Thermochemistry First law of thermochemistry Internal energy of
























- Slides: 36

Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different forms such as potential energy, electrical energy, heat, work, light … The total energy of a process = sum of all the different forms of energy produced. This is equal but opposite in sign to the change in potential energy of the system prior to the process



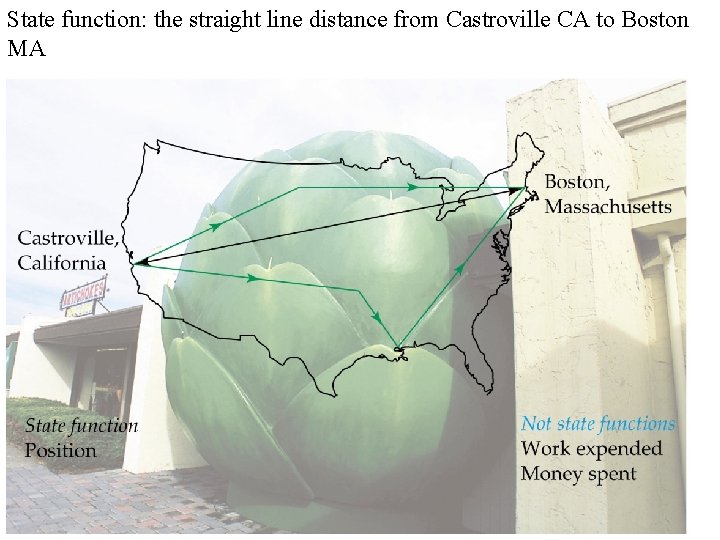
A state function is a property whose value is independent of the manner in which it is determined

State function: the straight line distance from Castroville CA to Boston MA

E = internal energy The change in internal energy of a system depends on the heat, q, given off and any work, w, that the system does. (conservation of energy) E = q + w Many processes that we perform are processes that occur in an open vessel Chemical reactions are sometimes used to do work. For example a car battery turns the crankshaft of the car in order to get it started.

Consider a piston in a car engine just before a spark ignited the gasoline air mixture. The fuel + air have some internal energy. After the spark ignites the fuel, products, CO 2 and H 2 O, also have some internal energy. In this case, the internal energy of the CO 2 and H 2 O is less than the original fuel. According to the first law, since energy can not be created or destroyed, the difference in energy between the fuel + air and the CO 2 and H 2 O, must be equal to the heat given off, q, and any work done, w. The work that is done is to cause the volume of the piston to expand. If we assume that the piston offers a constant resistance against expansion, we can relate this resistance to the pressure that must be exerted by the hot gases of combustion against the piston wall. Pressure = force/unit area therefore force = Pressure x area If the piston moves some distance d, then the work done: w = force x d = Pressure x unit area x d = P x volume change w = P V where volume change = V

The heat, q, and the work done must have the same sign since the system (fuel) has done work on its environment. Since q is negative (b definition, so the work must be negative

The change in internal energy, E = q +w If we designate the heat given off by the combustion as being negative, then the work done by the system in expanding the piston must also be negative since if there were no work done, more heat would have been given off. E = q - P V q = E + P V which is defined as H, the enthalpy of the system We use the term enthalpy, H, to describe the heat given off in any process that occurs at constant pressure, usually atmospheric pressure. H = E + P V

