Chemistry 2402 Thermodynamics Lecture 7 Entropy Lecture 8











- Slides: 20

Chemistry 2402 - Thermodynamics Lecture 7 : Entropy Lecture 8 : Converting Heat to Work Lecture 9: Free Energies

Aim The aim of this course is to develop the general principles that govern all equilibrium states. We shall base everything on two results from the statistics of energy distribution: 1. the equilibrium state of an isolated system is that which maximises the entropy. (2 nd Law of thermodynamics) 2. d. S = q/T where q is the (reversible) heat flow into the system

REFLECTIONS ON THE MOTIVE POWER OF FIRE AND ON MACHINES FITTED TO DEVELOP THIS POWER BY S. CARNOT 1824 This image is in the public domain because its copyright has expired. http: //commons. wikimedia. org/wiki/Image: Sadi_Carnot. jpeg

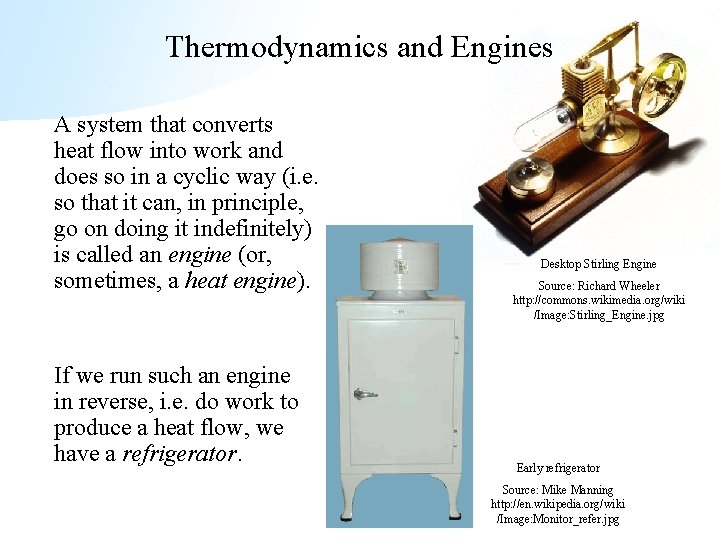
Thermodynamics and Engines A system that converts heat flow into work and does so in a cyclic way (i. e. so that it can, in principle, go on doing it indefinitely) is called an engine (or, sometimes, a heat engine). If we run such an engine in reverse, i. e. do work to produce a heat flow, we have a refrigerator. Desktop Stirling Engine Source: Richard Wheeler http: //commons. wikimedia. org/wiki /Image: Stirling_Engine. jpg Early refrigerator Source: Mike Manning http: //en. wikipedia. org/wiki /Image: Monitor_refer. jpg

The Newcomen Engine The first application of steam engines was to pump water from mines. James Watt realised that reheating the cooled cylinder wasted heat. He introduced a separate condenser - the increase in efficiency contributed to the Industrial Revolution. This image is in the public domain because its copyright has expired. http: //en. wikipedia. org/wiki/Image: Newcomen 6325. png

A Simple Engine Th A Th D B Tc Tc C A - an isothermal (i. e. constant temperature) expansion at Th against a fixed pressure PA B - cooling from Th to Tc at a fixed volume C - an isothermal compression at Tc at the new fixed pressure PC D - heating from Tc to Th at a fixed volume

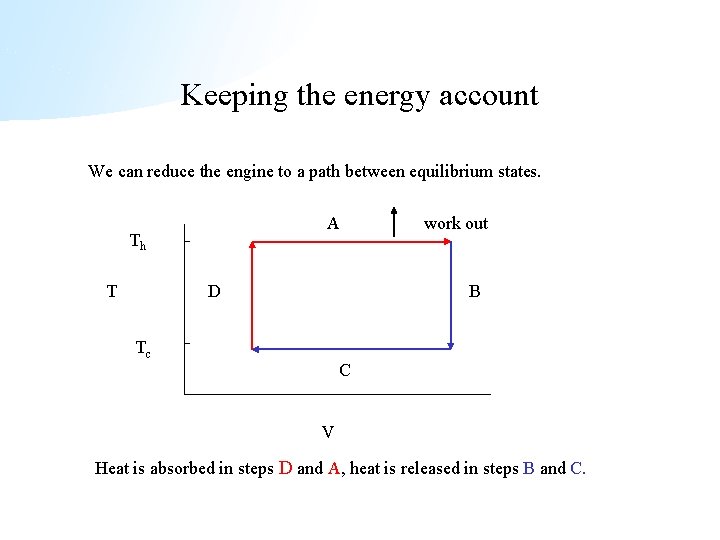
Keeping the energy account We can reduce the engine to a path between equilibrium states. A Th T D work out B Tc C V Heat is absorbed in steps D and A, heat is released in steps B and C.

