H 2 O (s) 2 Hg (l) + O 2 (g) H 2 O (l) 6. 2

Potential energy Heat

Potential energy Heat

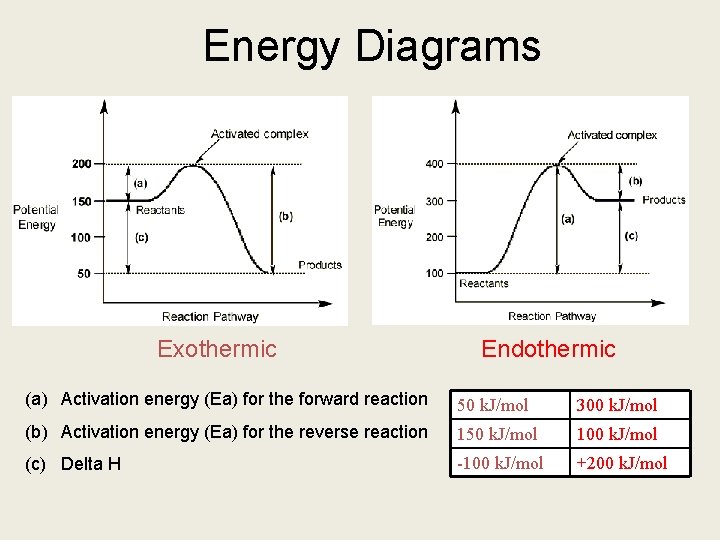
Energy Diagrams Exothermic Endothermic (a) Activation energy (Ea) for the forward reaction 50 k. J/mol 300 k. J/mol (b) Activation energy (Ea) for the reverse reaction 150 k. J/mol 100 k. J/mol (c) Delta H -100 k. J/mol +200 k. J/mol

Direction • 1. 2. 3. • • Every energy measurement has three parts. A unit ( Joules of calories). A number how many. and a sign to tell direction. negative - exothermic positive- endothermic

Surroundings System Energy E <0

Surroundings System Energy E >0

Chemical energy • Reaction • http: //www. youtube. com/watch? v=n. N 8 x. D_b v 2 a. Q

calorimetry

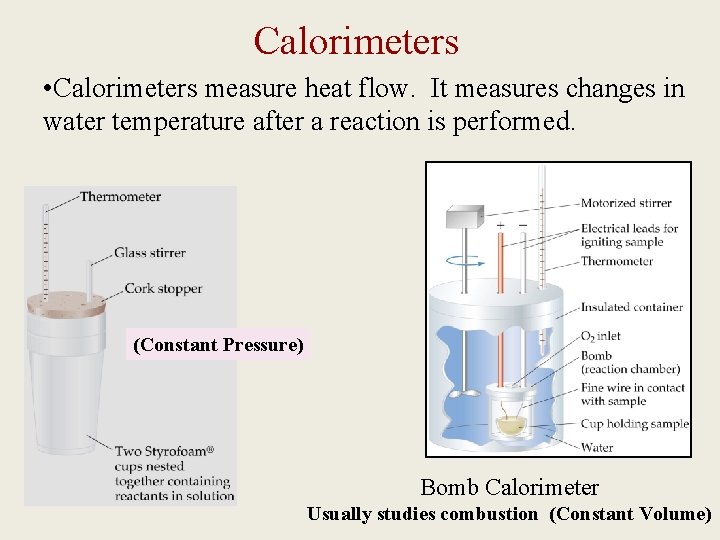
Calorimeters • Calorimeters measure heat flow. It measures changes in water temperature after a reaction is performed. (Constant Pressure) Bomb Calorimeter Usually studies combustion (Constant Volume)

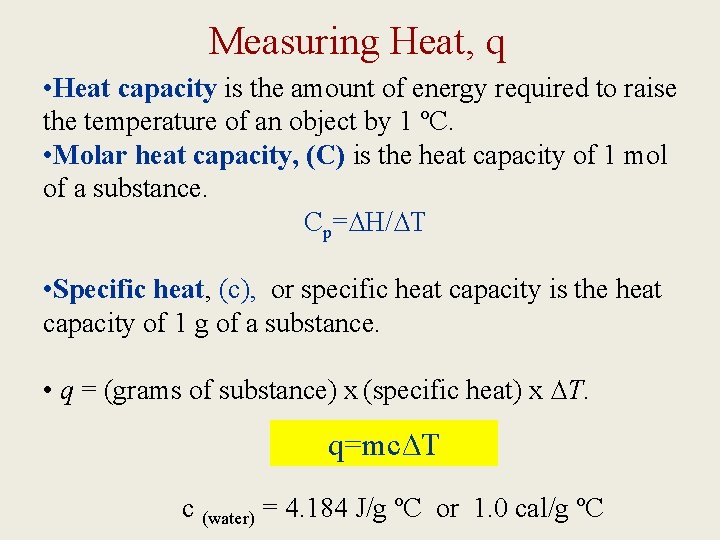
Measuring Heat, q • Heat capacity is the amount of energy required to raise the temperature of an object by 1 ºC. • Molar heat capacity, (C) is the heat capacity of 1 mol of a substance. Cp=∆H/∆T • Specific heat, (c), or specific heat capacity is the heat capacity of 1 g of a substance. • q = (grams of substance) x (specific heat) x ∆T. q=mc∆T c (water) = 4. 184 J/g ºC or 1. 0 cal/g ºC

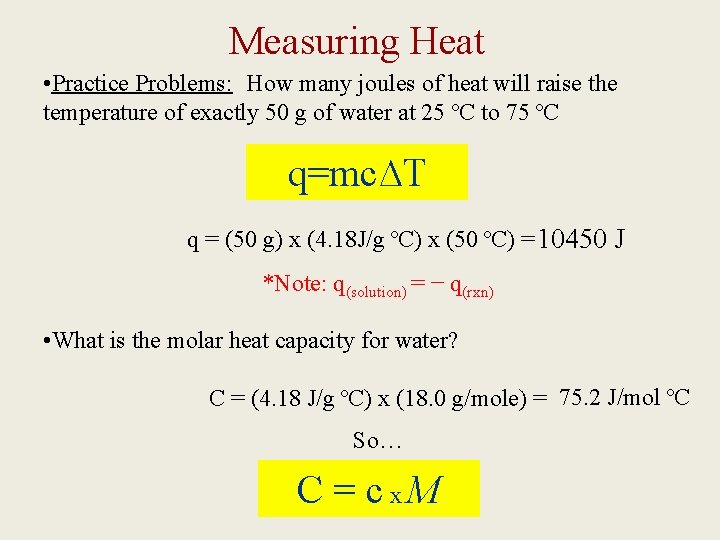
Measuring Heat • Practice Problems: How many joules of heat will raise the temperature of exactly 50 g of water at 25 ºC to 75 ºC q=mc∆T q = (50 g) x (4. 18 J/g ºC) x (50 ºC) = 10450 J *Note: q(solution) = − q(rxn) • What is the molar heat capacity for water? C = (4. 18 J/g ºC) x (18. 0 g/mole) = 75. 2 J/mol ºC So… C = cx. M

Enthalpy Hesse’s law

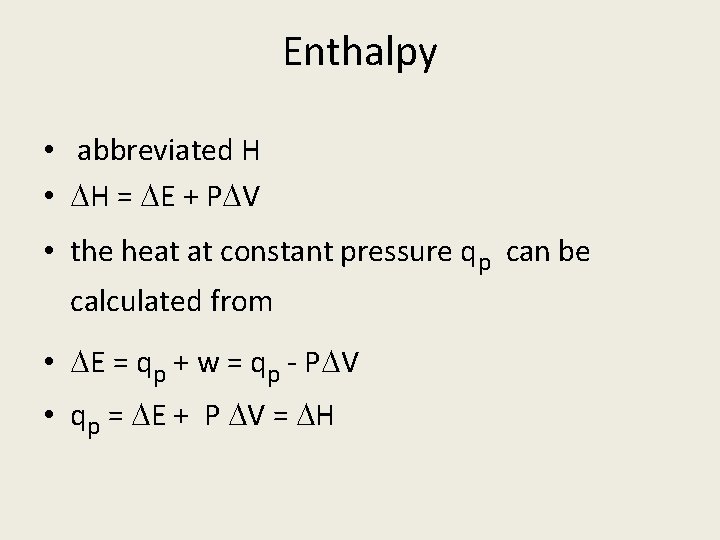
Enthalpy • abbreviated H • H = E + P V • the heat at constant pressure qp can be calculated from • E = qp + w = qp - P V • qp = E + P V = H

