Chemistry 2402 Thermodynamics Lecture 13 Non Ideal Solutions






- Slides: 13

Chemistry 2402 - Thermodynamics Lecture 13 : Non Ideal Solutions and Activity Lecture 14 : Chemical Equilibria Lecture 15 : Kinetic Coefficients & the Transition State

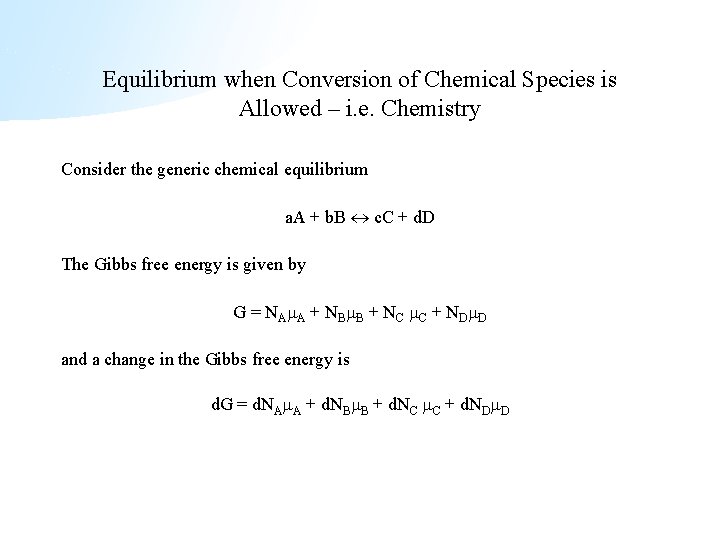
Equilibrium when Conversion of Chemical Species is Allowed – i. e. Chemistry Consider the generic chemical equilibrium a. A + b. B c. C + d. D The Gibbs free energy is given by G = NA A + NB B + NC C + ND D and a change in the Gibbs free energy is d. G = d. NA A + d. NB B + d. NC C + d. ND D

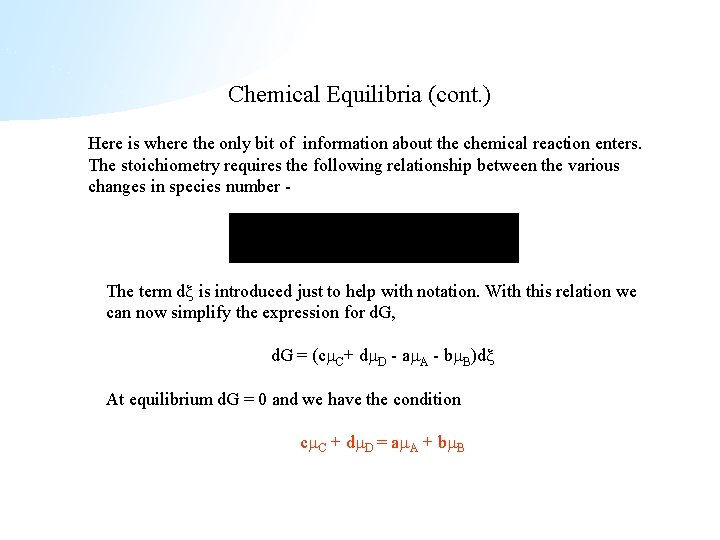
Chemical Equilibria (cont. ) Here is where the only bit of information about the chemical reaction enters. The stoichiometry requires the following relationship between the various changes in species number - The term d is introduced just to help with notation. With this relation we can now simplify the expression for d. G, d. G = (c C+ d D - a A - b B)d At equilibrium d. G = 0 and we have the condition c C + d D = a A + b B

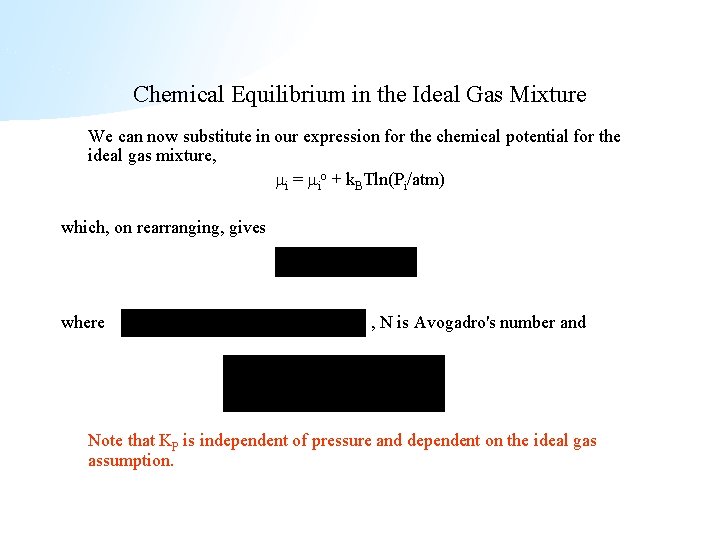
Chemical Equilibrium in the Ideal Gas Mixture We can now substitute in our expression for the chemical potential for the ideal gas mixture, i = io + k. BTln(Pi/atm) which, on rearranging, gives where , N is Avogadro's number and Note that KP is independent of pressure and dependent on the ideal gas assumption.