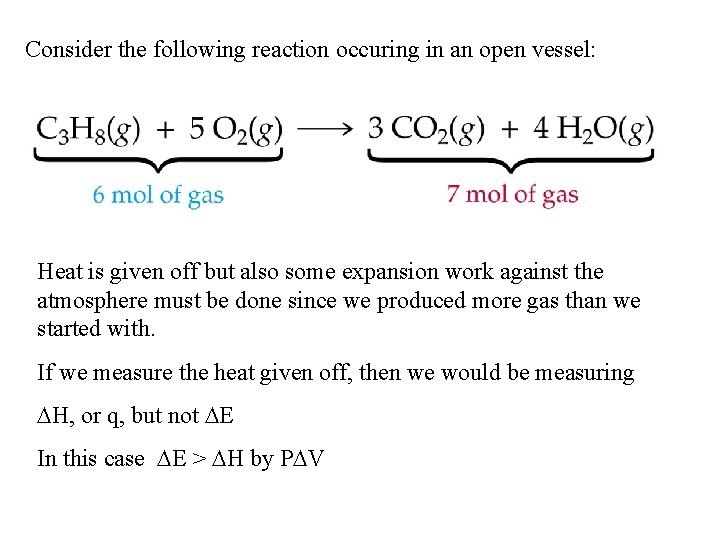
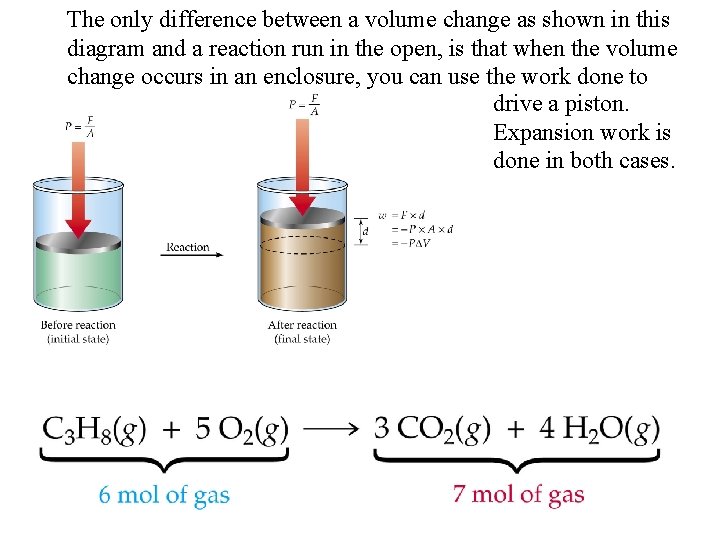
Consider the following reaction occuring in an open vessel: Heat is given off but also some expansion work against the atmosphere must be done since we produced more gas than we started with. If we measure the heat given off, then we would be measuring H, or q, but not E In this case E > H by P V

Consider the following reaction: In this case the atmosphere is doing work for us. If we measured the enthalpy of the reaction, H, or q, this quantity would be greater the E


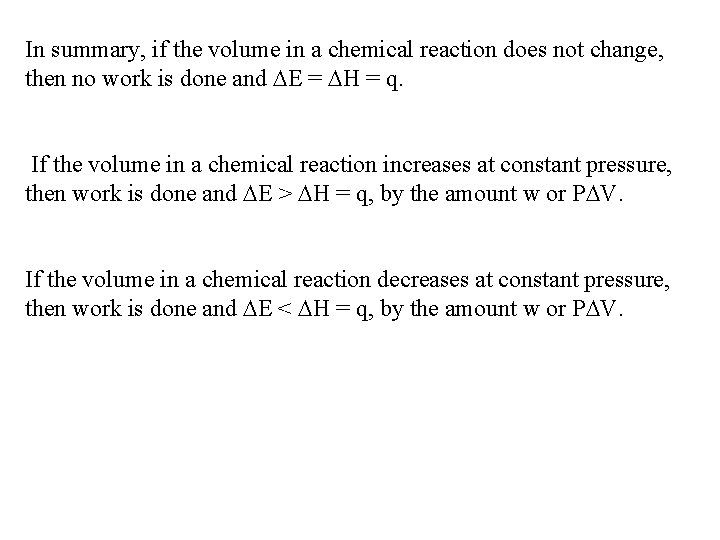
In summary, if the volume in a chemical reaction does not change, then no work is done and E = H = q. If the volume in a chemical reaction increases at constant pressure, then work is done and E > H = q, by the amount w or P V. If the volume in a chemical reaction decreases at constant pressure, then work is done and E < H = q, by the amount w or P V.

The only difference between a volume change as shown in this diagram and a reaction run in the open, is that when the volume change occurs in an enclosure, you can use the work done to drive a piston. Expansion work is done in both cases.