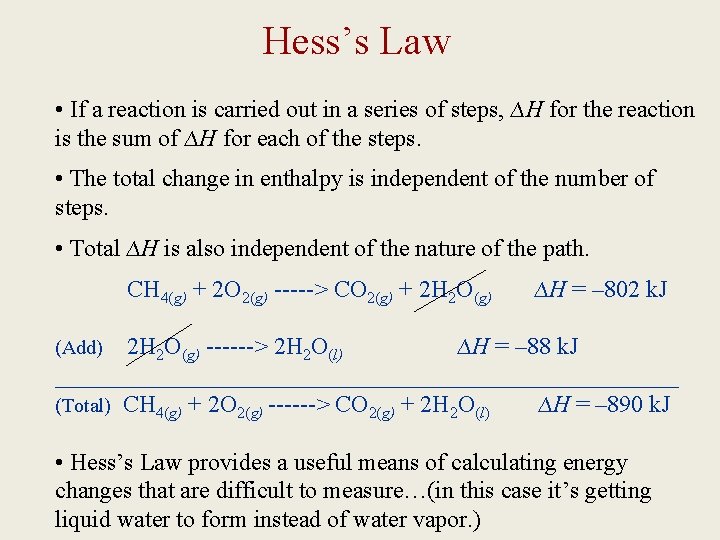
Hess’s Law • If a reaction is carried out in a series of steps, ∆H for the reaction is the sum of ∆H for each of the steps. • The total change in enthalpy is independent of the number of steps. • Total ∆H is also independent of the nature of the path. CH 4(g) + 2 O 2(g) -----> CO 2(g) + 2 H 2 O(g) ∆H = – 802 k. J 2 H 2 O(g) ------> 2 H 2 O(l) ∆H = – 88 k. J __________________________ (Total) CH 4(g) + 2 O 2(g) ------> CO 2(g) + 2 H 2 O(l) ∆H = – 890 k. J (Add) • Hess’s Law provides a useful means of calculating energy changes that are difficult to measure…(in this case it’s getting liquid water to form instead of water vapor. )

Entalphy for changes of state of matter

Heat of Fusion & Heat of Vaporization • Molar heat of fusion (∆Hfus) : the amount of energy required to take 1 mole of a solid to the liquid state. Example: H 2 O(s) H 2 O(l) ∆Hfus = 6. 01 k. J -Heat of fusion is usually greater for ionic solids than molecular solids since ionic solids are more strongly held together. For ice to water … ∆Hfus= 6. 01 k. J/mol • Molar heat of vaporization (∆H vap): the amount of energy required to take 1 mole of a liquid to the gaseous state. Example: H 2 O(l) H 2 O(g) ∆Hvap = 40. 67 k. J For water to steam … ∆Hvap= 40. 67 k. J/mol

Entalphy of formation and reaction

Enthalpies of Formation • If a compound is formed from its constituent elements, then the enthalpy change for the reaction is called the enthalpy of formation, ∆Hf. Example: C(s) + O 2(g) CO 2(g) ∆Hf = − 393. 5 k. J Standard state (standard conditions) refer to the substance at: 1 atm and 25ºC (298 K) (We will assume that reactants and products are both at 25 ºC unless otherwise stated. ) • Standard enthalpy, ∆Hº, is the enthalpy measured when everything is in its standard state. • Standard enthalpy of formation of a compound, ∆Hºf , is the enthalpy change for the formation of 1 mole of compound with all substances in their standard states.

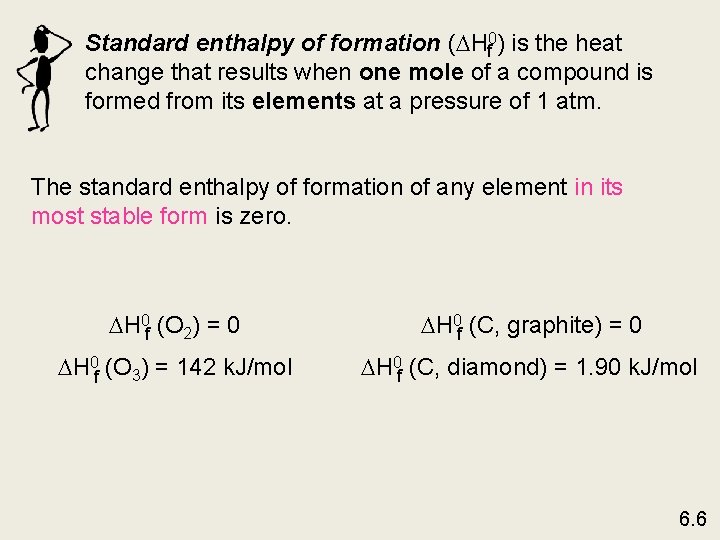
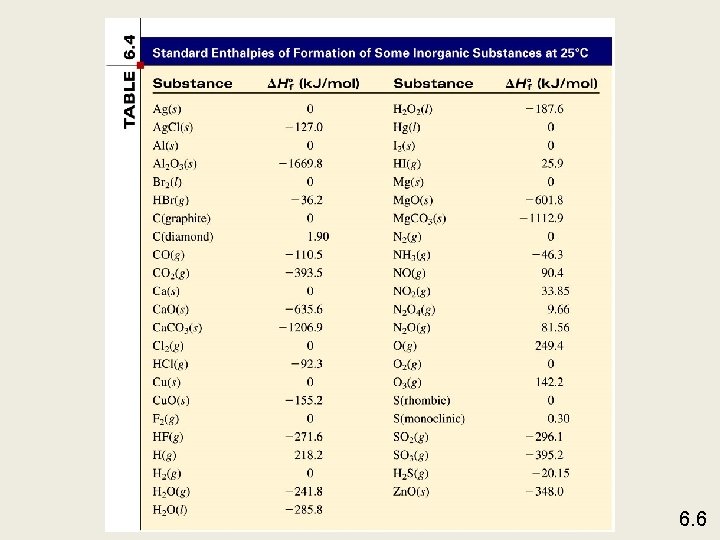
Standard enthalpy of formation ( Hf 0) is the heat change that results when one mole of a compound is formed from its elements at a pressure of 1 atm. The standard enthalpy of formation of any element in its most stable form is zero. H 0 f (O 2) = 0 H 0 f (C, graphite) = 0 H 0 f (O 3) = 142 k. J/mol H 0 f (C, diamond) = 1. 90 k. J/mol 6. 6

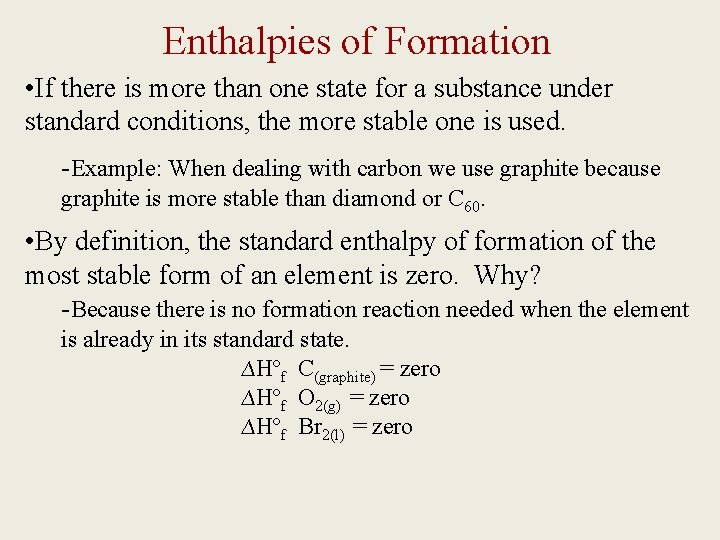
Enthalpies of Formation • If there is more than one state for a substance under standard conditions, the more stable one is used. -Example: When dealing with carbon we use graphite because graphite is more stable than diamond or C 60. • By definition, the standard enthalpy of formation of the most stable form of an element is zero. Why? -Because there is no formation reaction needed when the element is already in its standard state. ∆Hºf C(graphite) = zero ∆Hºf O 2(g) = zero ∆Hºf Br 2(l) = zero

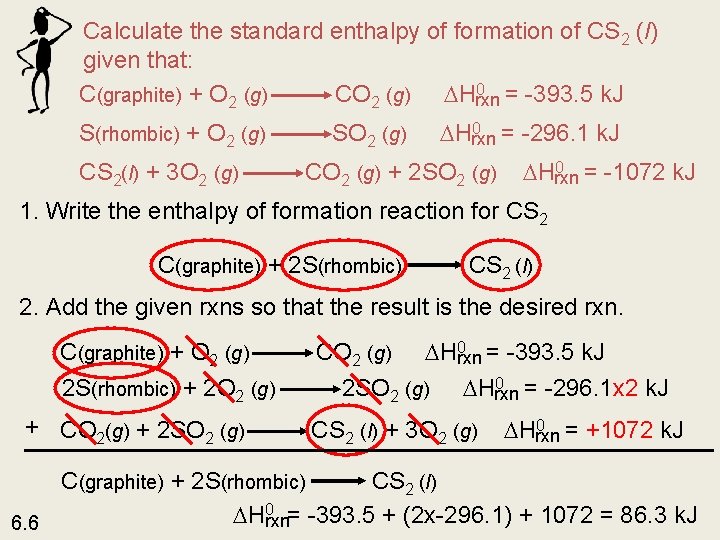
Calculate the standard enthalpy of formation of CS 2 (l) given that: 0 = -393. 5 k. J C(graphite) + O 2 (g) CO 2 (g) Hrxn S(rhombic) + O 2 (g) CS 2(l) + 3 O 2 (g) SO 2 (g) 0 = -296. 1 k. J Hrxn CO 2 (g) + 2 SO 2 (g) 0 = -1072 k. J Hrxn 1. Write the enthalpy of formation reaction for CS 2 C(graphite) + 2 S(rhombic) CS 2 (l) 2. Add the given rxns so that the result is the desired rxn. C(graphite) + O 2 (g) 2 S(rhombic) + 2 O 2 (g) + CO 2(g) + 2 SO 2 (g) 0 = -393. 5 k. J CO 2 (g) Hrxn 0 = -296. 1 x 2 k. J 2 SO 2 (g) Hrxn CS 2 (l) + 3 O 2 (g) 0 = +1072 k. J Hrxn C(graphite) + 2 S(rhombic) CS 2 (l) 0 = -393. 5 + (2 x-296. 1) + 1072 = 86. 3 k. J H rxn 6. 6

