Quote of the day Only entropy comes easy




- Slides: 14

Quote of the day: Only entropy comes easy. Anton Chekhov Announcements • If you don’t have a clicker with you, sign in after class • Chapter 19 Homework has been assigned in OWL • Exam #1: • One week from today • Covers Chapter 5, 8. 4, and 19 (up to page 9 -14) • Study guide posted next week

Spontaneity= Will a reaction happen under these conditions? A ball rolls downhill Na metal + H 2 O => Na. OH + H 2 Ice melts at 25°C (ΔH= +6. 01 k. J/mol) Water freezes at -10°C (ΔH= -6. 01 k. J/mol) Does ΔH favor spontaneity?

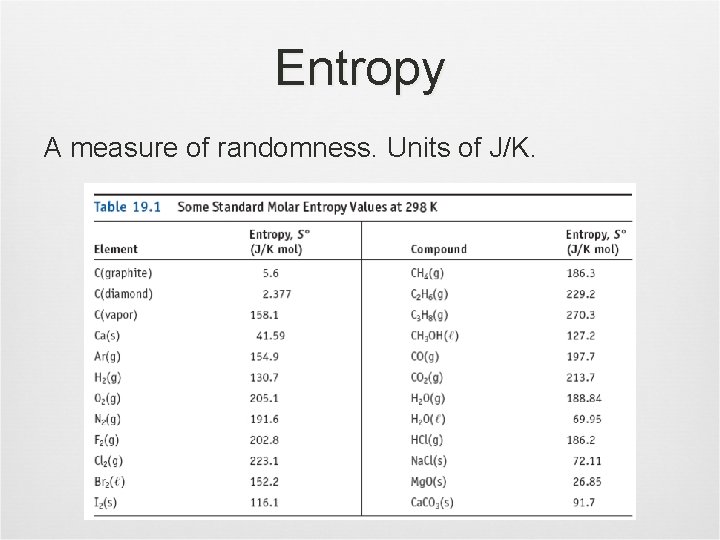
Entropy A measure of randomness. Units of J/K.

Trends in entropy Physical State Size Temperature Gas

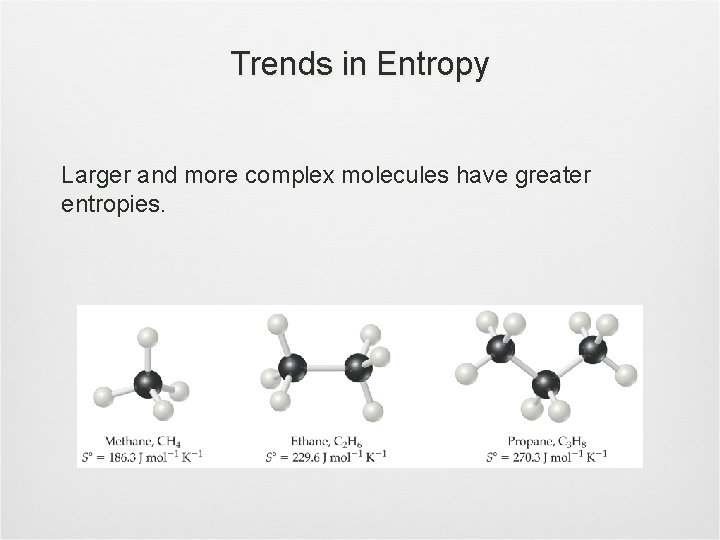
Trends in Entropy Larger and more complex molecules have greater entropies.