Heat capacity: energy necessary to raise a substance’s temperature Specific heat (spht): the amount of heat needed to raise one gram one degree centigrade. H = q = spht x m x T spht (H 2 O) = 4. 184 J/g How much heat does it take to heat 1 kg of H 2 O from 25 to 100 °C? H = 1000*4. 184*75 This is also the amount of heat that would be released into the environment when 1 kg of water at 100 °C is cooled to 25 °C.


Heat associated with phase changes QT

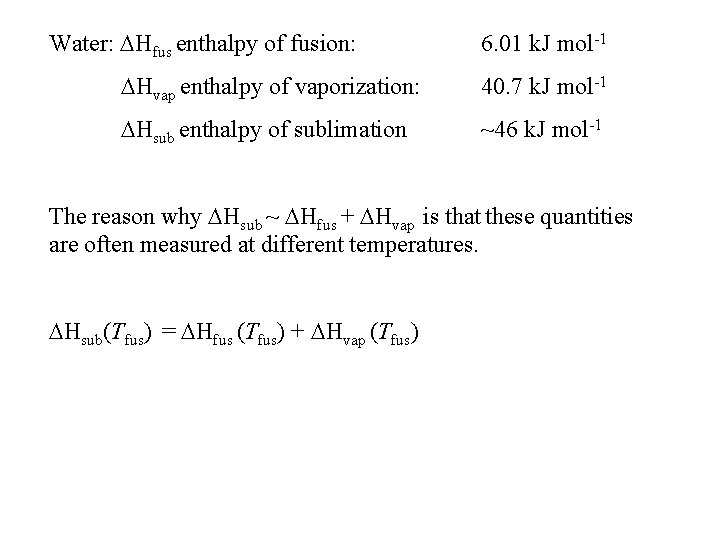
Water: Hfus enthalpy of fusion: 6. 01 k. J mol-1 Hvap enthalpy of vaporization: 40. 7 k. J mol-1 Hsub enthalpy of sublimation ~46 k. J mol-1 The reason why Hsub ~ Hfus + Hvap is that these quantities are often measured at different temperatures. Hsub(Tfus) = Hfus (Tfus) + Hvap (Tfus)

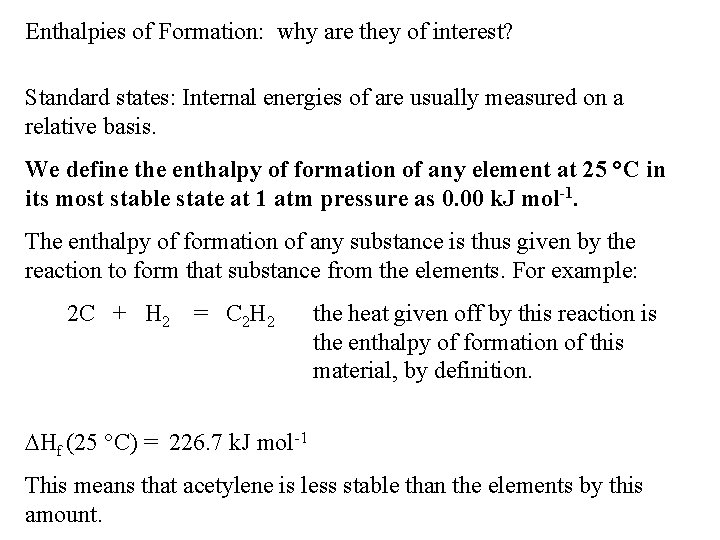
Enthalpies of Formation: why are they of interest? Standard states: Internal energies of are usually measured on a relative basis. We define the enthalpy of formation of any element at 25 °C in its most stable state at 1 atm pressure as 0. 00 k. J mol-1. The enthalpy of formation of any substance is thus given by the reaction to form that substance from the elements. For example: 2 C + H 2 = C 2 H 2 the heat given off by this reaction is the enthalpy of formation of this material, by definition. Hf (25 °C) = 226. 7 k. J mol-1 This means that acetylene is less stable than the elements by this amount.