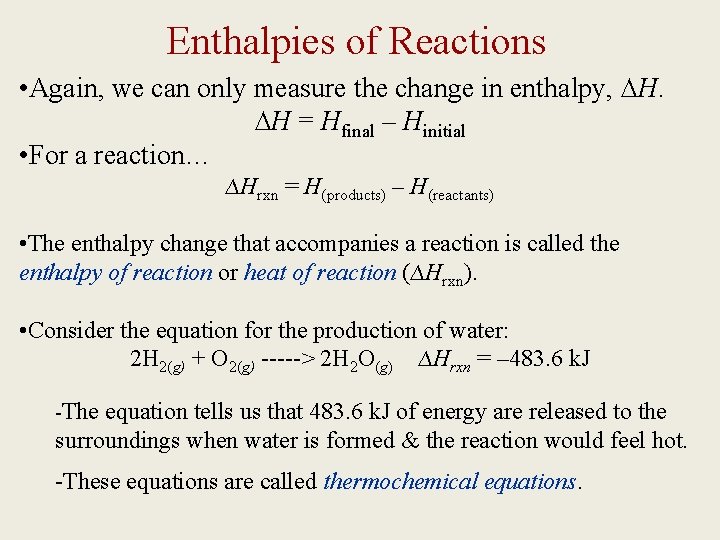
Enthalpies of Reactions • Again, we can only measure the change in enthalpy, ∆H. ∆H = Hfinal – Hinitial • For a reaction… ∆Hrxn = H(products) – H(reactants) • The enthalpy change that accompanies a reaction is called the enthalpy of reaction or heat of reaction (∆Hrxn). • Consider the equation for the production of water: 2 H 2(g) + O 2(g) -----> 2 H 2 O(g) ∆Hrxn = – 483. 6 k. J -The equation tells us that 483. 6 k. J of energy are released to the surroundings when water is formed & the reaction would feel hot. -These equations are called thermochemical equations.

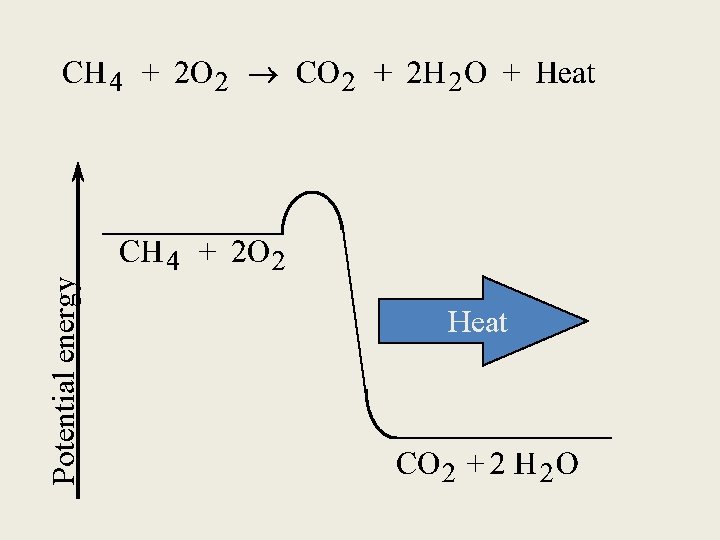
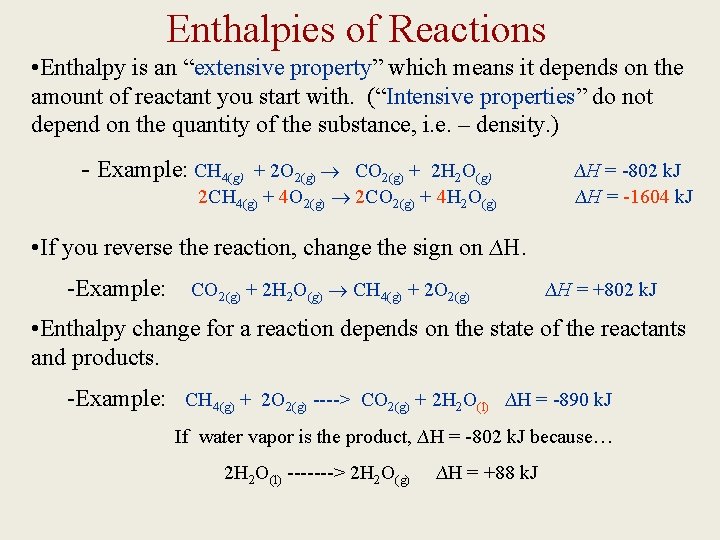
Enthalpies of Reactions • Enthalpy is an “extensive property” which means it depends on the amount of reactant you start with. (“Intensive properties” do not depend on the quantity of the substance, i. e. – density. ) - Example: CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) 2 CH 4(g) + 4 O 2(g) 2 CO 2(g) + 4 H 2 O(g) ∆H = -802 k. J ∆H = -1604 k. J • If you reverse the reaction, change the sign on ∆H. -Example: CO 2(g) + 2 H 2 O(g) CH 4(g) + 2 O 2(g) ∆H = +802 k. J • Enthalpy change for a reaction depends on the state of the reactants and products. -Example: CH 4(g) + 2 O 2(g) ----> CO 2(g) + 2 H 2 O(l) ∆H = -890 k. J If water vapor is the product, ∆H = -802 k. J because… 2 H 2 O(l) -------> 2 H 2 O(g) ∆H = +88 k. J

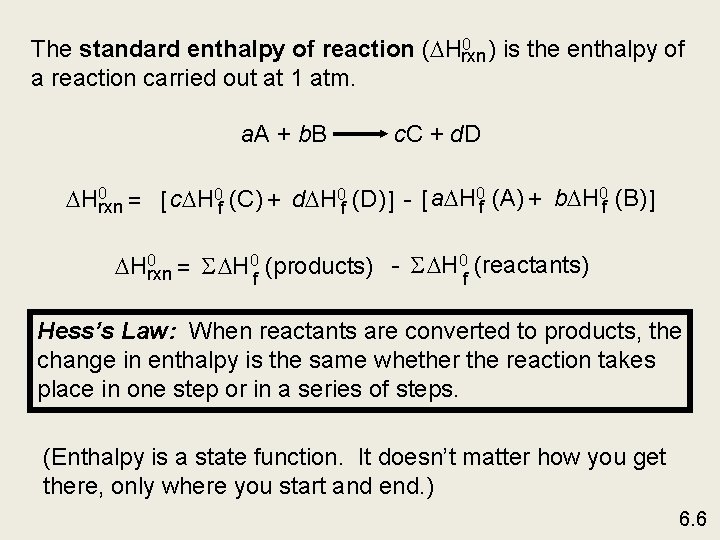
0 ) is the enthalpy of The standard enthalpy of reaction ( Hrxn a reaction carried out at 1 atm. a. A + b. B c. C + d. D H 0 rxn = [ c H 0 f (C) + d H 0 f (D) ] - [ a H 0 f (A) + b H 0 f (B) ] H 0 rxn = S H 0 f (products) - S H 0 f (reactants) Hess’s Law: When reactants are converted to products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps. (Enthalpy is a state function. It doesn’t matter how you get there, only where you start and end. ) 6. 6