Converting Heat to Work All engines can be described as follows. hot bath T h qin wout engine qout cold bath T c From conservation of energy, wout = qin –qout Why does there have to be any qout at all?

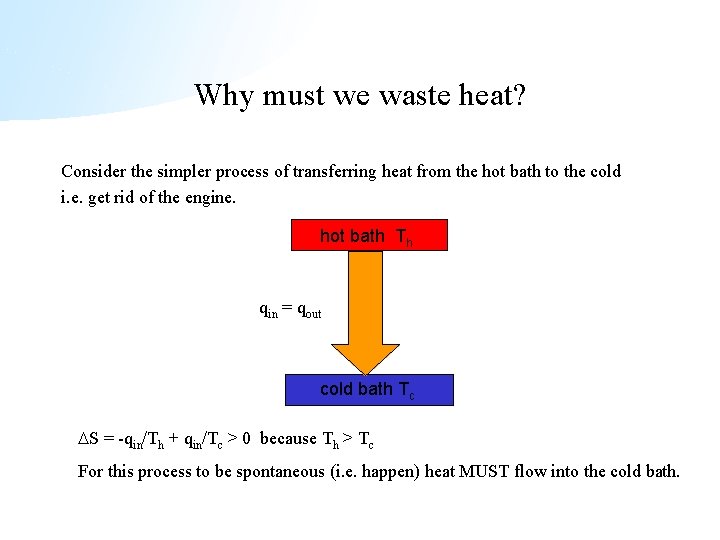
Why must we waste heat? Consider the simpler process of transferring heat from the hot bath to the cold i. e. get rid of the engine. hot bath Th qin = qout cold bath Tc S = -qin/Th + qin/Tc > 0 because Th > Tc For this process to be spontaneous (i. e. happen) heat MUST flow into the cold bath.

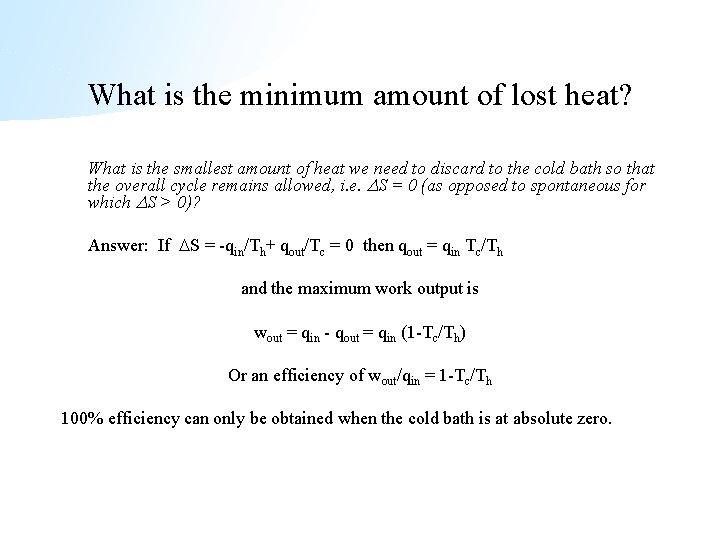
What is the minimum amount of lost heat? What is the smallest amount of heat we need to discard to the cold bath so that the overall cycle remains allowed, i. e. S = 0 (as opposed to spontaneous for which S > 0)? Answer: If S = -qin/Th+ qout/Tc = 0 then qout = qin Tc/Th and the maximum work output is wout = qin - qout = qin (1 -Tc/Th) Or an efficiency of wout/qin = 1 -Tc/Th 100% efficiency can only be obtained when the cold bath is at absolute zero.

Kelvin’s Temperature Scale In fact, the relation qin/qout = Th /Tc is only satisfied by an absolute temperature scale. This is how Kelvin settled on this scale in the first place. "heavier-than-air flying machines are impossible" Kelvin 1895 "There is nothing new to be discovered in physics now. All that remains is more and more precise measurement. " Kelvin 1900 This image is in the public domain because its copyright has expired.