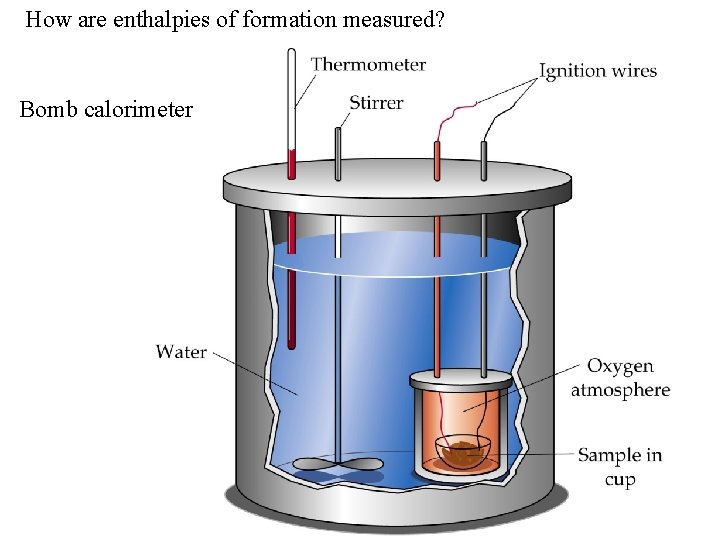
How are enthalpies of formation measured? Bomb calorimeter

C (graphite, 25 °C) + O 2 = CO 2 Hf (25 °C) = -393. 5 k. J mol-1 Hf (l, 25 °C) = -241. 8 k. J mol-1 H 2 + ½ O 2 = H 2 O Let’s calculate the heat of formation of acetylene at 25 ° C When 1 mol of C 2 H 2 is burned, the heat ( H) given off at constant pressure is – 2511 k. J mol-1 C 2 H 2 + 2. 5 O 2 = 2 CO 2 + H 2 O Hc (25 ° C) = – 2711 k. J mol-1 2 CO 2 = 2 C (graphite) + 2 O 2 = 2(393. 5) k. J mol-1 H 2 O = = 241. 8 C 2 H 2 2 C + H 2 = H 2 + ½ O 2 2 C + H 2 = C 2 H 2 - Hf (25 ° C) = - 226. 7 k. J mol-1 Hf (25 ° C) = 226. 7 k. J mol-1



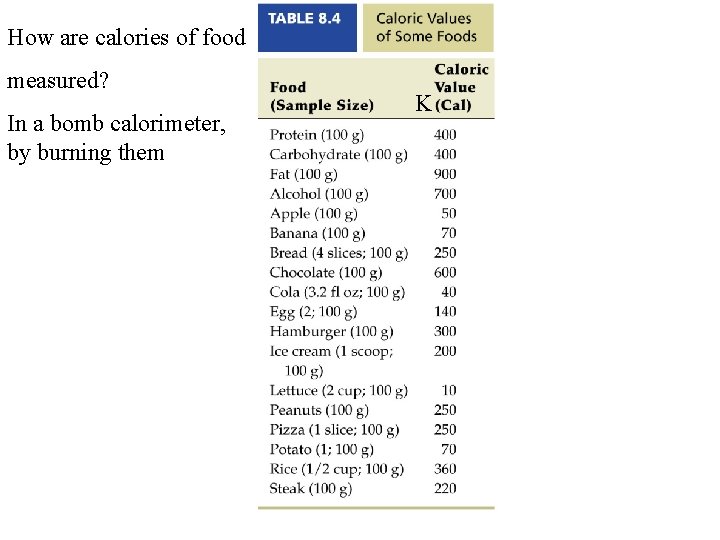
How are calories of food measured? In a bomb calorimeter, by burning them K