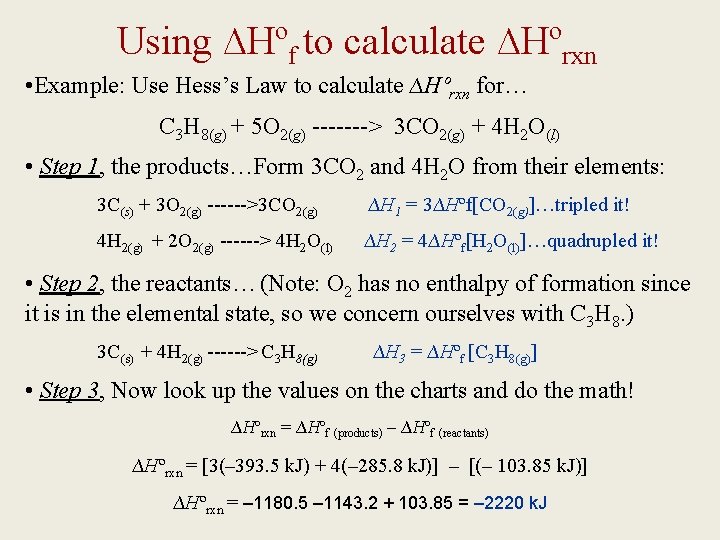
Using ∆Hºf to calculate ∆Hºrxn • Example: Use Hess’s Law to calculate ∆Hºrxn for… C 3 H 8(g) + 5 O 2(g) -------> 3 CO 2(g) + 4 H 2 O(l) • Step 1, the products…Form 3 CO 2 and 4 H 2 O from their elements: 3 C(s) + 3 O 2(g) ------>3 CO 2(g) ∆H 1 = 3∆Hºf[CO 2(g)]…tripled it! 4 H 2(g) + 2 O 2(g) ------> 4 H 2 O(l) ∆H 2 = 4∆Hºf[H 2 O(l)]…quadrupled it! • Step 2, the reactants…(Note: O 2 has no enthalpy of formation since it is in the elemental state, so we concern ourselves with C 3 H 8. ) 3 C(s) + 4 H 2(g) ------> C 3 H 8(g) ∆H 3 = ∆Hºf [C 3 H 8(g)] • Step 3, Now look up the values on the charts and do the math! ∆Hºrxn = ∆Hºf (products) – ∆Hºf (reactants) ∆Hºrxn = [3(– 393. 5 k. J) + 4(– 285. 8 k. J)] – [(– 103. 85 k. J)] ∆Hºrxn = – 1180. 5 – 1143. 2 + 103. 85 = – 2220 k. J

6. 6

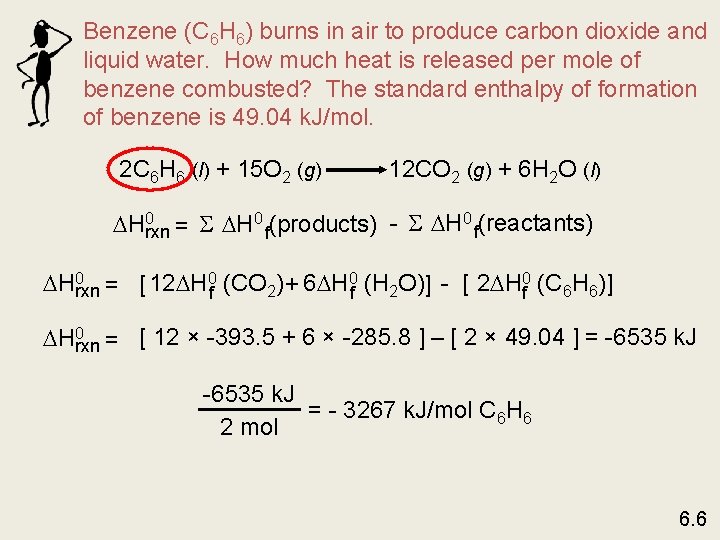
Benzene (C 6 H 6) burns in air to produce carbon dioxide and liquid water. How much heat is released per mole of benzene combusted? The standard enthalpy of formation of benzene is 49. 04 k. J/mol. 2 C 6 H 6 (l) + 15 O 2 (g) 12 CO 2 (g) + 6 H 2 O (l) H 0 rxn = S H 0 f(products) - S H 0 f(reactants) H 0 rxn = [ 12 H 0 f (CO 2) + 6 H 0 f (H 2 O)] - [ 2 H 0 f (C 6 H 6)] H 0 rxn = [ 12 × -393. 5 + 6 × -285. 8 ] – [ 2 × 49. 04 ] = -6535 k. J = - 3267 k. J/mol C 6 H 6 2 mol 6. 6

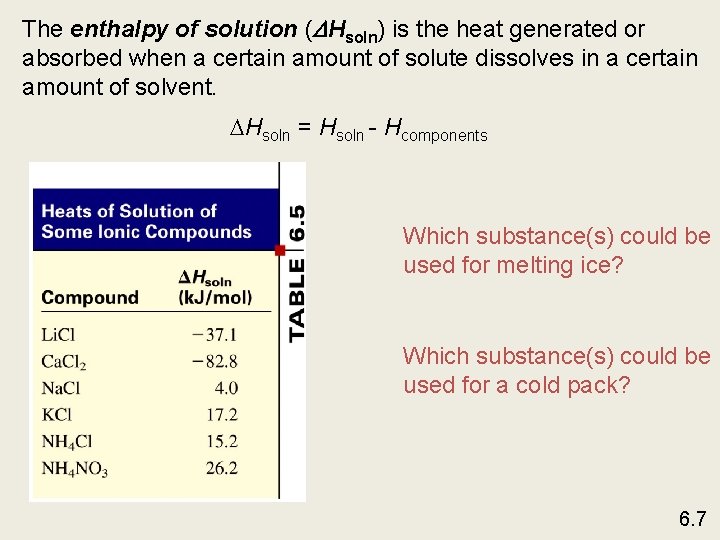
The enthalpy of solution ( Hsoln) is the heat generated or absorbed when a certain amount of solute dissolves in a certain amount of solvent. Hsoln = Hsoln - Hcomponents Which substance(s) could be used for melting ice? Which substance(s) could be used for a cold pack? 6. 7

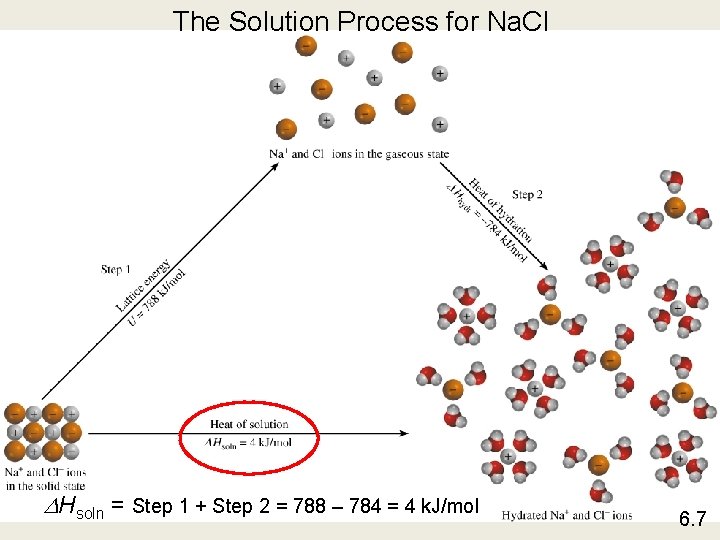
The Solution Process for Na. Cl DHsoln = Step 1 + Step 2 = 788 – 784 = 4 k. J/mol 6. 7

Chapt 17 THERMODYNAMICS AH AND AG

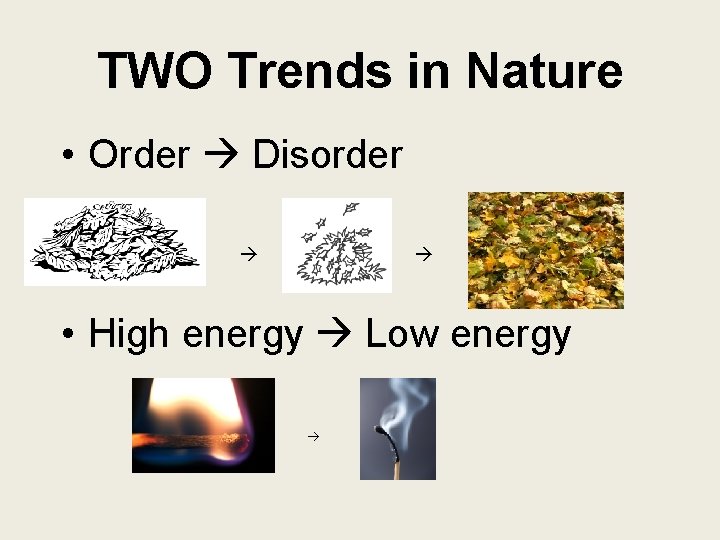
TWO Trends in Nature • Order Disorder • High energy Low energy
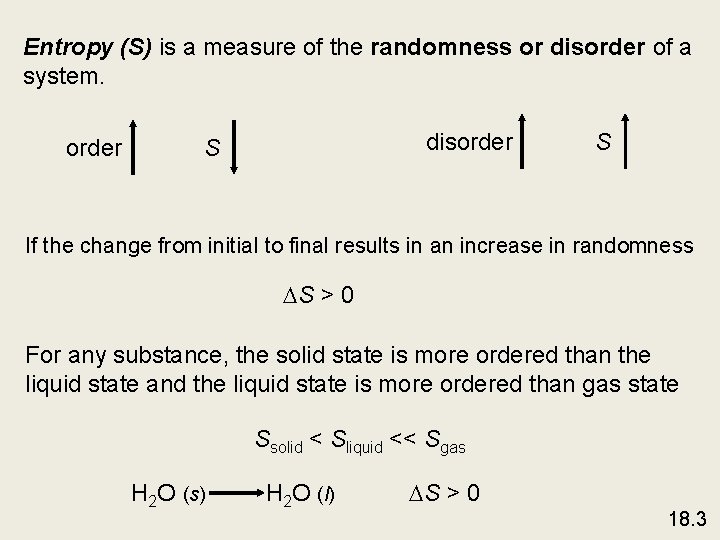
Entropy (S) is a measure of the randomness or disorder of a system. order disorder S S If the change from initial to final results in an increase in randomness S > 0 For any substance, the solid state is more ordered than the liquid state and the liquid state is more ordered than gas state Ssolid < Sliquid << Sgas H 2 O (s) H 2 O (l) S > 0 18. 3