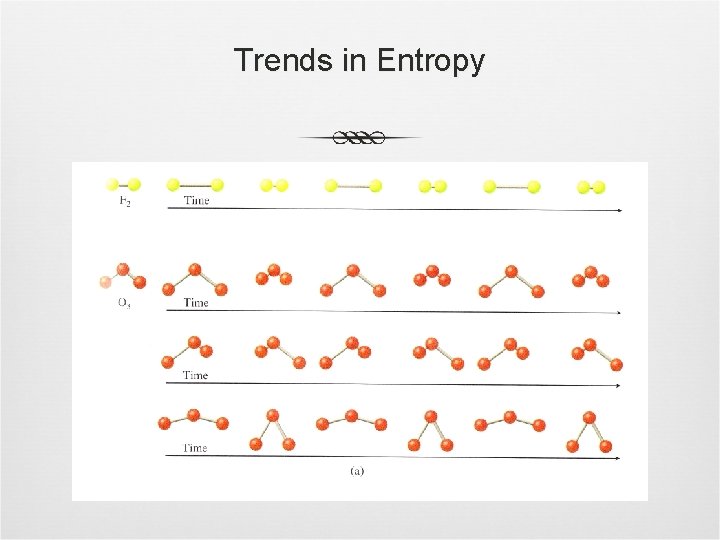
Trends in Entropy

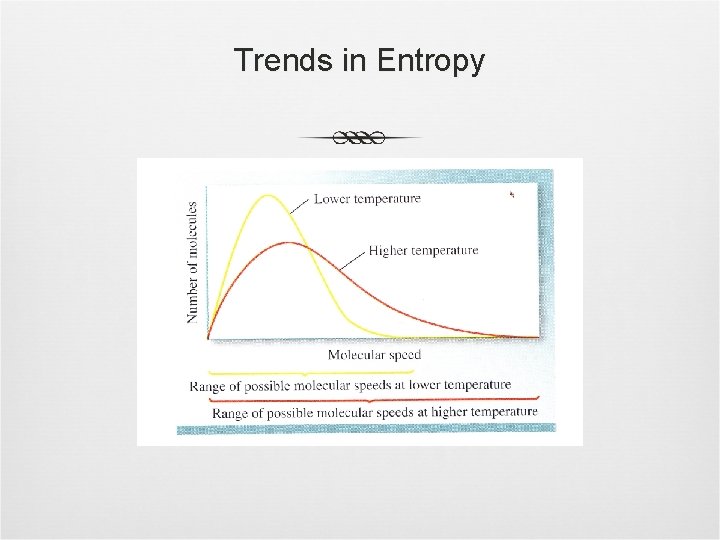
Trends in Entropy

Trends in entropy Physical State Size Temperature Gas

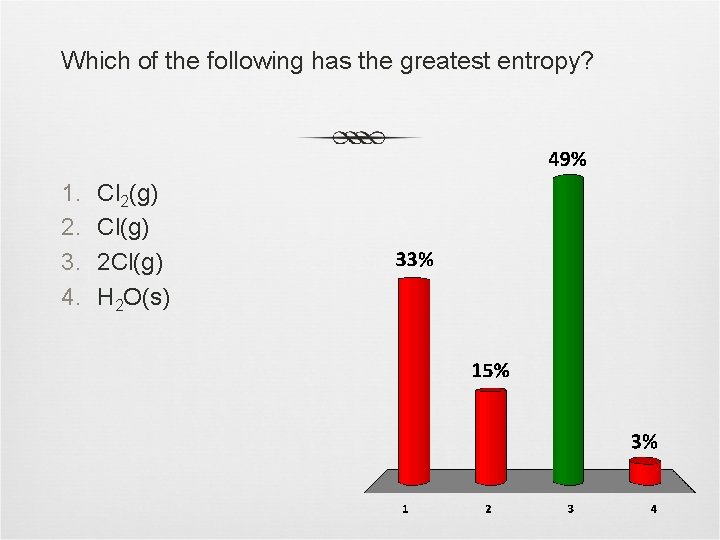
Which of the following has the greatest entropy? 1. 2. 3. 4. Cl 2(g) Cl(g) 2 Cl(g) H 2 O(s)

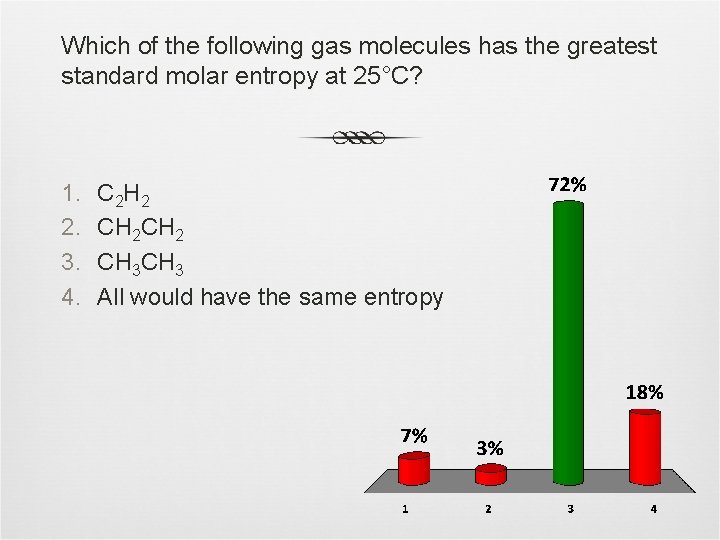
Which of the following gas molecules has the greatest standard molar entropy at 25°C? 1. 2. 3. 4. C 2 H 2 CH 3 All would have the same entropy