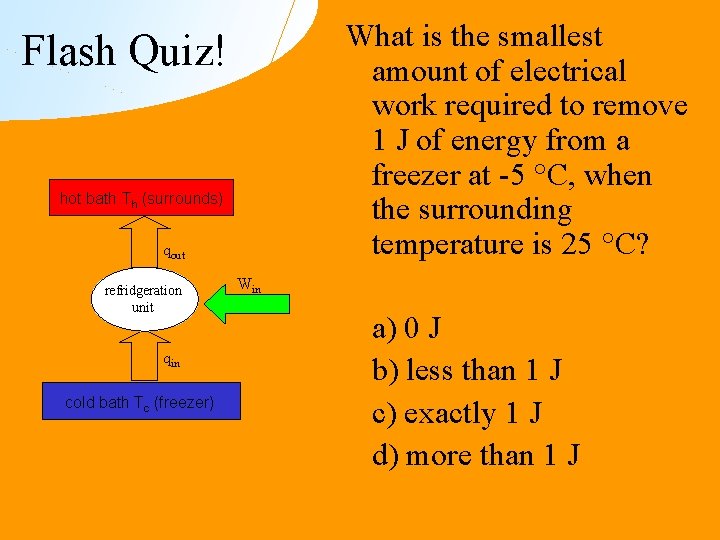
What is the smallest amount of electrical work required to remove 1 J of energy from a freezer at -5 °C, when the surrounding temperature is 25 °C? Flash Quiz! hot bath Th (surrounds) qout refridgeration unit qin cold bath Tc (freezer) Win a) 0 J b) less than 1 J c) exactly 1 J d) more than 1 J

Hints What is the smallest amount of electrical work required to remove 1 J of energy from a freezer at -5 °C, when the surrounding temperature is 25 °C? “smallest energy” → most efficient, so S = +qout/Th - qin/Tc = 0 hot bath Th (surrounds) qout refridgeration unit qin cold bath Tc (freezer) Win conservation of energy (first law) also means win + qin = qout

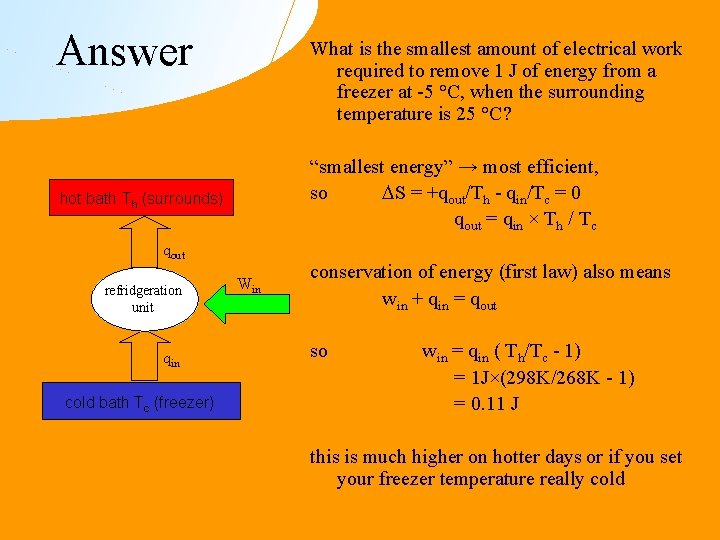
Answer What is the smallest amount of electrical work required to remove 1 J of energy from a freezer at -5 °C, when the surrounding temperature is 25 °C? “smallest energy” → most efficient, so S = +qout/Th - qin/Tc = 0 qout = qin × Th / Tc hot bath Th (surrounds) qout refridgeration unit qin cold bath Tc (freezer) Win conservation of energy (first law) also means win + qin = qout so win = qin ( Th/Tc - 1) = 1 J×(298 K/268 K - 1) = 0. 11 J this is much higher on hotter days or if you set your freezer temperature really cold

How do we make an engine that runs at maximum efficiency? Consider the work obtained from an expansion at constant temperature. Let’s hold a piston+cylinder at volume V 1 with a pressure P 1 and then drop the pressure to P 2 let it expand freely to V 2 The work obtained is the shaded area.

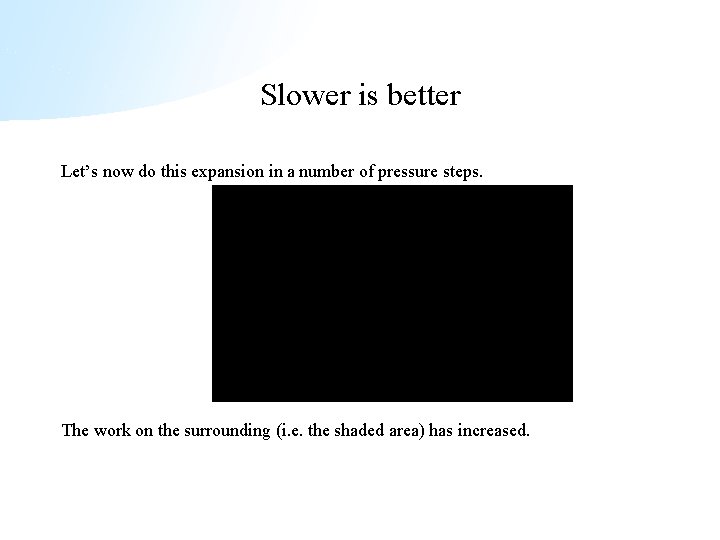
Slower is better Let’s now do this expansion in a number of pressure steps. The work on the surrounding (i. e. the shaded area) has increased.