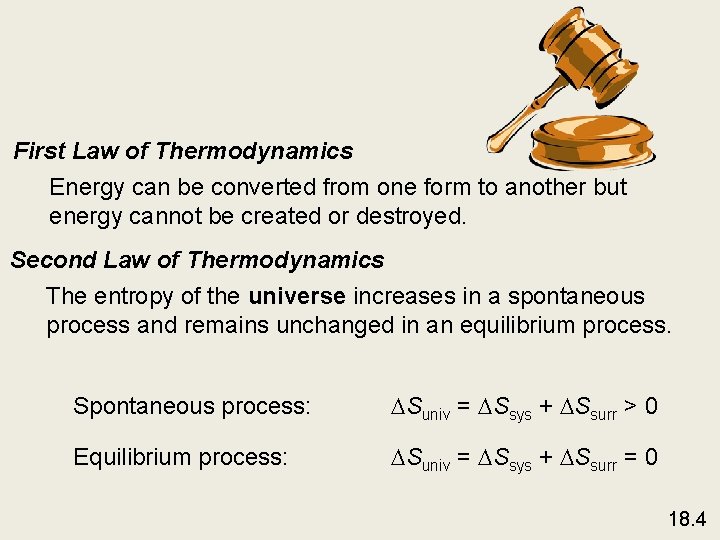
First Law of Thermodynamics Energy can be converted from one form to another but energy cannot be created or destroyed. Second Law of Thermodynamics The entropy of the universe increases in a spontaneous process and remains unchanged in an equilibrium process. Spontaneous process: Suniv = Ssys + Ssurr > 0 Equilibrium process: Suniv = Ssys + Ssurr = 0 18. 4

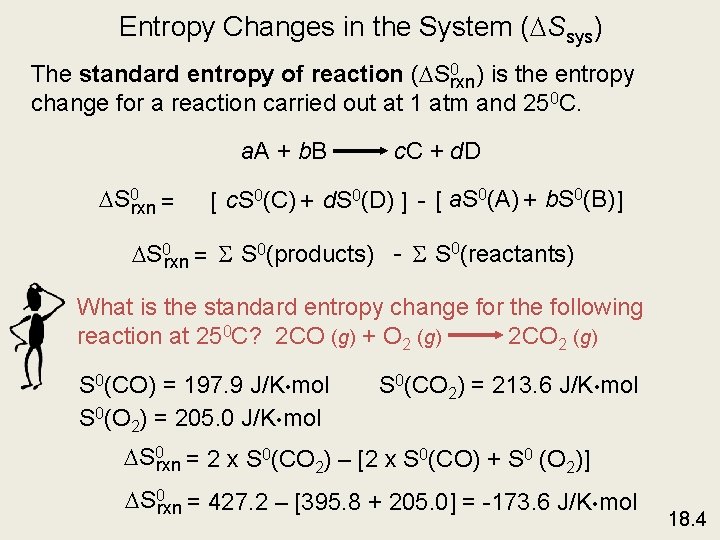
Entropy Changes in the System ( Ssys) The standard entropy of reaction ( S 0 rxn) is the entropy change for a reaction carried out at 1 atm and 250 C. a. A + b. B S 0 rxn = c. C + d. D [ c. S 0(C) + d. S 0(D) ] - [ a. S 0(A) + b. S 0(B) ] S 0 rxn = S S 0(products) - S S 0(reactants) What is the standard entropy change for the following reaction at 250 C? 2 CO (g) + O 2 (g) 2 CO 2 (g) S 0(CO) = 197. 9 J/K • mol S 0(O 2) = 205. 0 J/K • mol S 0(CO 2) = 213. 6 J/K • mol S 0 rxn = 2 x S 0(CO 2) – [2 x S 0(CO) + S 0 (O 2)] S 0 rxn = 427. 2 – [395. 8 + 205. 0] = -173. 6 J/K • mol 18. 4

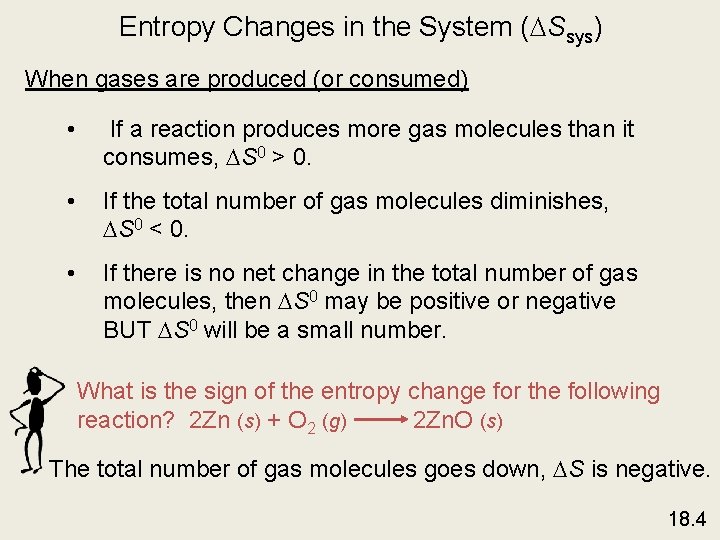
Entropy Changes in the System ( Ssys) When gases are produced (or consumed) • If a reaction produces more gas molecules than it consumes, S 0 > 0. • If the total number of gas molecules diminishes, S 0 < 0. • If there is no net change in the total number of gas molecules, then S 0 may be positive or negative BUT S 0 will be a small number. What is the sign of the entropy change for the following reaction? 2 Zn (s) + O 2 (g) 2 Zn. O (s) The total number of gas molecules goes down, S is negative. 18. 4

Spontaneous Physical and Chemical Processes • A waterfall runs downhill • A lump of sugar dissolves in a cup of coffee • At 1 atm, water freezes below 0 0 C and ice melts above 0 0 C • Heat flows from a hotter object to a colder object • A gas expands in an evacuated bulb • Iron exposed to oxygen and water forms rust spontaneous nonspontaneous 18. 2

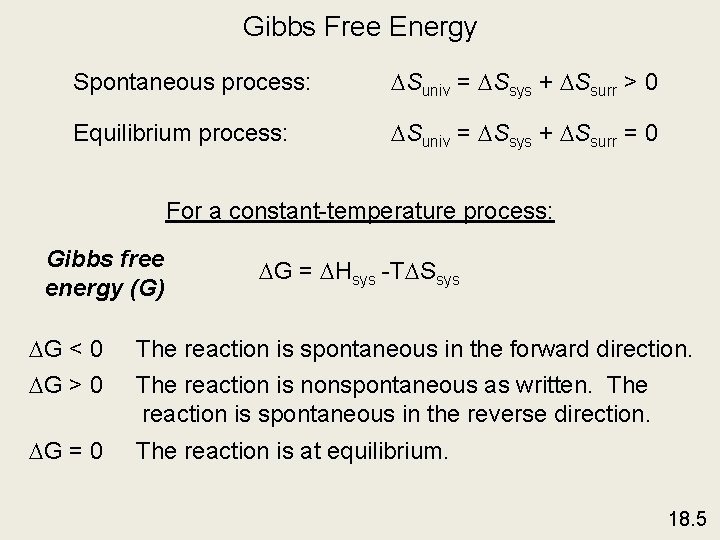
Gibbs Free Energy Spontaneous process: Suniv = Ssys + Ssurr > 0 Equilibrium process: Suniv = Ssys + Ssurr = 0 For a constant-temperature process: Gibbs free energy (G) G = Hsys -T Ssys G < 0 The reaction is spontaneous in the forward direction. G > 0 The reaction is nonspontaneous as written. The reaction is spontaneous in the reverse direction. G = 0 The reaction is at equilibrium. 18. 5