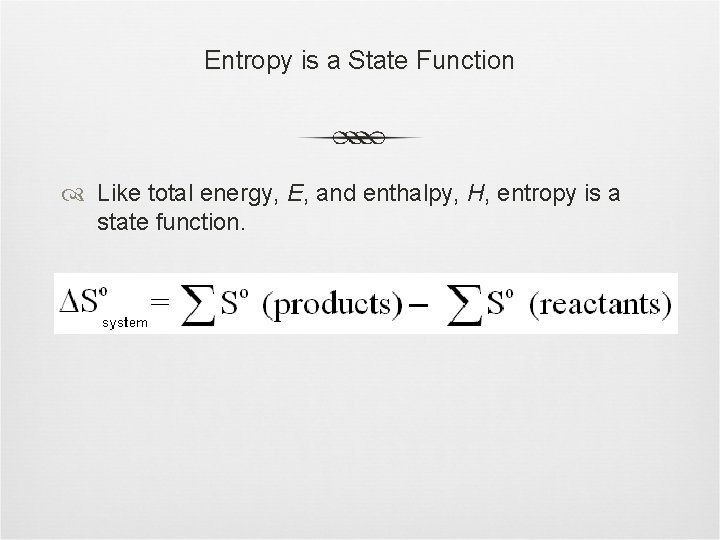
Entropy is a State Function Like total energy, E, and enthalpy, H, entropy is a state function.

Entropy and Physical States Entropy increases with the freedom of motion of molecules. Therefore, S(g) > S(l) > S(s)

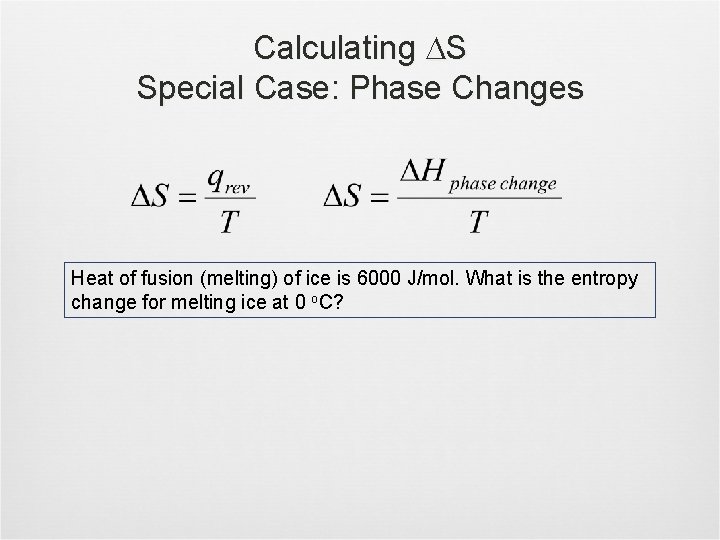
Calculating S Special Case: Phase Changes Heat of fusion (melting) of ice is 6000 J/mol. What is the entropy change for melting ice at 0 o. C?

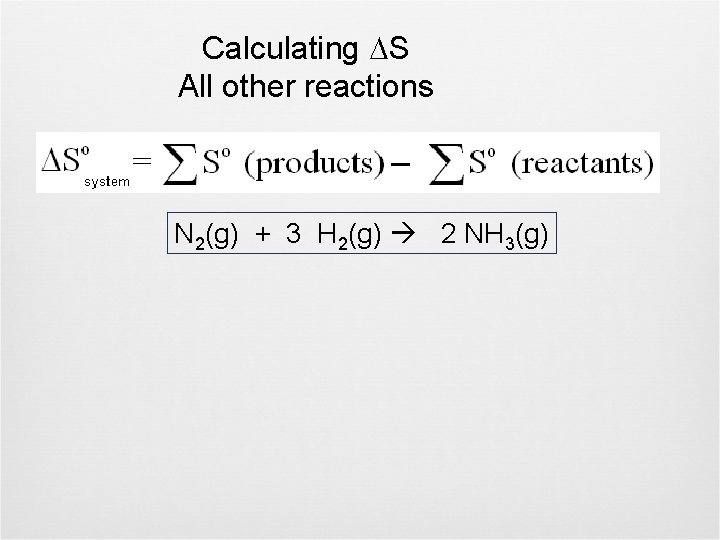
Calculating S All other reactions N 2(g) + 3 H 2(g) 2 NH 3(g)
 Only entropy comes easy
Only entropy comes easy Day 1 day 2 day 3 day 4
Day 1 day 2 day 3 day 4 Physical science chapter 6 review answers
Physical science chapter 6 review answers Day 1 day 2 day 817
Day 1 day 2 day 817 Every quiz has been easy therefore the quiz will be easy
Every quiz has been easy therefore the quiz will be easy Deductive reasoning examples
Deductive reasoning examples Deductive reasoning
Deductive reasoning Dialogue quote vs flow quote
Dialogue quote vs flow quote Integrate quotes
Integrate quotes Dancing with demons yelena garcia
Dancing with demons yelena garcia How to quote a quote apa
How to quote a quote apa Ap style quote punctuation
Ap style quote punctuation Body paragraphs example
Body paragraphs example First comes love then comes marriage
First comes love then comes marriage Steve only likes easy courses
Steve only likes easy courses