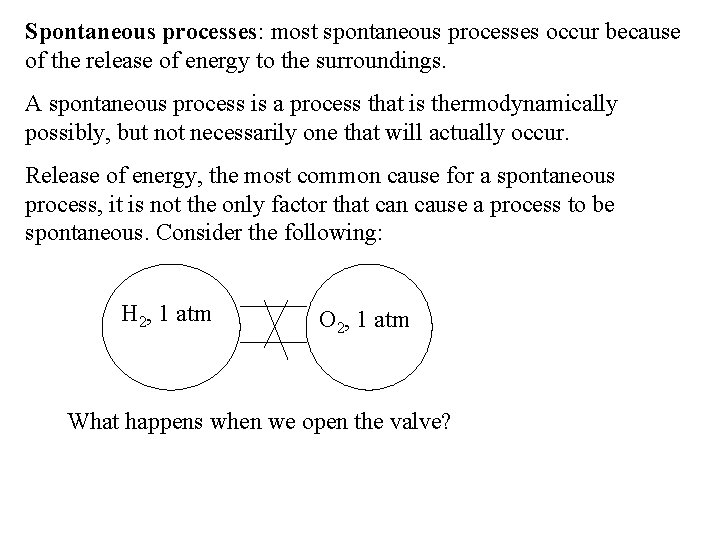
Spontaneous processes: most spontaneous processes occur because of the release of energy to the surroundings. A spontaneous process is a process that is thermodynamically possibly, but not necessarily one that will actually occur. Release of energy, the most common cause for a spontaneous process, it is not the only factor that can cause a process to be spontaneous. Consider the following: H 2, 1 atm O 2, 1 atm What happens when we open the valve?

Spontaneous processes: most spontaneous processes occur because of the release of energy to the surroundings. However, release of energy, although the most common cause for a spontaneous process, it is not the only factor that can cause a process to be spontaneous. Consider the following: H 2, 0. 5 atm O 2, 0. 5 atm Is this process spontaneous? Was any heat involved?

Entropy: S Processes that involve an increase in entropy can also be spontaneous, provided that there is not a large enthalpic barrier ( a large positive enthalpy). Entropy: is related to an increase in randomness Gibb’s free energy = G G = H - T S If G < 0 (negative) , a process is spontaneous If G > 0 (positive), the process will not occur but the reverse process is spontaneous If G = 0 the process is at equilibrium Just because a process is spontaneous, it doesn’t mean it will occur immediately (example: burning of paper)

Definition of spontaneous in a thermodynamic sense: the process can occur; not necessarily that it will occur immediately or that it will occur. G = H - T S If G < 0 (negative) , a process is spontaneous If G > 0 (positive), the process will not occur but the reverse process is spontaneous If G = 0 the process is at equilibrium (no net change occurs) High temperature favors processes with positive (increase in randomness) entropy

Factors favoring an increase in entropy: increase in the number of particles expansion of a gas phase change from solid to liquid or from liquid to gas

G = H - T S High temperatures favors formation of small molecules

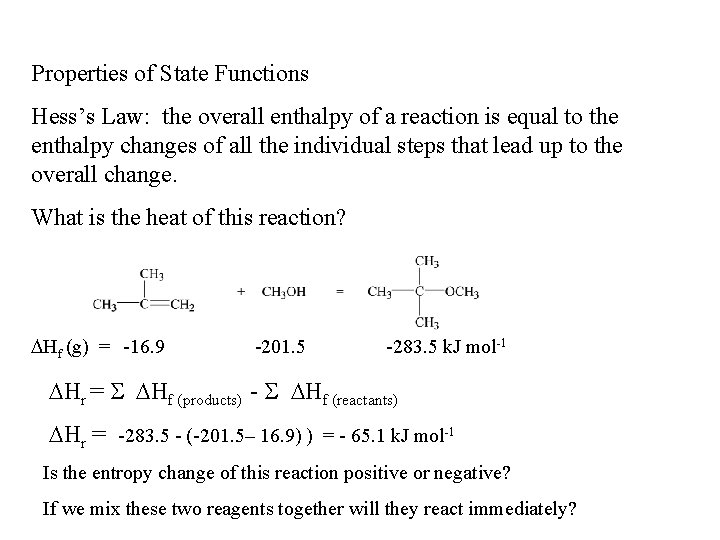
Properties of State Functions Hess’s Law: the overall enthalpy of a reaction is equal to the enthalpy changes of all the individual steps that lead up to the overall change. What is the heat of this reaction? Hf (g) = -16. 9 -201. 5 -283. 5 k. J mol-1 Hr = Hf (products) - Hf (reactants) Hr = -283. 5 - (-201. 5– 16. 9) ) = - 65. 1 k. J mol-1 Is the entropy change of this reaction positive or negative? If we mix these two reagents together will they react immediately?