Reversible and Irreversible Processes In the limit of infinitely many small pressure steps we find the state of the gas in the cylinder always remains very close to the equilibrium volume for the pressure and the maximum work is obtained. This infinitely gradual process is called a quasi-static or reversible process. All real processes – i. e. ones that occur in a finite time – are irreversible.

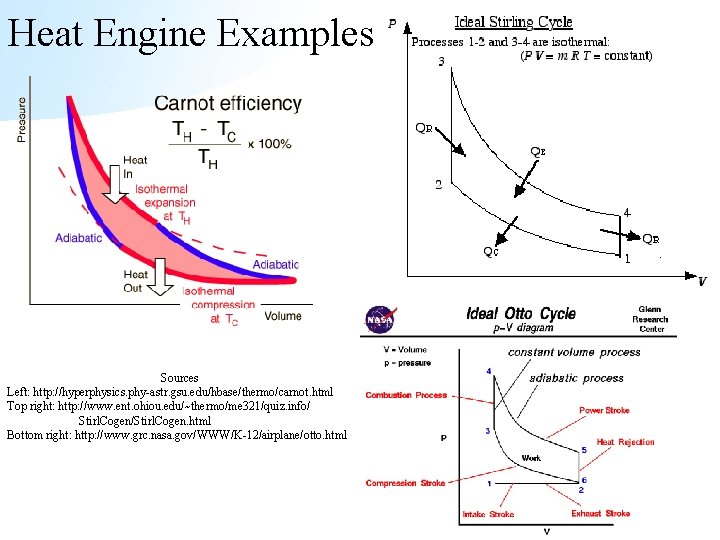
Heat Engine Examples Sources Left: http: //hyperphysics. phy-astr. gsu. edu/hbase/thermo/carnot. html Top right: http: //www. ent. ohiou. edu/~thermo/me 321/quiz. info/ Stirl. Cogen/Stirl. Cogen. html Bottom right: http: //www. grc. nasa. gov/WWW/K-12/airplane/otto. html

Sample exam questions from previous years • Provide a brief explanation of each of the following terms: – heat engine – reversible process

Summary You should now • Be able to explain the role of heat and work in an engine • Understand the significance of the Kelvin temperature scale • Be able to represent an engine by a thermodynamic cycle • Know the connection between a path on a P-V plot, and the work generated by an engine carrying out that path • Be able to calculate the maximum efficiency for an ideal engine or refrigerator • Explain thermodynamic meaning of a reversible process Next Lecture • Free energies
 Thermodynamic second law
Thermodynamic second law What is entropy in thermodynamics
What is entropy in thermodynamics Entropy in thermodynamics
Entropy in thermodynamics Ap chemistry spontaneity entropy and free energy
Ap chemistry spontaneity entropy and free energy Statistical thermodynamics in chemistry
Statistical thermodynamics in chemistry 11th chemistry thermodynamics lec 10
11th chemistry thermodynamics lec 10 11th chemistry thermodynamics lec 13
11th chemistry thermodynamics lec 13 Thermodynamics ap chemistry
Thermodynamics ap chemistry Third law of thermodynamics derivation
Third law of thermodynamics derivation Ap chemistry thermochemistry
Ap chemistry thermochemistry 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Lightning elves
Lightning elves Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Molar entropy trends
Molar entropy trends Positive gibbs
Positive gibbs Hrxn formula
Hrxn formula Law of entropy
Law of entropy Yeytex
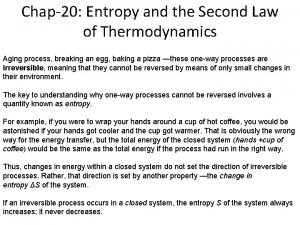
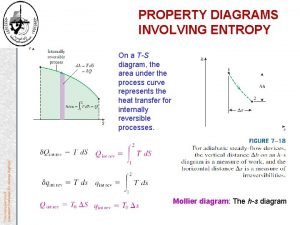
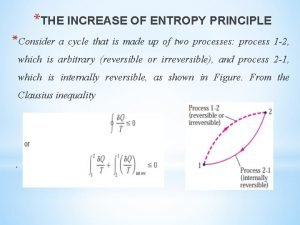
Yeytex Increase in entropy principle
Increase in entropy principle