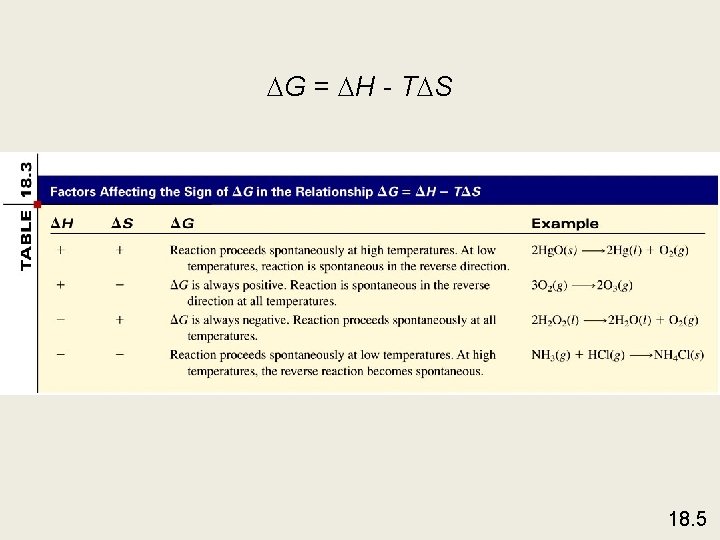
G = H - T S 18. 5

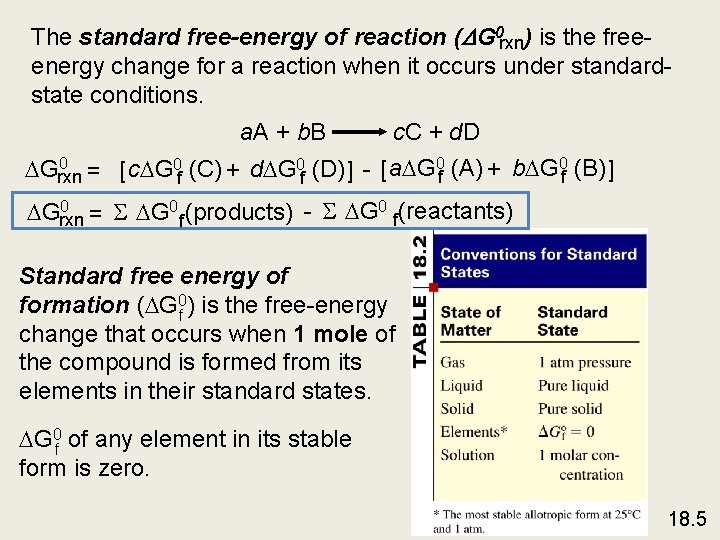
The standard free-energy of reaction ( G 0 rxn) is the freeenergy change for a reaction when it occurs under standardstate conditions. a. A + b. B c. C + d. D 0 Grxn = [ c G 0 f (C) + d G 0 f (D) ] - [ a G 0 f (A) + b G 0 f (B) ] 0 Grxn = S G 0 f (products) - S G 0 f(reactants) Standard free energy of formation ( G 0 f ) is the free-energy change that occurs when 1 mole of the compound is formed from its elements in their standard states. G 0 f of any element in its stable form is zero. 18. 5

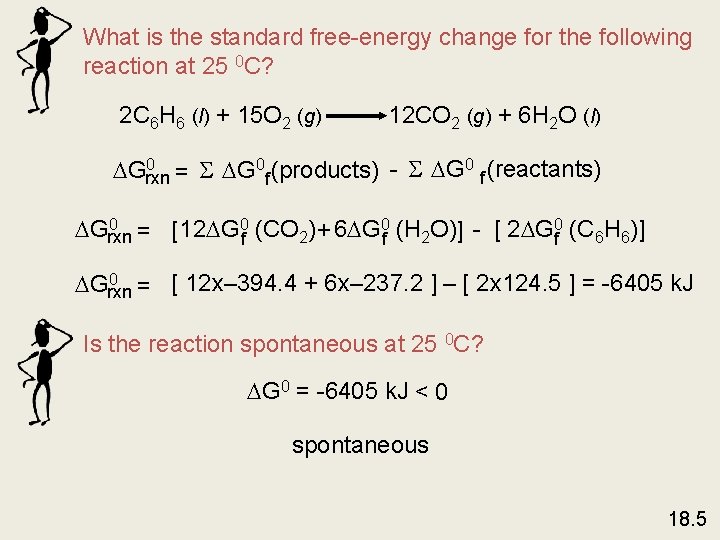
What is the standard free-energy change for the following reaction at 25 0 C? 2 C 6 H 6 (l) + 15 O 2 (g) 12 CO 2 (g) + 6 H 2 O (l) 0 Grxn = S G 0 f (products) - S G 0 f (reactants) 0 Grxn = [12 G 0 f (CO 2) + 6 G 0 f (H 2 O)] - [ 2 G 0 f (C 6 H 6)] 0 Grxn = [ 12 x– 394. 4 + 6 x– 237. 2 ] – [ 2 x 124. 5 ] = -6405 k. J Is the reaction spontaneous at 25 0 C? G 0 = -6405 k. J < 0 spontaneous 18. 5

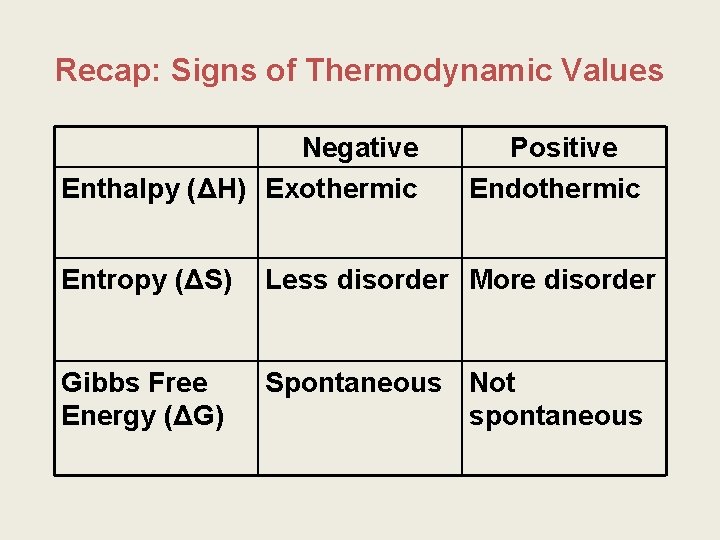
Recap: Signs of Thermodynamic Values Negative Enthalpy (ΔH) Exothermic Positive Endothermic Entropy (ΔS) Less disorder More disorder Gibbs Free Energy (ΔG) Spontaneous Not spontaneous

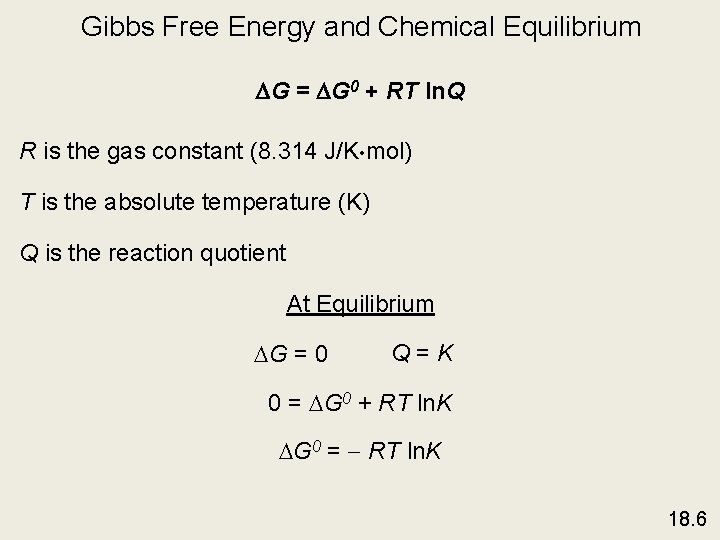
Gibbs Free Energy and Chemical Equilibrium DG = DG 0 + RT ln. Q R is the gas constant (8. 314 J/K • mol) T is the absolute temperature (K) Q is the reaction quotient At Equilibrium G = 0 Q=K 0 = G 0 + RT ln. K G 0 = - RT ln. K 18. 6

![The specific heat s most books use lower case c of a substance is The specific heat (s) [most books use lower case c] of a substance is](https://slidetodoc.com/presentation_image_h2/abeb172a6b910b76451f092c3feb5974/image-57.jpg)
The specific heat (s) [most books use lower case c] of a substance is the amount of heat (q) required to raise the temperature of one gram of the substance by one degree Celsius. The heat capacity (C) of a substance is the amount of heat (q) required to raise the temperature of a given quantity (m) of the substance by one degree Celsius. C = ms Heat (q) absorbed or released: q = ms t q = C t t = tfinal - tinitial 6. 5
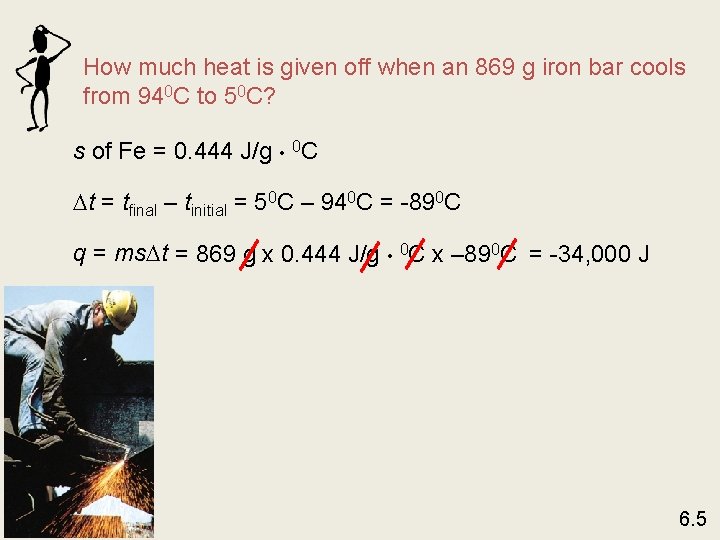
How much heat is given off when an 869 g iron bar cools from 940 C to 50 C? s of Fe = 0. 444 J/g • 0 C t = tfinal – tinitial = 50 C – 940 C = -890 C q = ms t = 869 g x 0. 444 J/g • 0 C x – 890 C = -34, 000 J 6. 5