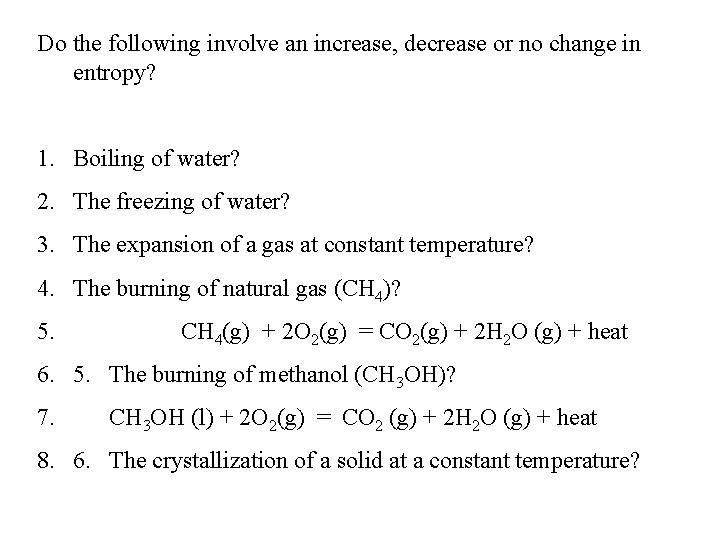
Do the following involve an increase, decrease or no change in entropy? 1. Boiling of water? 2. The freezing of water? 3. The expansion of a gas at constant temperature? 4. The burning of natural gas (CH 4)? 5. CH 4(g) + 2 O 2(g) = CO 2(g) + 2 H 2 O (g) + heat 6. 5. The burning of methanol (CH 3 OH)? 7. CH 3 OH (l) + 2 O 2(g) = CO 2 (g) + 2 H 2 O (g) + heat 8. 6. The crystallization of a solid at a constant temperature?

Which will give you a worst burn, water at 100 °C or water vapor at 100 °C?

How much energy is required to convert 100 m. L of water from the liquid at room temperature (25 ° C) to a gas at 100 °C? Heat capacity of water = 4. 184 J/g °C Vaporization enthalpy(∆Hv(373 K) =2260 J/g 1. Heat the water from 25 to 100 °C 2. 4. 184 J/g °C* (100 -25) *100 g = 31380 J 2. Vaporize the water 100 ml *1 g/m. L*2260 J/g = 226000 J 3. 31380+ 226000 = 257380 J Which will give you a worst burn, water at 100 °C or water vapor at 100 °C?

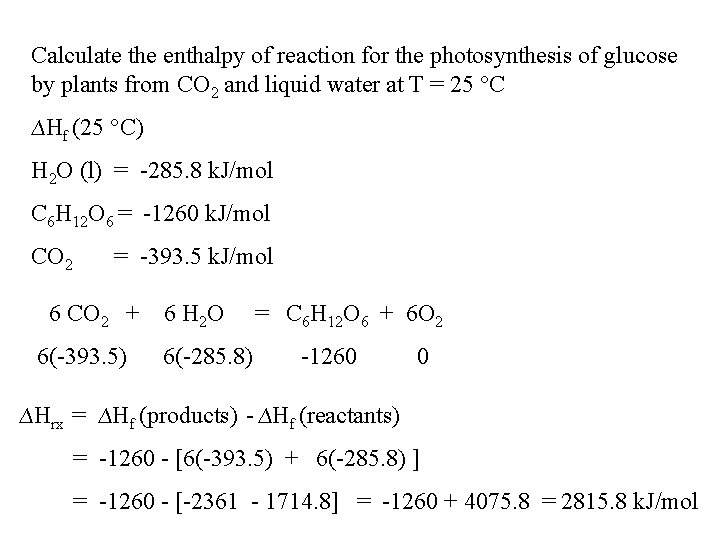
Calculate the enthalpy of reaction for the photosynthesis of glucose by plants from CO 2 and liquid water at T = 25 °C ∆Hf (25 °C) H 2 O (l) = -285. 8 k. J/mol C 6 H 12 O 6 = -1260 k. J/mol CO 2 = -393. 5 k. J/mol 6 CO 2 + 6(-393. 5) 6 H 2 O 6(-285. 8) = C 6 H 12 O 6 + 6 O 2 -1260 0 ∆Hrx = ∆Hf (products) - ∆Hf (reactants) = -1260 - [6(-393. 5) + 6(-285. 8) ] = -1260 - [-2361 - 1714. 8] = -1260 + 4075. 8 = 2815. 8 k. J/mol