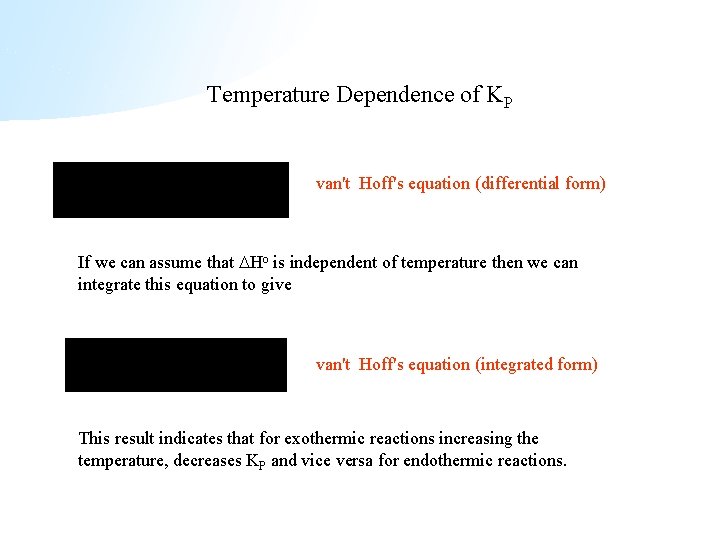
Temperature Dependence of KP van't Hoff's equation (differential form) If we can assume that Ho is independent of temperature then we can integrate this equation to give van't Hoff's equation (integrated form) This result indicates that for exothermic reactions increasing the temperature, decreases KP and vice versa for endothermic reactions.

Flash Quiz! Using the example of an exothermic reaction, explain how van’t Hoff’s equation is consistent with the predictions of Le Châtelier’s principle.

Answer Using the example of an exothermic reaction, explain how van’t Hoff’s equation is consistent with the predictions of Le Châtelier’s principle. If a change is applied to a chemical system at equilibrium; the equilibrium will shift in order to partially counteract the imposed change. • Exothermic → DHo < 0 • Therefore Kp goes down with increased temperature • ↑T → reaction to shift to cool the system (i. e. in the endothermic direction) • For an exothermic reaction, this shifts toward the reactants, so Kp goes down

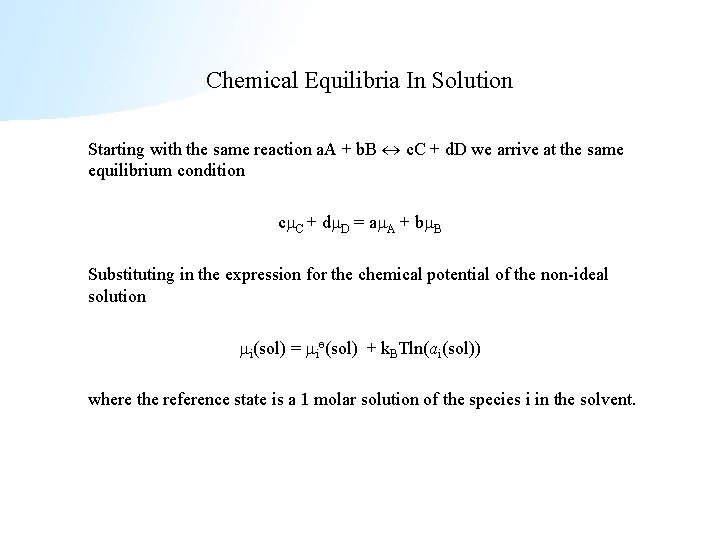
Chemical Equilibria In Solution Starting with the same reaction a. A + b. B c. C + d. D we arrive at the same equilibrium condition c C + d D = a A + b B Substituting in the expression for the chemical potential of the non-ideal solution i(sol) = iө(sol) + k. BTln(ai(sol)) where the reference state is a 1 molar solution of the species i in the solvent.