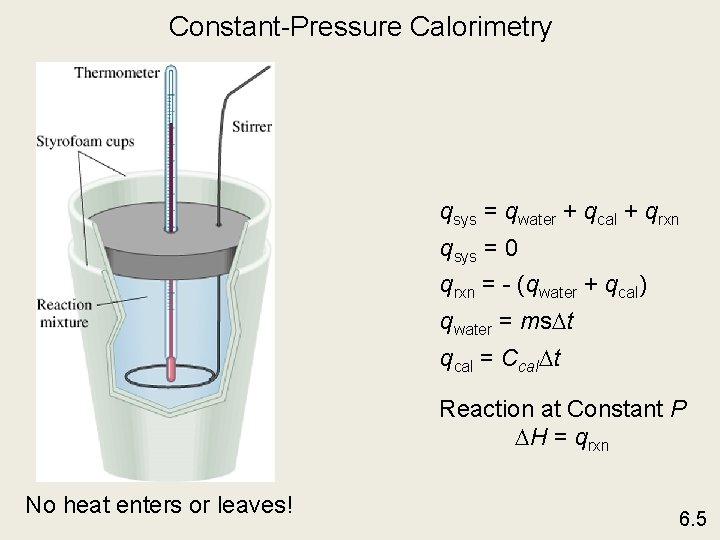
Constant-Pressure Calorimetry qsys = qwater + qcal + qrxn qsys = 0 qrxn = - (qwater + qcal) qwater = ms t qcal = Ccal t Reaction at Constant P H = qrxn No heat enters or leaves! 6. 5

6. 5

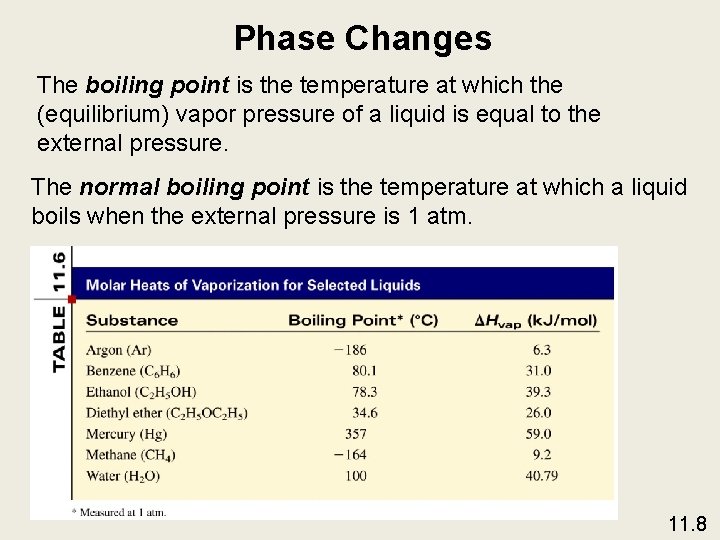
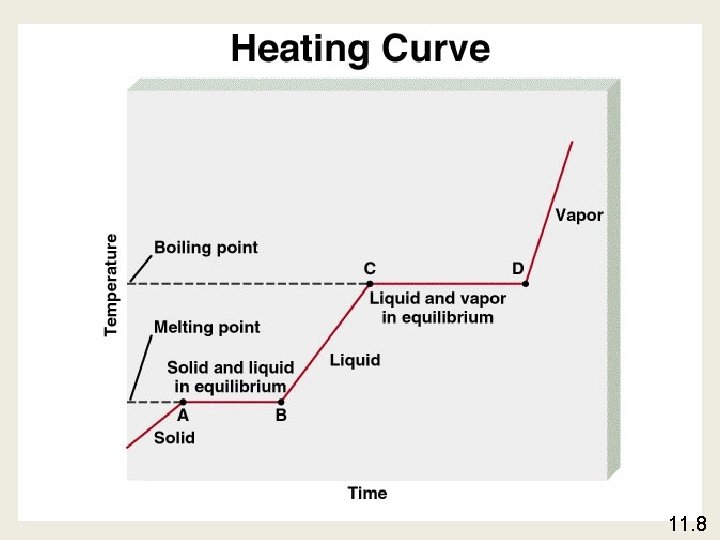
Phase Changes The boiling point is the temperature at which the (equilibrium) vapor pressure of a liquid is equal to the external pressure. The normal boiling point is the temperature at which a liquid boils when the external pressure is 1 atm. 11. 8

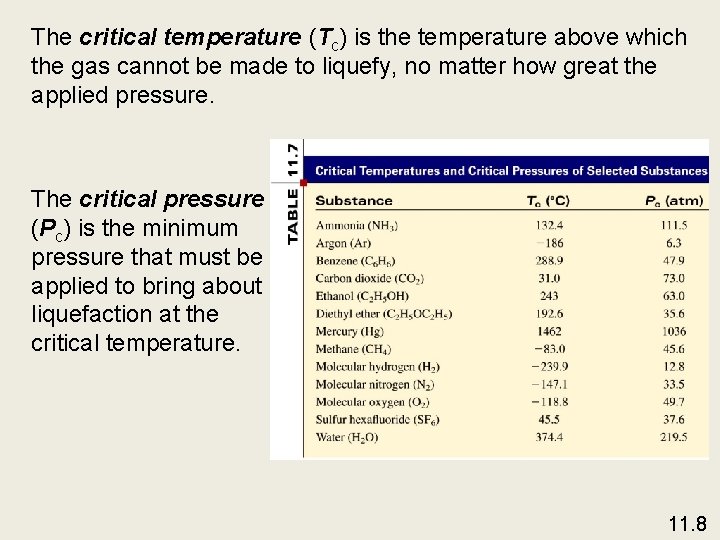
The critical temperature (Tc) is the temperature above which the gas cannot be made to liquefy, no matter how great the applied pressure. The critical pressure (Pc) is the minimum pressure that must be applied to bring about liquefaction at the critical temperature. 11. 8

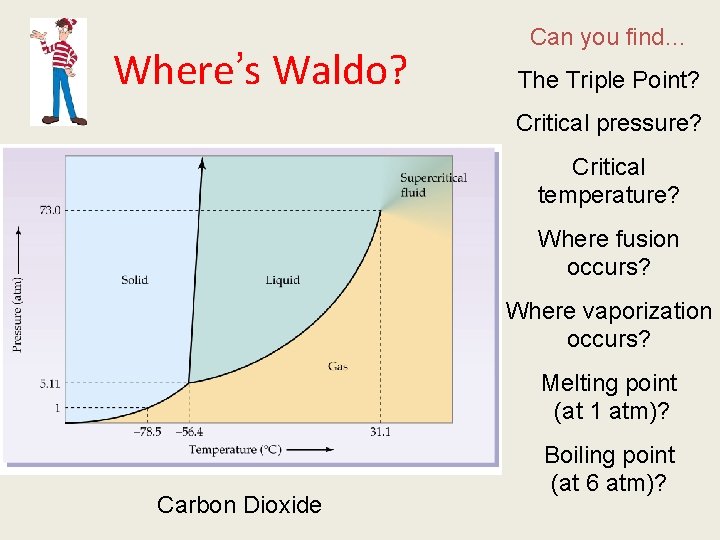
Where’s Waldo? Can you find… The Triple Point? Critical pressure? Critical temperature? Where fusion occurs? Where vaporization occurs? Melting point (at 1 atm)? Carbon Dioxide Boiling point (at 6 atm)?