Suppose we have ice and water at equilibrium, what is G ? G = 0 If the melting of ice requires +6. 01 k. J/mol, what is S for this process? G = H - T S; S = H/T; S = 6010/273 (J/mol K)
 Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law First law analysis of an internal combustion engine
First law analysis of an internal combustion engine Kirchhoff's law of thermochemistry
Kirchhoff's law of thermochemistry Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry Conservation of energy thermodynamics
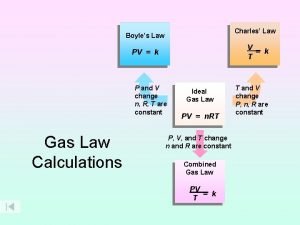
Conservation of energy thermodynamics Boyle's law charles law avogadro's law
Boyle's law charles law avogadro's law Avogadro's law constant
Avogadro's law constant Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Explain the concept of internal control
Explain the concept of internal control Features of vouching
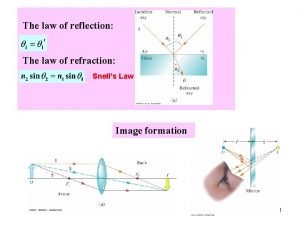
Features of vouching Total internal reflection snell's law
Total internal reflection snell's law Total internal reflection snell's law
Total internal reflection snell's law Gauss law
Gauss law Entropy equation
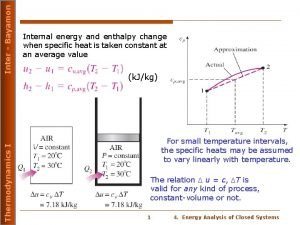
Entropy equation Constant specific heats
Constant specific heats What is internal energy
What is internal energy Thermodynamic potentials
Thermodynamic potentials The principal source of earth's internal energy is
The principal source of earth's internal energy is First law of thermodynamics
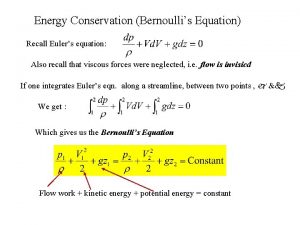
First law of thermodynamics Bernoulli equation in terms of head
Bernoulli equation in terms of head Internal energy formula
Internal energy formula Enthalpy vs internal energy
Enthalpy vs internal energy First law of thermodynamics control mass
First law of thermodynamics control mass Internal energy formula
Internal energy formula Internal energy ppt
Internal energy ppt Internal energy of matter
Internal energy of matter Internal thermal energy
Internal thermal energy Internal thermal energy
Internal thermal energy Partition function
Partition function Gibbs free energy
Gibbs free energy Heat and internal energy
Heat and internal energy Thermochemistry
Thermochemistry Chapter 17 thermochemistry practice problems answers
Chapter 17 thermochemistry practice problems answers Thermochemistry is the study of
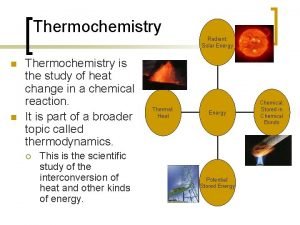
Thermochemistry is the study of