Chemical Equilibria in Solutions (cont. ) This results in the following relation ΔGө = - RTln. Ka where Gө = N(cμCө + dμDө - aμAө -bμBө) and or Note that the expression for the equilibrium constant in terms of concentrations is only valid for the ideal solution.

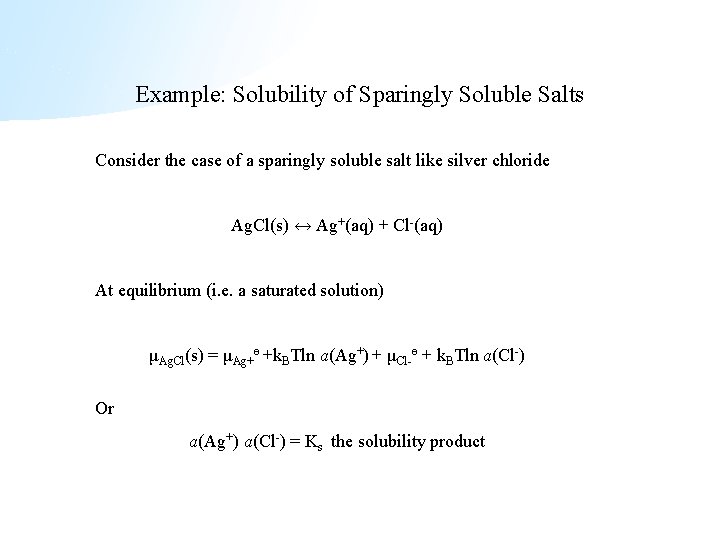
Example: Solubility of Sparingly Soluble Salts Consider the case of a sparingly soluble salt like silver chloride Ag. Cl(s) ↔ Ag+(aq) + Cl-(aq) At equilibrium (i. e. a saturated solution) μAg. Cl(s) = μAg+ө +k. BTln a(Ag+) + μCl-ө + k. BTln a(Cl-) Or a(Ag+) a(Cl-) = Ks the solubility product

Sparingly Soluble Salts (cont. ) Writing the solubility product in terms of activity coefficients gives Ks = [γ(Ag+) γ(Cl-)] x(Ag+) x(Cl-) Since the concentration of dissolved salt is low we typically find the activity coefficients are close to 1. If, however, we add extra salt with no ion in common e. g. KNO 3 then we find that the activity coefficients are less than 1. Since Ks is a constant, this means the concentration of ions must increase – i. e. the solubility increases due to the added salt.

Sample exam questions from previous years • At room temperature (25 °C), calcium carbonate has an aqueous solubility product of Ksp = 4. 47× 10‑ 9 M 2. – Calculate the calcium concentration in a saturated solution of calcium carbonate in pure water at room temperature. State any assumptions or approximations you make. – Sea water has a high concentration of aqueous Na. Cl. This reduces the activity ratio of calcium ions to 0. 405, and carbonate ions to 0. 370. Calculate the calcium concentration in a saturated solution of calcium carbonate in sea water at room temperature. • Using the example of an exothermic reaction, explain how van’t Hoff’s equation is consistent with the predictions of Le Châtelier’s principle. • The equilibrium constant for the gas phase reaction H 2 + I 2 2 HI is 45. 6 at 764 K and 60. 8 at 667 K. Assuming that the heat of reaction is constant over this temperature range, calculate the enthalpy change which accompanies one mole of the forward reaction.

Summary You should now • Understand the connection between the standard free energy of a reaction, and its equilibrium constant • Calculate one from the other • Understand the conditions under which the integrated form of Van’t Hoff’s equation is valid • Apply Van’t Hoff’s equation to calculate the change in an equilibrium constant as temperature is changed • Explain how “spectator ions” can affect the solubility of a salt in a non-ideal solution Next Lecture • Kinetic Coefficients & the Transition State
 Vapour pressure
Vapour pressure Ideal solution thermodynamics
Ideal solution thermodynamics First law of thermodynamics
First law of thermodynamics 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Law of thermodynamics in chemistry
Law of thermodynamics in chemistry Statistical thermodynamics in chemistry
Statistical thermodynamics in chemistry 11th chemistry thermodynamics lec 13
11th chemistry thermodynamics lec 13 Thermodynamics ap chemistry
Thermodynamics ap chemistry Thermodynamics ap chemistry
Thermodynamics ap chemistry 11th chemistry thermodynamics lec 10
11th chemistry thermodynamics lec 10 A first course in atmospheric thermodynamics solutions
A first course in atmospheric thermodynamics solutions Veux tu briser du péché le pouvoir parole
Veux tu briser du péché le pouvoir parole Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Lightning elves
Lightning elves