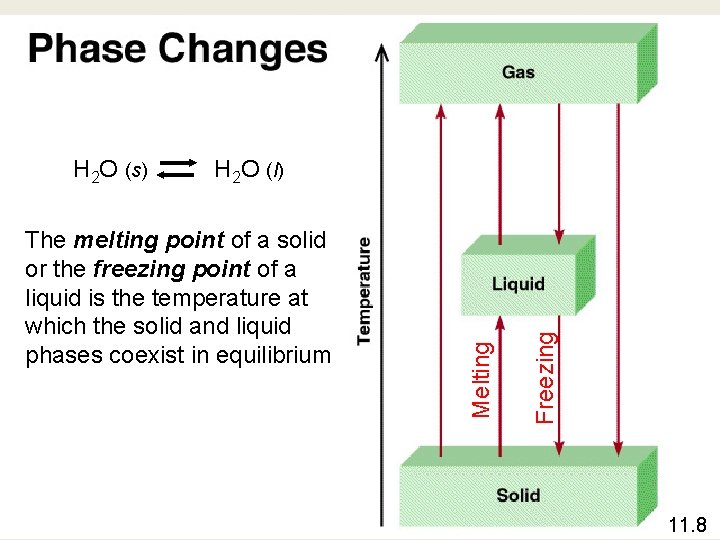
The melting point of a solid or the freezing point of a liquid is the temperature at which the solid and liquid phases coexist in equilibrium Freezing H 2 O (l) Melting H 2 O (s) 11. 8

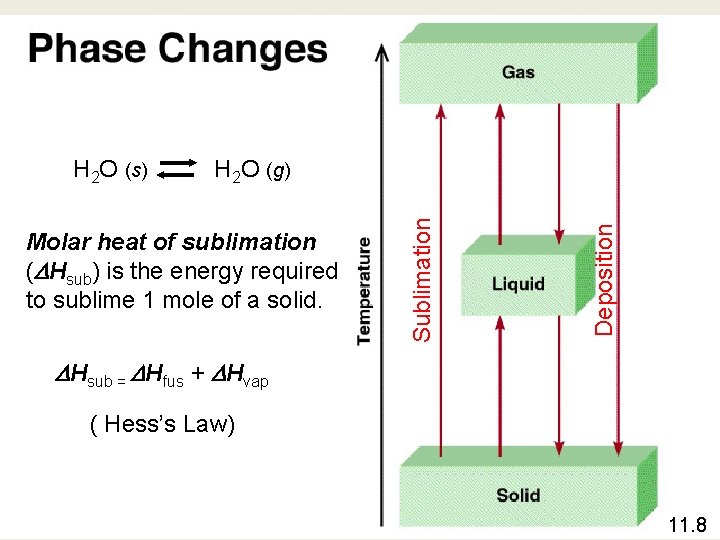
Molar heat of sublimation ( Hsub) is the energy required to sublime 1 mole of a solid. Deposition H 2 O (g) Sublimation H 2 O (s) Hsub = Hfus + Hvap ( Hess’s Law) 11. 8

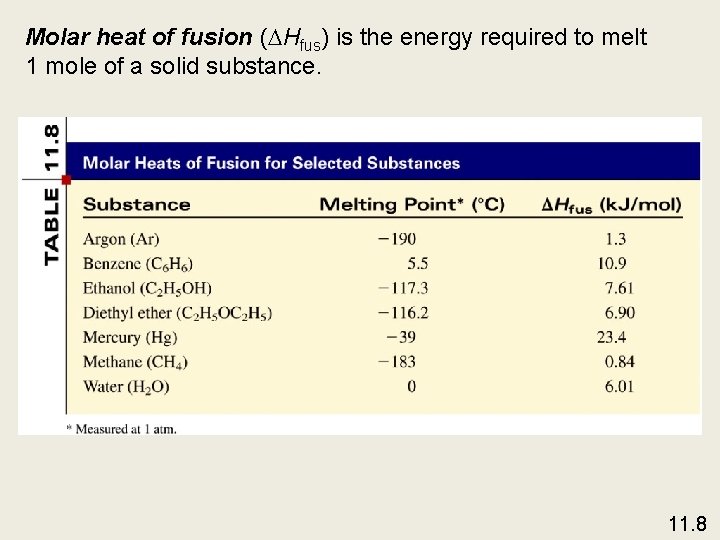
Molar heat of fusion ( Hfus) is the energy required to melt 1 mole of a solid substance. 11. 8

11. 8

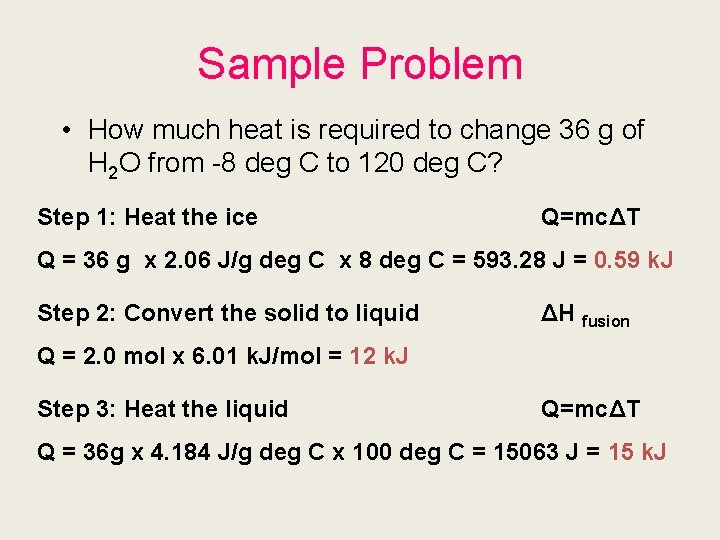
Sample Problem • How much heat is required to change 36 g of H 2 O from -8 deg C to 120 deg C? Step 1: Heat the ice Q=mcΔT Q = 36 g x 2. 06 J/g deg C x 8 deg C = 593. 28 J = 0. 59 k. J Step 2: Convert the solid to liquid ΔH fusion Q = 2. 0 mol x 6. 01 k. J/mol = 12 k. J Step 3: Heat the liquid Q=mcΔT Q = 36 g x 4. 184 J/g deg C x 100 deg C = 15063 J = 15 k. J

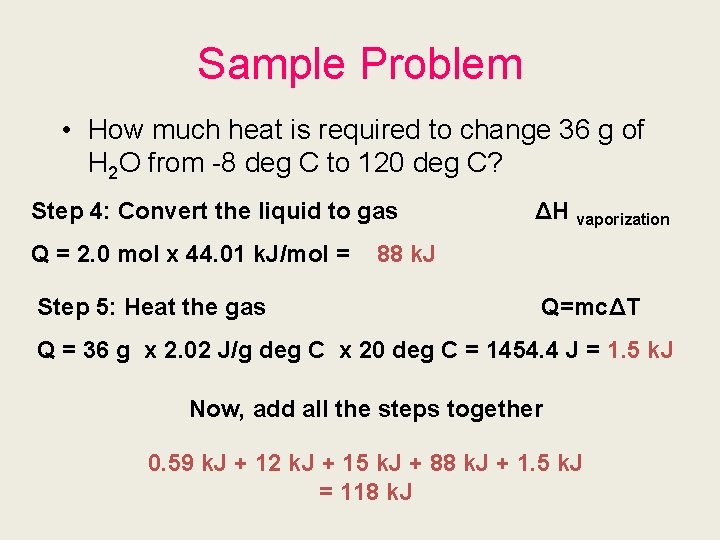
Sample Problem • How much heat is required to change 36 g of H 2 O from -8 deg C to 120 deg C? Step 4: Convert the liquid to gas Q = 2. 0 mol x 44. 01 k. J/mol = Step 5: Heat the gas ΔH vaporization 88 k. J Q=mcΔT Q = 36 g x 2. 02 J/g deg C x 20 deg C = 1454. 4 J = 1. 5 k. J Now, add all the steps together 0. 59 k. J + 12 k. J + 15 k. J + 88 k. J + 1. 5 k. J = 118 k. J
 Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Thermochemistry conversions
Thermochemistry conversions Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Glasgow thang điểm
Glasgow thang điểm Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Số nguyên tố là số gì
Số nguyên tố là số gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi To kill a mockingbird chapter 10 and 11 summary
To kill a mockingbird chapter 10 and 11 summary To kill a mockingbird questions chapter 1-4
To kill a mockingbird questions chapter 1-4 Noughts and crosses summary by chapters
Noughts and crosses summary by chapters Sparknotes gatsby chapter 3
Sparknotes gatsby chapter 3 Based on chapters 3 and 4 of the strange case
Based on chapters 3 and 4 of the strange case The children were too flabbergasted
The children were too flabbergasted Summary of chapter 11 to kill a mockingbird
Summary of chapter 11 to kill a mockingbird The scarlet letter chapters
The scarlet letter chapters Pride and prejudice chapter 18
Pride and prejudice chapter 18 Lord of the flies chapter 9-10
Lord of the flies chapter 9-10 Scarlet letter chapter 21 and 22 summary
Scarlet letter chapter 21 and 22 summary Gibbs free energy unit
Gibbs free energy unit Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of: *
Thermochemistry is the study of: * Nn
Nn Introduction to thermochemistry
Introduction to thermochemistry Gaussian thermochemistry
Gaussian thermochemistry Thermochemical equation
Thermochemical equation Thermochemistry cartoon
Thermochemistry cartoon Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Thermochemistry is concerned with the study of
Thermochemistry is concerned with the study of Thermochemistry equations
Thermochemistry equations